Abstract
Hyperhomocysteinemia plays an etiologic role in the pathogenesis of disorders, including homocystinuria and neurodegenerative and cardiovascular diseases. In the present study, we studied the effect of acute administration of homocysteine, similar to that found in homocystinuria, on parameters of inflammation such as cytokines (TNF-α, IL-1β and IL-6), chemokine CCL2 (MCP-1), nitrite and acute phase-proteins (C-reactive protein and α1-Acid glycoprotein) levels in brain and blood of rats. In addition, a differential count of blood leukocytes was performed. Wistar rats, aged 29 days, received a single subcutaneous injection of saline (control) or homocysteine (0.6 µmol/g body weight). Fifteen minutes, 1 h, 6 h or 12 h after the injection, the rats were sacrificed and serum, hippocampus and cerebral cortex were used. Results showed that homocysteine significantly increased proinflammatory cytokines (TNF-α, IL-1β and IL-6) and chemokine CCL2 (MCP-1) in serum, hippocampus and cerebral cortex. Nitrite levels also increased in hippocampus and cerebral cortex at 15 min, 1 h and 6 h, but not 12 h after homocysteine administration. Acute phase-protein levels were not altered by homocysteine. The percentage of neutrophils and monocytes significantly increased in blood at 15 min and 1 h, but not at 6 h and 12 h after acute hyperhomocysteinemia, when compared to the control group. Our results showed that acute administration of homocysteine increased inflammatory parameters, suggesting that inflammation might be associated, at least in part, with the neuronal and cardiovascular dysfunctions observed in homocystinuric patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Elevated homocysteine (Hcy) levels can be found in several disorders, such as homocystinuria, and neurodegenerative and neuroinflammatory diseases (Mudd et al. 2001; Tyagi et al. 2009). Homocystinuria is an inborn error of metabolism caused by severe deficiency of cystathionine β-synthase (CBS, EC 4.2.1.22) activity. Affected patients present alterations in various organs and systems, especially the central nervous and the vascular systems and present manifestations that include mental retardation, seizures and atherosclerosis (Mudd et al. 2001), whose underlying mechanisms are still obscure.
Inflammation often elicits a generalized sequence of events, known as the acute phase response (Suffredini et al. 1999), which is mediated by the generation of early response cytokines, such as interleukin (IL)-1β, IL-6 and tumor necrosis factor-alpha (TNF-α), acute-phase proteins, expression of cell-surface adhesion molecules, as well as chemotactic molecules production (Keane and Strieter 2000). Although several cell types of the central nervous system (CNS) are able to secrete cytokines including microglia, astrocytes and neurons, there is evidence that peripherally-derived cells can contribute to brain inflammation and injury (Ghirnikar et al. 1998; Giulian et al. 1989). It has been shown that cytokines cross the blood-brain barrier (BBB), probably either by active transport or through leaky regions of endothelia, when BBB is compromised by a pathological condition. Thus, the CNS can be affected not only by inflammatory mediators produced in the brain, but also by the actions of mediators from the periphery (Pollmacher et al. 2002).
It has been shown that exposure of cultured endothelial cells (ECs) to Hcy leads to endothelial activation, resulting in the increased expression of chemokines (Poddar et al. 2001) and adhesion molecules (Wang et al. 2002). On the other hand, Upchurch et al. (1997) suggest that endothelial injury, caused by Hcy, may be due to oxidative stress, nitric oxide (NO) and disturbances in the anti-thrombotic activities of the endothelium. In this context, we have previously reported that an acute experimental model of hyperhomocysteinemia in rats, similar to that found in homocystinuria, induces oxidative stress, reducing antioxidant defenses and increasing lipid peroxidation (Wyse et al. 2002; Matté et al. 2004, 2009).
In order to verify whether high Hcy levels could alter inflammatory markers, in the present study we evaluated the effect of acute Hcy administration on inflammatory parameters such as cytokines (TNF-α, IL-1β and IL-6), chemokine CCL2 (MCP-1), nitrite and acute-phase proteins [(C-reative protein (CRP) and α1-Acid glycoprotein)] levels in hippocampus, cerebral cortex and serum. Differential leukocytes counts in the blood of rats were also performed.
Materials and methods
Animals and reagents
Sixty-five Wistar rats were obtained from the Central Animal House of the Departamento de Bioquímica, Instituto de Ciências Básicas da Saúde, Universidade Federal do Rio Grande do Sul, Porto Alegre, RS, Brazil. Animals were maintained on a 12/12 h light/dark cycle in an air-conditioned constant room temperature (22 ± 1°C) colony room. Rats had free access to a 20% (w/w) protein commercial chow and water. The NIH “Guide for the Care and Use of Laboratory Animals” (NIH Publication No. 80–23, revised 1996), and the official governmental guidelines in compliance with the Federação das Sociedades Brasileiras de Biologia Experimental were followed in all experiments. All chemicals were obtained from Sigma Chemical Co., St. Louis, MO, USA.
