Abstract
Erythropoietin (EPO) has a neuroprotective effect and can resist aging, which most likely occur through EPO increasing the activity of antioxidant enzymes and scavenging free radicals. In this study, we verified the anti-aging function of EPO and discussed the mechanism occurring through the extracellular signal-regulated kinase (ERK)/NF-E2-related factor 2 (Nrf2)-ARE pathway. A rat model of aging was induced by the continuous subcutaneous injection of 5 % d-galactose for 6 weeks. At the beginning of the sixth week, physiological saline or EPO was administered twice per day through a lateral ventricle system for a total of 7 days. In one group, 2 μl PD98059 was administered 30 min before EPO. Learning and memory ability were analyzed with the Morris water maze system. HE staining was used to observe the morphological changes in the neurons in the hippocampus, and immunohistochemical staining as well as Western blots were carried out to detect the expression of ERK for each group of rats and the expression of phosphorylated-ERK (P-ERK), Nrf2, and superoxide dismutase (SOD). Real-Time PCR was carried out to detect the amount of Nrf2 mRNA and the KEAP1 mRNA expression. EPO can significantly improve learning and memory ability in aging rats and can provide protection against aging by improving the hippocampus morphology. Immunohistochemical staining and Western blots showed P-ERK, Nrf2, and Cu-Zn SOD decreases in aging rats compared to the normal group, while the expression for those proteins increased after EPO intervention. PD98059 inhibited the enhanced expression of P-ERK, Nrf2, and Cu-Zn SOD induced by EPO. Real-Time PCR results suggested that the trend of Nrf2mRNA expression was the same as that for the proteins, which confirmed that the enhancement occurred at the gene level. As such, EPO can significantly resist or delay aging and protect the brain by reducing oxidative stress. The most likely mechanism is that EPO can promote the ERK/Nrf2-ARE pathway in aging rats and that PD98059 can inhibit that process. These findings may facilitate further studies on the mechanism of aging and applications for the neuroprotective properties of EPO for clinical treatments.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Aging is the natural course of life but is also associated with many age-related diseases. Recent studies have found that aging is a disease that can be treated (Butler et al. 2008; Gassen et al. 2016). Many mechanisms are involved in aging. Free radical theory is one of the most important theories related to aging (Liochev 2015; Sanz 2016). The theory states that increased age leads to reduced antioxidant enzymes in the body; these enzymes lose the ability to scavenge for free radicals, which can attack normal cells and lead to oxidative damage and aging. Erythropoietin (EPO) is a cytokine used to promote erythropoiesis. Recent studies found that EPO also has neuroprotective effects (Merelli et al. 2013; Merelli et al. 2015; Sargin et al. 2010) and confirmed that EPO plays a role in anti-aging activities by increasing the activity of antioxidant enzymes and scavenging free radicals. However, the pathway associated with those activities is unclear. Nuclear factor-E2-related factor 2 (Nrf2)-ARE is an important antioxidant pathway (Buendia et al. 2016; Kume et al. 2016), and extracellular signal-regulated kinase (ERK) is an important upstream kinase for regulating the expression of Nrf2 (Costa et al. 2016). Our previous study confirmed that EPO played a role in anti-aging of the nervous system (Wu et al. 2015). In this study, we will further study the signaling pathways associated with anti-aging mechanisms.
Materials and Methods
Animal Groups and Administration
For this study, 90 SD male rats weighing 250–300 g (Experimental Animal Center of Xi'an Jiaotong University) were divided into six groups, including a normal control group (group A), a normal + EPO group (group B), an aging group (group C), an aging + EPO intervention group (group D), an aging + inhibitor PD98059 group (group E), and an aging + EPO + inhibitor PD98059 group (group F). There were 15 rats in each group. A solution of 5 % d-galactose (125 mg/kg day) was continuously injected subcutaneously for 6 weeks to establish the aging model. At the beginning of the sixth week, 10 μl physiological saline was administered to groups A and C in a lateral ventricle system. Using the same system, 10 μl EPO (100 U/rat) was administered to groups B and D. Two microliters of PD98059 (2 mmol/l) was administered to group E, and 2 μl PD98059 was administered to group F 30 min before they were administered 10 μl EPO twice per day for a total of 7 days.