Acute homocysteine treatment
Wistar rats, aged 29 days, received a single subcutaneous injection of saline solution (control) or Hcy (0.6 µmol/g body weight). D,L-Hcy was dissolved in 0.9% NaCl solution (saline) and buffered to pH 7.4. Plasma Hcy concentration in rats subjected to this treatment achieved levels similar to those found in homocystinuric patients (Streck et al. 2002; Matté et al. 2009; Mudd et al. 2001). Rats were sacrificed by decapitation without anesthesia 15 min, 1 h, 6 h or 12 h after the injection; serum was separated and brain was quickly removed and hippocampus and cerebral cortex were dissected.
Tissue preparation
For acquisition of serum, whole blood was centrifuged at 1,000×g for 5 min and the serum was immediately removed. Hippocampus and cerebral cortex were homogenized 1:5 (w/v) in saline solution (0.9% NaCl). The homogenate was centrifuged at 800×g for 10 min at 4°C and the supernatant was used in assays.
Cytokines (TNF-α, IL-1β, IL-6) and chemokine CCL2 (MCP-1) assay
TNF-α, IL-1β, IL-6 and MCP-1 levels in hippocampus, cerebral cortex and serum were quantified by a rat high-sensitivity enzyme-linked immunoabsorbent assays (ELISA) with commercially-available kits (Biosource®, Camarillo, CA).
Nitrite assay
Nitrite levels were measured using the Griess reaction; 100 µL of supernatant of hippocampus and cerebral cortex were mixed with 100 µL Griess reagent (1:1 mixture of 1% sulfanilamide in 5% phosphoric acid and 0.1% naphthylethylenediamine dihydrochloride in water) and incubated in 96-well plates for 10 min at room temperature. The absorbance was measured on a microplate reader at a wavelength of 543 nm. Nitrite concentration was calculated using sodium nitrite standards (Green et al. 1982).
Acute-phase protein assay
Acute-phase proteins (CRP and α1-Acid glycoprotein) in serum were determined by a colorimetric assay with commercially available kits (BioSystems® and Bioclin®, Brazil).
Differential leukocyte count
Cytological slide smears stained with May-Grunwald/Giemsa were used for leukocyte differential counts with a light microscope, and total leukocytes were diluted in Thoma solution (1:20) and counted in a Neubauer chamber by light microscopy (Frode-Saleh et al. 1999).
Protein determination
Protein concentration was measured by the method of Lowry et al. (1951) using bovine serum albumin as standard.
Statistical determination
Data were analyzed by the Student’s t test for unpaired samples. All analyses were performed using the Statistical Package for the Social Sciences (SPSS) software. Differences were considered statistically significant if p < 0.05.
Results
Firstly, we evaluated the effect of acute Hcy administration on cytokine levels (TNF-α, IL-1β and IL-6) and chemokine CCL2 (MCP-1) in the hippocampus of rats. Figure 1 shows that Hcy significantly increased the levels of TNF-α (15 min: [t(6) = 8.17; p < 0.05]; 1 h: [t(6) = 3.92; p < 0.001], but not at 6 h: [t(6) = 0.67; p > 0.05] after acute administration. IL-1β and IL-6 levels also increased 15 min, 1 h and 6 h after Hcy injection, as compared to control (15 min: [t(6) = 5.72; p < 0.01]; 1 h: [t(6) = 10.11; p < 0.01]; 6 h: [t(6) = 8.49; p < 0.01]); and (15 min: [t(6) = 10.30; p < 0.01], 1 h: [t(6) = 3.90; p < 0.05]; 6 h: [t(6) = 4.71; p < 0.01]), respectively. We also observed that MCP-1 levels significantly increased (15 min: [t(6) = 11.17; p < 0.001]; 1 h: [t(6) = 8.31; p < 0.001] and 6 h: [t(6) = 24.17; p < 0.001]) in the hippocampus of rats after Hcy administration. Twelve hours after acute Hcy administration, no alterations in the levels of cytokines and chemokine CCL2 were observed (TNF-α [t(6) = 0.83; p > 0.05]; IL-1β [t(6) = 0.36; p > 0.05]; IL-6 [t(6) = 1.08; p > 0.05] and MCP-1 [t(6) = 0.94; p > 0.05]).
Effect of acute administration of homocysteine on cytokines (TNF-α, IL-1β, IL-6) and chemokine CCL2 (MCP-1) levels in the hippocampus of rats. Results are expressed as mean±SD for six animals in each group. Different from control, * p < 0.05; ** p < 0.01; *** p < 0.001 (Student’s t-test). Hcy: homocysteine; TNF-α: tumor necrosis factor alpha; IL-1 β: interleukin-1 beta; IL-6: interleukin-6; MCP-1: monocyte chemoattractant protein-1
We also investigated the effect of acute administration of Hcy on cytokines (TNF-α, IL-1β and IL-6) and chemokine CCL2 (MCP-1) levels in the cerebral cortex of rats. Figure 2 shows that Hcy significantly increased TNF-α (15 min: [t(6) = 4.09; p < 0.05]; 1 h: [t(6) = 8.88; p < 0.001]; IL-1β (15 min: ([t(6) = 6.35; p < 0.01]; 1 h: [t(6) = 5.24; p < 0.05] and 6 h: [t(6) = 4.49; p < 0.05]), and IL-6 levels (15 min: [t(6) = 5.52; p < 0.01]; 1 h: [t(6) = 3.69; p < 0.05] and 6 h: [t(6) = 3.24; p < 0.05]) after acute administration, when compared to control. We also observed that Hcy administration significantly increased MCP-1 levels (15 min: [t(6) = 7.84; p < 0.001]; 1 h: [t(6) = 11.52; p < 0.001]). However, at 6 h after Hcy administration, we did not observe any alterations in TNF-α [t(6) = 1.47; p > 0.05] and MCP-1 [t(6) = 2.24; p > 0.05]. In addition, at 12 h after hyperhomocysteinemia the inflammatory parameters studied were not altered (TNF-α [t(6) = 0.06; p > 0.05]; IL-1β [t(6) = 1.08; p > 0.05]; IL-6 [t(6) = 0.71; p > 0.05] and MCP-1 [t(6) = 0.05; p > 0.05]).
Effect of acute administration of homocysteine on cytokines (TNF-α, IL-1β, IL-6) and chemokine CCL2 (MCP-1) levels in the cerebral cortex of rats. Results are expressed as mean±SD for six animals in each group. Different from control, * p < 0.05; ** p < 0.01; *** p < 0.001 (Student’s t-test). Hcy: homocysteine; TNF-α: tumor necrosis factor alpha; IL-1 β: interleukin-1 beta; IL-6: interleukin-6; MCP-1: monocyte chemoattractant protein-1
Next, we measured the nitrite levels after acute Hcy administration, in hippocampus and cerebral cortex of rats. Figure 3 shows that Hcy significantly increased the nitrite levels in hippocampus and cerebral cortex at 15 min, 1 h and 6 h after Hcy injection, as compared to control (15 min: [t(6) = 5.18; p < 0.01]; 1 h: [t(6) = 4.14; p < 0.05]; 6 h: [t(6) = 3.65; p < 0.01]) and (15 min: [t(6) = 8.43; p < 0.001]; 1 h: [t(6) = 3.35; p < 0.05]; 6 h: [t(6) = 3.82; p < 0.05]), respectively. Nitrite levels were not altered in the hippocampus [t(6) = 1.28; p > 0.05] and cerebral cortex [t(6) = 0.32; p > 0.05] of rats at 12 h after acute hyperhomocysteinemia.
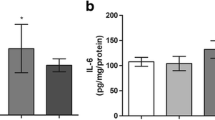
We also evaluated the effect of acute administration of Hcy on cytokine (TNF-α, IL-6) levels in the serum of rats. Figure 4 shows that rats sacrificed at 15 min and 1 h after Hcy injection presented a significant increase in TNF-α levels in the serum, when compared to the control group, [t(6) = 2.76; p < 0.05] and [t(6) = 3.82; p < 0.05], respectively. However, animals sacrificed at 6 h: [t(6) = 1.26; p > 0.05] and 12 h: [t(6) = 0.32; p > 0.05] after Hcy administration did not present any alteration in this parameter. In addition, IL-6 levels were increased at 15 min: [t(6) = 5.69; p < 0.01], 1 h: [t(6) = 6.34; p < 0.01] and 6 h: [t(6) = 10.13; p < 0.001], but not at 12 h: [t(6) = 1.29; p > 0.05] after Hcy administration.