Main Reagents
d-galactose was purchased from Sigma, ERK and P-ERK antibodies were purchased from CST, and the Nrf2 and Cu-Zn superoxide dismutase (SOD) antibodies came from Abcam. The PD98059 was from CST. The immunohistochemical kit and the DAB kit were from Zhong Shan.
Stereotaxic Surgery and EPO Injection
A solution of 350 mg/kg 10 % chloral hydrate was intraperitoneally administered for anesthesia. The rats were then stereotaxically implanted with a cannula in the left lateral ventricle (from bregma, 1.1 mm anteroposterior, 1.5 mm lateral, and 4.5 mm below the dura) for intracerebroventricular injection. The animals were given 7 days to recover after surgery before the injection protocol was initiated. The EPO (100 U/rat) was injected at a flow rate of 1 ml/min into the lateral ventricle over a period of 5 min twice per day for a total of 7 days. For group E, 2 μl PD98059 was administered. For group F, 2 μl PD98059 (2 mmol/l) was administered 30 min before EPO was provided.
Learning and Memory Ability Tests
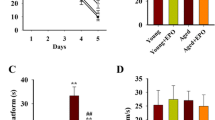
The Morris water maze test was performed to investigate the learning and memory of rats. The Morris water maze included a navigation experiment and a space-searching test. The indexes used were escape latency and platform cross times. Escape latency was the time required to find a hidden platform under the water, which was recorded in the navigation experiment. After removing the platform, the time required for the rats to swim to the quadrant where the platform was originally located was recorded as the platform cross time. The rats were placed in the pool facing the pool wall and were put into the water at four quadrants. The time required to find the platform was recorded. In the previous navigation trial, if the rats took over 120 s to swim to the platform, they were guided to the platform and allowed to stay for 10 s. The rats were trained for 5 days in a row, and each rat was trained four times per day. A 15–20-min interval was provided between training sessions. On the sixth day, every rat’s escape latency time was tested, and the platform was removed and the platform cross times were tested for 60 s. The platform was removed on the sixth day, and the platform cross times were recorded. Each rat lasted 60 s each time. After each test, the rats were wiped dry and put back into their cages.
HE and Immunohistochemical Staining
After the Morris water maze test, five rats from each group were anesthetized with 10 % chloral hydrate. The brain was irrigated with 4 % paraformaldehyde and immersed in fixative for 24–72 h. The tissues were dehydrated at varying levels of ethanol, cleared in xylene anhydrous ethanol mixture, and then immersed in wax, embedded, and sliced using conventional techniques. HE staining and immunohistochemical staining were carried out. Using the SABA method for immunohistochemical staining, the slices were dewaxed into water, microwave antigen retrieval was conducted, and the slices were incubated in 3 % hydrogen peroxide deionized water for 10 min, blocked with serum for 15 min, and incubated in a primary antibody (rabbit anti-rat P-ERK for 1:1000, rabbit anti-rat Nrf2 for 1:1000, Cu-Zn SOD for 1:200) at 4 °C for one night. The samples were incubated in a second antibody for 30 min, and horseradish enzyme mark streptomycin avid in working liquid was added for 15 min. DAB was developed for 5 min. PBS with displaced primary antibody was used as the negative control. Cells were selected that had a relatively constant expression in the middle CA1 area under a light microscope (×40), and three non-overlapping views from each slice were selected to count and average the number of P-ERK, Nrf2, and Cu-Zn SOD-positive cells.
Western Blot Analysis
For Western blots, six rats from each group were sacrificed after anesthesia. The hippocampus was collected for liquid nitrogen freezing and stored at −80 °C. The tissues were tested for MeCP2 and phosphorylated MeCP2. RIPA cracking liquid and protease inhibitors were joined to frozen hippocampus tissue and ground until the tissue cracked. After centrifugation at 12,000 rpm and 4 °C for 5 min, a spectrophotometer was used to measure protein concentrations. The SDS-PAGE loading buffer (reduction, 5×) was blended with the protein sample at a 1:4 ratio and put in a boiling water bath for 3 to 5 min. The samples were cooled to room temperature and stored at −20 °C. The proteins in the samples were separated using electrophoresis and transferred to a membrane using conventional lab techniques. They were then blocked in 5 % skim milk in TBST for 2 h and incubated with the rabbit antibody against ERK (1:1000; Cell Signaling Technology), the rabbit antibody against P-ERK (1:2000; Cell Signaling Technology), the rabbit antibody against Nrf2 (1:1000; Abcam), the rabbit antibody against Cu-Zn SOD (1:1000; Abcam), or the mouse antibody against β-actin (1:2000; Santa Cruz Biotechnology) at 4 °C for one night followed by a 1-h incubation with a secondary antibody (goat anti-rabbit immunoglobulin G [lgG], 1:10,000, goat anti-mouse lgG, 1:5000). Protein levels were analyzed in comparison to β-actin for each group by densitometry. Experiments were independently repeated three times.
RNA Extraction and Quantitative Real-Time PCR
Total mRNA was extracted from the hippocampus of rats using TRIzol reagent (Life Technologies), and complementary DNA was then reverse-transcribed from 1 μg of total RNA with a reverse transcription kit (Takara Biotechnology, China) according to the manufacturer’s instructions. PCR was performed using SYBR Green Master Mix (Takara Biotechnology, China), and GAPDH served as the internal control. The relative expression levels of Nrf2 and KEAP1 were determined using the 2−ΔΔCt method and shown as fold change compared to the controls. The primers were 5′-GAACGAGCTTCGCTGAG-3′(forward) and 5′-ATGACCTTGGGGTGGATG-3′ (reverse) for Nrf2, 5′-CACCAGGGCAGGATCTAC-3′ (forward) and 5′-TTGCTTCCGACAGGGTTC-3′ (reverse) for Keap1, and 5′-TACCCACGGCAAGTTCAACG-3′ (forward) and 5′-CACCAgCATCACCCCATTTg-3′ (reverse) for GAPDH. All primers were designed and synthesized by Sangon Biotech (Shanghai, China). The cycling conditions included one cycle at 94 °C for 5 min, 30 cycles at 94 °C for 1 min, 58 °C for 1 min, and 72 °C for 1 min, followed by one cycle at 72 °C for 10 min. The specificity of amplification was assessed via melting curve analysis and gel electrophoresis.
Statistical Analysis
All data were analyzed using SPSS (ver. 19.0) software. Measurement data are shown as the means ± SD. One-way analysis of variance (ANOVA) was used to assess statistical significance. Enumeration data were compared using a chi-square test between groups. P ≤ 0.05 was considered statistically significant.
Results
EPO Improved the Learning and Memory Abilities of Aging Rats
The water maze experiment showed no significance differences in the escape latency (EL) or platform cross times between groups A and B (P > 0.05). The EL of group B was longer, and the platform times were lower than those for group A (P < 0.05). Group D had shorter EL times and lower platform cross times than group C (P < 0.05). There were no significant differences in EL or cross-platform times between groups C and E (P > 0.05). The escape latency of groups E and F was longer than group D, and the cross-platform times were higher (P < 0.05) (Table 1).
EPO Ameliorated the Hippocampal Morphological Changes in Rats with Vascular Dementia
The hippocampus CA1 areas were observed with alight microscope. Hippocampus neurons of the normal group had no obvious pathological changes. The neurons were close, the dye was clear, and the cell shapes were regular. In the aging group, the cells were sparsely arranged, the cellular structures were unclear, and the cells were irregularly shaped. In the EPO group, the shape and structure of hippocampus neurons more closely resembled the normal group (Fig. 1).