Effect of acute administration of homocysteine on cytokine (TNF-α, IL-6) levels in the serum of rats. Results are expressed as mean±SD for six animals in each group. Different from control, * p < 0.05; ** p < 0.01; *** p < 0.001 (Student’s t-test). Hcy: homocysteine; TNF-α: tumor necrosis factor alpha; IL-6: interleukin-6
The effects of acute administration of Hcy on acute-phase proteins (CRP and α1-Acid glycoprotein) in the serum of rats were also evaluated. Table 1 shows that serum CRP and α1-Acid glycoprotein levels were not altered at any time tested after acute Hcy administration (15 min: [t(8) = 0.14; p > 0.05]; 1 h: [t(8) = 0.54; p > 0.05]; 6 h: [t(8) = 1.41; p > 0.05]; 12 h: [t(8) = 0.11; p > 0.05]) and (15 min: [t(8) = 0.28; p > 0.05]; 1 h: [t(8) = 1.82; p > 0.05]; 6 h: [t(8) = 2.10; p > 0.05]; 12 h: [t(8) = 0.04; p > 0.05]), respectively.
Finally, we investigated the effect of acute administration of Hcy on the differential count of blood leukocytes in rats. Table 2 shows a significant increase in the percentage of cells with a predominance of neutrophils in the blood (15 min: [t(8) = 2.89; p < 0.01]; 1 h: [t(8) = 2.27; p < 0.05]) and monocytes (15 min: [t(8) = 2.39; p < 0.05]; 1 h: [t(8) = 2.48; p < 0.05]) after Hcy administration, when compared to the control group. In addition, the percentage of neutrophils and monocytes was not altered after acute hyperhomocysteinemia (6 h: [t(8) = 0.22; p > 0.05]; 12 h: [t(8) = 1.97; p > 0.05]) and (6 h: [t(8) = 0.49; p > 0.05] and 12 h: [t(8) = 0.82; p > 0.05]), respectively. Nevertheless, the total number of leukocytes did not change significantly after Hcy administration, as compared to the control group (data not shown).
Discussion
Homocystinuria is an inborn error of metabolism, characterized by a severe deficiency of cystathionine β-synthase activity. Affected patients present tissue accumulation of Hcy and a variable symptomatology, including mental retardation, epilepsy, seizures and atherosclerosis, whose pathophysiology is poorly understood (Mudd et al. 2001).
Since Hcy and inflammatory parameters seem to be associated with the pathogenesis of several diseases (Gori et al. 2005; Weiss et al. 2003; Welch and Loscalzo 1998), in the present study, we evaluated the effects of acute hyperhomocysteinemia on important markers of inflammation such as cytokines, chemokine CCL2, nitrite levels and acute-phase proteins in hippocampus, cerebral cortex and serum of rats. In addition, in order to evaluate the blood leucocyte profile, differential counts of leukocytes were also performed. Firstly, we investigated the effect of Hcy administration on the hippocampus and cerebral cortex of rats. Results showed that Hcy increases the cytokines IL-1β and IL-6, chemokine CCL2 (MCP-1) and nitrite levels in both cerebral structures studied from rats sacrificed at 15 min, 1 h and 6 h, whereas TNF-α was increased only at 15 min and 1 h after acute administration. However, 12 h after acute administration of Hcy, onwards, there was no effect on cytokines and chemokine CCL2. It is possible that the increase in the levels of TNF-α, IL-1β, IL-6, MCP-1 and nitrite probably depend on Hcy, since a previous study showed that this amino acid presents a peak in the brain at 15 min after injection, returning to baseline levels after 12 h (Streck et al. 2002)
It has been reported that proinflammatory cytokines and other mediators play an essential role in CNS inflammation (Rothwell and Luheshi 2000). Activated microglia may secrete a diverse range of proinflammatory cytokines and neurotoxic factors, such as NO and reactive species of oxygen (ROS), which contribute to neuronal damage in neurodegenerative diseases (Liu and Hong 2003; Lerouet et al. 2002). In this context, hyperhomocysteinemia has been demonstrated to induce neuronal death, often associated with increased levels of ROS formation (Lipton et al. 1997). Indeed, we have previously demonstrated that chronic Hcy treatment induces oxidative stress in the cerebrum of rats, increasing lipid peroxidation and reducing enzymatic and non-enzymatic antioxidant defenses (Matté et al. 2007, 2009; Streck et al. 2003). Therefore, these findings may be closely related to the increase in proinflammatory cytokines and nitrite levels elicited by Hcy in brain, observed in the present study, since proinflammatory cytokines are often produced in response to oxidative stress and, conversely, may act to cause oxidative stress in their target cells (Halliwell and Gutteridge 2007).