The hippocampus CA1 area of rats observed with HE staining. a–f Normal, normal + EPO, aging, aging + EPO, aging + PD98059, and aging + EPO + PD98059 groups, respectively. The images shown are representative of three experiments. a–c For magnification ×400. d–f For magnification ×400. Bar = 100 μm in a–c. Bar = 10 μm in d, c, h
An Anti-Aging Effect of Erythropoietin Was Mediated via the ERK/Nrf2-ARE Pathway in Aging Rats
The target proteins were stained brown using immunohistochemical staining. The staining showed that P-ERK and SOD were both located in the nucleus and cytoplasm, while Nrf2 was located only in the cytoplasm. We counted the number of cells that could express the proteins P-ERK, Nrf2, and SOD. The activity of these proteins decreased in the aging group compared to the normal control group and normal + EPO group. Compared to the aging group, the activity of these proteins increased in the aging + EPO intervention group, and there was little difference between the aging + EPO intervention group and the normal group. Additionally, this difference also decreased in the aging + inhibitor PD98059 group and the aging + EPO + inhibitor PD98059 group compared to the aging + EPO intervention group and showed little difference compared to the aging group(Figs. 2, 3, and 4).
Distribution of P-ERK in immunohistochemistry experiments. a Hippocampi were stained with P-ERK after stimulation and fixation. a–f The normal, normal + EPO, aging, aging + EPO, aging + PD98059, and aging + EPO + PD98059 groups, respectively (×400). b The number of P-ERK-positive cells was counted under high magnification. The images shown are representative of three experiments. Magnification ×400. Data are the means ± SD for three independent experiments (*P < 0.05 vs. the normal group; black up-pointed triangle, P < 0.05 vs. the aging + EPO group), bar = 10 μm
Distribution of P-ERK in immunohistochemistry experiments. a Hippocampi were stained with Nrf2 after stimulation and fixation. a–f The normal, normal + EPO, aging, aging + EPO, aging + PD98059, and aging + EPO + PD98059 groups, respectively (×400). b The number of Nrf2-positive cells was counted under high magnification. The images shown are representative of three experiments. Magnification ×400. Data are the means ± SD for three independent experiments (*P < 0.05 vs. the normal group; black up-pointed triangle: P < 0.05 vs. the aging + EPO group), bar = 10 μm
Distribution of SOD in the immunohistochemistry experiments. a Hippocampi were stained with SOD after stimulation and fixation. a–f The normal, normal + EPO, aging, aging + EPO, aging + PD98059, and aging + EPO + PD98059 groups, respectively (×400). b The number of SOD-positive cells was counted under high magnification. The images shown are representative of three experiments. Magnification ×400. Data are the means ± SD for three independent experiments (*P < 0.05 vs. the normal group; black up-pointed triangle: P < 0.05 vs. the aging + EPO group), bar = 10 μm
Western blots showed that the expression of ERK changed little in each group. The expression trends of P-ERK, SOD, and Nrf2 had the same immunohistochemical results. The expressions of these proteins in the normal, normal + EPO, and the aging intervention groups were higher than the aging, aging + inhibitor PD98059, and aging + EPO + inhibitor PD98059 groups. The expression differences between groups A (normal group), B (normal + EPO group), and D (aging + EPO group) were not statistically significant, and the differences between groups C (aging group), E (aging + PD98059 group), and F (aging + EPO+ PD98059 group) were not statistically significant (Figs. 5, 6, and 7).
a Expression of T-ERK and P-ERK following erythropoietin and PD98059 detected using Western blot. The expression of P-ERK was markedly enhanced by erythropoietin and inhibited by PD98059, and the expression of T-ERK was not influenced by erythropoietin or PD98059. b The relative P-ERK protein level showed that erythropoietin enhanced the expression of P-ERK and was significantly suppressed by PD98059. c Relative T-ERK protein levels showed that erythropoietin and PD98059 had little influence. Data are presented as the means ± SEM for three independent experiments (*P < 0.05 vs. the normal group; black up-pointed triangle: P < 0.05 vs. the aging + EPO group)
a Expression of nucleus Nrf2 and total Nrf2 following erythropoietin and PD98059 detected using Western blot. The expressions of nucleus Nrf2 and total Nrf2 were markedly enhanced by erythropoietin and inhibited by PD98059. b, c Relative nucleus Nrf2 and total Nrf2 protein levels were enhanced by erythropoietin, and the enhanced expression of nucleus Nrf2 and total Nrf2 was significantly suppressed by PD98059. Data are presented as the means ± SEM for three independent experiments (*P < 0.05 vs. the normal group; black up-pointed triangle, P < 0.05 vs. the aging + EPO group)
a Expression of SOD following erythropoietin and PD98059 treatment detected using Western blot. Expression of SOD was markedly enhanced by erythropoietin and inhibited by PD98059. b The relative nucleus SOD protein level was significantly enhanced by erythropoietin and suppressed by PD98059. Data are presented as the means ± SEM for three independent experiments (*P < 0.05 vs. the normal group; black up-pointed triangle: P < 0.05 vs. the aging + EPO group)
The real-time PCR results showing a trend in Nrf2mRNA expression changes were the same as those for immunohistochemical staining and Western blot. These results showed that at the gene level, EPO increased the expression of Nrf2 in antioxidant pathways and lowered the expression of the negative regulation factor KEAP1. Additionally, EPO may play a role in nerve protection through the ERK/Nrf2 pathway (Fig. 8).
Expression of Nrf2 and KEAP1 following stimulation. a, b After stimulation, total RNA was extracted, and the relative expression of Nrf2 and KEAP1 was determined using quantitative real-time PCR. Relative mRNA expression was calculated using the 2−ΔΔCt method and shown as the percent change from the controls. Data are the means ± SEM for three independent experiments (*P < 0.05 vs. normal group; black up-pointed triangle, P < 0.05 vs. the aging + EPO group). c, d Melting curve and amplification curve of KEAP1. e, f Melting curve and amplification curve of Nrf2. g, h Melting curve and amplification curve of NADPH
Discussion
Aging-related diseases have recently attracted substantial attention (Bundy and He 2016; Chetelat et al. 2016; Engel 2016), and directly treating aging may represent a new method to address several issues. The anti-aging effects of EPO were herein demonstrated for the first time. After continuous intracerebroventricular injection of EPO for 1 week, the escape latency period of rats in the Morris water maze experiment decreased and the time required to cross the platform increased in the aging group; the differences were statistically significant (P < 0.05), which indicated that the learning and memory ability of aging rats notably improved. The pathology signs of HE staining had the same results.
Oxidative stress, which refers to an excessive generation of reactive oxygen species (ROS), plays a key role in the process of aging (Koltover 2016). Nrf2 is one of the critical regulators of endogenous antioxidant defense, which promote the transcription of a wide variety of antioxidant genes (Lu et al. 2016). Nrf2 contains six well-conserved Nrf2-ECH homologous (Neh) domains that support molecular functions. The cap “n” collar (CNC) and DNA binding regions are located in the Neh1 domain, as is the Maf dimerization site. Neh4 and Neh5 are necessary for recruitment of transcription factors and other canonical proteins required for gene expression (Zhang 2006). Emerging evidence has demonstrated that activation of Nrf2 and its target genes may protect the brain, and therapies aimed at increasing Nrf2 activity appear to be beneficial to alleviate brain injury in aging through the suppression of oxidative stress (Herrera-Arozamena et al. 2016).
Based on the theory, this study discussed the mechanism of the protective effects of EPO. Previous studies (Li et al. 2015) found that EPO can enhance the ability of antioxidant enzymes by removing excess free radicals (Khalil et al. 2016); however, the specific mechanism is unclear. Studies (Jin et al. 2011; Koskenkorva-Frank et al. 2013) showed that the KEAP1-Nrf2 pathway is one of the key pathways for EPO to enhance the ability of antioxidant enzymes in an ischemic hypoxic cell model. Using molecular biology methods and a SH SY5Y cell model (Genc et al. 2010), researchers observed the expression of Nrf2 and HO-1 after EPO intervention. EPO regulated the Nrf2 pathway, which led to an enhanced expression of HO-1 and increased antioxidant capacity. Further applications of PI3K and MAPK inhibitors show that EPO regulation of the Nrf2 pathway was closely related to PI3K and MAPK. Thus, EPO can be assumed to play a role in anti-aging by regulating the Nrf2 pathway.
Western blots and immunohistochemistry showed decreased P-ERK, Nrf2, and Cu-Zn SOD protein expression in aging rats. After cell senescence, material and energy metabolism in the cells declined, organelles experienced dysfunction, and cell receptors had reduced activity. While under oxidative stress, the cells could not produce sufficient signal transduction molecules for the signal transduction pathway to effectively cope with the stimulation. The EPO expressions in a senile rat’s cerebral cortex hippocampus CA1 pyramidal cell layer and the dentate gyrus granular layer were significantly reduced compared to those in adult rats (Juul 2012; Juul and Pet 2015; Morishita et al. 1997), which showed that aging may reduce the content of many antioxidants in the body. This could lead to a lack of activation of downstream molecules that play a protective role. After EPO interference, Nrf2 and SOD expression increased, which may prompt EPO to activate Nrf2 and SOD for anti-aging effects. P-ERK expression also relatively increased, which may have prompted P-ERK to activate upstream molecules for the activation of Nrf2. Following PD98059 administration (the specific MEK inhibiting the phosphorylation of ERK activation), downstream Nrf2 molecule activation was reduced, as was the nuclear transfer of Nrf2. This ultimately reduced antioxidant enzyme SOD expression. From the above results, we speculate that the anti-aging mechanism of EPO maybe its combination with an EPO receptor to phosphorylate the downstream molecule JAK-2 (Digicaylioglu and Lipton 2001), which activates the ERK pathway (Kilic et al. 2005). P-ERK, an Nrf2 molecular activator, can promote Nrf2 dissociation from the Nrf2/KEAP1 polymer. Thus, Nrf2 can be transported to the nucleus to become active Nrf2 and then combine with the MafG protein into Nrf2/MafG dimers (Katsuoka and Yamamoto 2016). The Nrf2/MafG dimers can combine with ARE (Katsuoka et al. 2005) and then activate the transcription and expression of target genes to augment several antioxidant enzyme expressions and play a role in the antioxidant effect. Nrf2mRNA and KEAP1mRNA were measured using RT-PCR, and the change in Nrf2mRNA had the same trend as Nrf2 protein expression, which decreased in the aging group compared to the normal group and increased in the EPO intervention group compared to the aging group. After using an inhibitor, Nrf2mRNA decreased compared to that in the EPO group. In contrast, the KEAP1 gene expression in the EPO intervention group decreased compared to the aging group. These results confirmed the effects of EPO on the Nrf2/KEAP1 pathway at the gene level.
This study included a normal + EPO group to determine whether EPO plays a role in oxidation resistance in normal rats. After EPO intervention, the learning and memory abilities of rats as well as their hippocampus morphology had few differences from the normal control group. This lack of difference may have occurred because the EPO receptors in the rat hippocampus of the normal group were fixed despite the lack of antioxidant pathway enhancement following the addition of exogenous EPO. Thus, the expression of the downstream molecules P-ERK, Nrf2, and Cu-Zn SOD protein showed little difference compared to those in the normal control group. This study also included an aging + PD98059 group to determine whether the inhibitor PD98059 could cause oxidative stress and affect the expression of antioxidant enzymes. We excluded the toxic effects of DMSO, which was the solute of the inhibitors and may have affected the experimental results. The learning and memory abilities, hippocampus morphology, and expression of P-ERK, Nrf2, and Cu-Zn SOD in the aging + PD98059 group changed very little compared to the aging group. The reason for the small amount of change may be that aging altered the signal transduction pathway in a manner that affected the expression of many endogenous antioxidants and increased the expression of endogenous EPO and its receptors. However, the increase in endogenous EPO and its receptors may be reduced, thereby preventing the anti-aging effect. Thus, inhibiting one of the EPO pathways may not produce obvious effects. These results showed that at race injection of DMSO was not toxic in rats; the effects of DMSO could be ignored.
EPO plays a role in slowing the process of aging in rats caused by d-galactose, and the primary mechanism of this anti-aging effect is through activating the expression of antioxidant enzymes. Previous theory and the results of this study indicate that EPO may combine with an EPO receptor to facilitate the phosphorylation of downstream JAK2- and ERK-related molecules as well as increase Nrf2 gene expression. EPO also promoted Nrf2 dissociation from the Nrf2/KEAP1 polymer. Thus, Nrf2 can be transported to the nucleus to become active Nrf2, which can combine with the MafG protein to form Nrf2/MafG dimers, which leads to the expression of antioxidant enzyme Cu-Zn SOD to remove excess free radicals and thereby resist oxidative stress.
References
Buendia I, Michalska P, Navarro E, Gameiro I, Egea J, Leon R (2016) Nrf2-ARE pathway: an emerging target against oxidative stress and neuroinflammation in neurodegenerative diseases. Pharmacol Ther 157:84–104
Bundy JD, He J (2016) Hypertension and related cardiovascular disease burden in China. Ann Glob Health 82:227–233
Butler RN, Miller RA, Perry D, Carnes BA, Williams TF, Cassel C, Brody J, Bernard MA, Partridge L, Kirkwood T, Martin GM, Olshansky SJ (2008) New model of health promotion and disease prevention for the 21st century. BMJ 337:a399
Chetelat G, Ossenkoppele R, Villemagne VL, Perrotin A, Landeau B, Mezenge F, Jagust WJ, Dore V, Miller BL, Egret S, Seeley WW, van der Flier WM, La Joie R, Ames D, van Berckel BN, Scheltens P, Barkhof F, Rowe CC, Masters CL, de La Sayette V, Bouwman F, Rabinovici GD (2016) Atrophy, hypometabolism and clinical trajectories in patients with amyloid-negative Alzheimer’s disease. Brain 139:2528–2539
Costa LG, Garrick JM, Roque PJ, Pellacani C (2016) Mechanisms of neuroprotection by quercetin: counteracting oxidative stress and more. Oxidative Med Cell Longev 2016:2986796
Digicaylioglu M, Lipton SA (2001) Erythropoietin-mediated neuroprotection involves cross-talk between Jak2 and NF-kappaB signalling cascades. Nature 412:641–647
Engel PA (2016) Is age-related failure of metabolic reprogramming a principal mediator in idiopathic Parkinson’s disease? Implications for treatment and inverse cancer risk. Med Hypotheses 93:154–160
Gassen NC, Chrousos GP, Binder EB, Zannas AS (2016) Life stress, glucocorticoid signaling, and the aging epigenome: implications for aging-related diseases. Neurosci Biobehav Rev
Genc K, Egrilmez MY, Genc S (2010) Erythropoietin induces nuclear translocation of Nrf2 and heme oxygenase-1 expression in SH-SY5Y cells. Cell Biochem Funct 28:197–201
Herrera-Arozamena C, Marti-Mari O, Estrada M, de la Fuente Revenga M, Rodriguez-Franco MI (2016) Recent advances in neurogenic small molecules as innovative treatments for neurodegenerative diseases. Molecules 21
Jin W, Kong J, Lu T, Wang H, Ni H, Wu J, Dai Y, Jiang J, Liang W (2011) Erythropoietin prevents secondary brain injury induced by cortical lesion in mice: possible involvement of Nrf2 signaling pathway. Ann Clin Lab Sci 41:25–32
Juul S (2012) Neuroprotective role of erythropoietin in neonates. J Matern Fetal Neonatal Med 25(Suppl 4):105–107
Juul SE, Pet GC (2015) Erythropoietin and neonatal neuroprotection. Clin Perinatol 42:469–481
Katsuoka F, Yamamoto M (2016) Small Maf proteins (MafF, MafG, MafK): history, structure and function. Gene 586:197–205
Katsuoka F, Motohashi H, Engel JD, Yamamoto M (2005) Nrf2 transcriptionally activates the mafG gene through an antioxidant response element. J Biol Chem 280:4483–4490
Khalil SK, Amer HA, El Behairy AM, Warda M (2016) Oxidative stress during erythropoietin hyporesponsiveness anemia at end stage renal disease: molecular and biochemical studies. J Adv Res 7:348–358
Kilic E, Kilic U, Soliz J, Bassetti CL, Gassmann M, Hermann DM (2005) Brain-derived erythropoietin protects from focal cerebral ischemia by dual activation of ERK-1/-2 and Akt pathways. FASEB J 19:2026–2028
Koltover VK (2016) Free radical timer of aging: from chemistry of free radicals to systems theory of reliability. Curr Aging Sci
Koskenkorva-Frank TS, Weiss G, Koppenol WH, Burckhardt S (2013) The complex interplay of iron metabolism, reactive oxygen species, and reactive nitrogen species: insights into the potential of various iron therapies to induce oxidative and nitrosative stress. Free Radic Biol Med 65:1174–1194
Kume T, Suenaga A, Izumi Y, Akaike A (2016) Protective effect of dimethyl fumarate on an oxidative stress model induced by sodium nitroprusside in mice. Biol Pharm Bull 39:1055–1059
Li YP, Yang GJ, Jin L, Yang HM, Chen J, Chai GS, Wang L (2015) Erythropoietin attenuates Alzheimer-like memory impairments and pathological changes induced by amyloid beta42 in mice. Brain Res 1618:159–167
Liochev SI (2015) Which is the most significant cause of aging? Antioxidants (Basel) 4:793–810
Lu MC, Ji JA, Jiang ZY, You QD (2016) The Keap1-Nrf2-ARE pathway as a potential preventive and therapeutic target: an update. Med Res Rev 36:924–963
Merelli A, Czornyj L, Lazarowski A (2013) Erythropoietin: a neuroprotective agent in cerebral hypoxia, neurodegeneration, and epilepsy. Curr Pharm Des 19:6791–6801
Merelli A, Czornyj L, Lazarowski A (2015) Erythropoietin as a new therapeutic opportunity in brain inflammation and neurodegenerative diseases. Int J Neurosci 125:793–797
Morishita E, Masuda S, Nagao M, Yasuda Y, Sasaki R (1997) Erythropoietin receptor is expressed in rat hippocampal and cerebral cortical neurons, and erythropoietin prevents in vitro glutamate-induced neuronal death. Neuroscience 76:105–116
Sanz A (2016) Mitochondrial reactive oxygen species: do they extend or shorten animal lifespan? Biochim Biophys Acta 1857:1116–1126
Sargin D, Friedrichs H, El-Kordi A, Ehrenreich H (2010) Erythropoietin as neuroprotective and neuroregenerative treatment strategy: comprehensive overview of 12 years of preclinical and clinical research. Best Pract Res Clin Anaesthesiol 24:573–594
Wu H, Wang H, Zhang W, Wei X, Zhao J, Yan P, Liu C (2015) rhEPO affects apoptosis in hippocampus of aging rats by upregulating SIRT1. Int J Clin Exp Pathol 8:6870–6880
Zhang DD (2006) Mechanistic studies of the Nrf2-Keap1 signaling pathway. Drug Metab Rev 38:769–789
Acknowledgements
This study was supported by the National Natural Science Foundation of China (81170300).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wu, H., Zhao, J., Chen, M. et al. The Anti-Aging Effect of Erythropoietin via the ERK/Nrf2-ARE Pathway in Aging Rats. J Mol Neurosci 61, 449–458 (2017). https://doi.org/10.1007/s12031-017-0885-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-017-0885-1