Other reports are in agreement with our data showing a relationship between hyperhomocysteinemia and proinflammatory state (Gori et al. 2005; de Jong et al. 1997). Some authors have shown that Hcy treatment stimulates MCP-1 expression in several cell types (Sung et al. 2001, Wang et al. 2000, 2001). In addition, the pre-treatment of cells with nuclear factor Kappa β (NF-κβ) inhibitors can alleviate the stimulatory effect of Hcy on MCP-1 expression, supporting the notion that Hcy-stimulated chemokine expression is mediated via NF-κB activation (Wang et al. 2000, 2001; Au-Yeung et al. 2003). In turn, NF-κB plays a key role in the orchestration of inflammatory and immune responses by controlling transcription of genes encoding adhesion molecules and cytokines (Yun et al. 2009; Zhou et al. 2007; Dalal et al. 2003).
A number of studies have indicated that Hcy may contribute to the progression of atherosclerosis, in part by enhancing vascular inflammation (Sung et al. 2001; Wang et al. 2000, 2001). Hcy has been shown to promote a potent stimulation of IL-6 expression in cultured rat aorta vascular smooth muscle cells (VSMCs) (Zhang et al. 2006). In vitro studies have shown that Hcy is able to induce mRNA and protein expression of the proinflammatory cytokines, IL-8 and MCP-1 in cultured human aortic endothelial cells (HAECs) (Poddar et al. 2001; Sung et al. 2001). In contrast, it had no effect on the expression of other cytokines, such as TNF-α, IL-1β, granulocyte/macrophage colony-stimulating factor (GM-CSF) and transforming growth factor-β (TGF-β) (Poddar et al. 2001).
In order to verify the systemic effects of Hcy on inflammation, we also measured the levels of TNF-α and IL-6 in the serum of rats submitted to acute hyperhomocysteinemia. Results revealed that Hcy significantly increases these cytokines, where IL-6 levels increased at 15 min, 1 h and 6 h, whereas TNF-α was increased only at 15 min and 1 h after administration. Similarly to effects observed in the brain, we did not observe any alteration at 12 h after Hcy administration.
It has been shown that Hcy may promote endothelial dysfunction, probably associated with oxidative stress, which can lead to the activation of proinflammatory pathways in the vasculature (Weiss 2005; Zhang et al. 2001; Liu et al. 2008). TNF-α is one of the central mediators of tissue inflammation and induces synthesis of other inflammatory cytokines (Lucas et al. 2006), whilst elevated IL-6 levels are closely related to an increased risk of myocardial infarction (Lindmark et al. 2001; Ridker et al. 2000a); as such, our findings that demonstrate that Hcy increases both TNF-α and IL-6 levels in serum of rats suggest an important role of Hcy in the induction of a systemic inflammatory response.
On the other hand, the local release of TNF-α can lead activate neutrophils and endothelial cells to further upregulate the expression of adhesion molecules so that activated neutrophils can bind and migrate across the endothelial cell barrier (Argenbright and Barton 1992). Moreover, it has been demonstrated that Hcy in human can enhance the adhesion of leukocytes to the vascular endothelium and lead to leukocyte-mediated changes in endothelial integrity and function, ultimately resulting in thromboses and vascular lesion (Dudman et al. 1999). Together, these events facilitate the initiation and progression of atherosclerosis lesion (Weiss 2005; Napoli et al. 2001) and might explain why mild hyperhomocysteinemia has been described as an important risk factor for neurodegenerative and vascular diseases (Mattson and Shea 2003).
Acute-phase proteins are produced by the liver in large quantities during an inflammatory state (Patti et al. 2002). Furthermore, it has been suggested that acute-phase proteins have an essential role in the inhibition of extracellular proteases, blood clotting, fibrinolysis and modulation of immune cell function (Ridker et al. 2000b). Considering that we have previously reported that histological analysis reveals the presence of inflammatory infiltrate in liver tissue sections from hyperhomocysteinemic rats (Matté et al. 2009); in this study, acute-phase proteins, CRP and α1-Acid glycoprotein, were also evaluated. However, results showed that Hcy did not alter these parameters at any time tested. These data are in agreement with clinical findings from other investigators who did not observe alterations in the CRP levels in older subjects with hyperhomocysteinemia (Gori et al. 2005).
Regarding the blood leucocyte profile, the analysis of differential counts of leukocytes showed an increase in the relative number of neutrophils and monocytes at 15 min and 1 h, but not at 6 h or 12 h, after Hcy administration. However, the total number of leukocytes did not change (data not shown). It has been reported that cytokines can induce neutrophilic inflammation (Jatakanon et al. 1999); on the other hand, neutrophils can also be important sources of cytokines such as TNF-α (Thomas et al. 1995). Interestingly, we found that Hcy increased TNF-α levels at the same times point that neutrophilia was observed (15 min and 1 h after Hcy injection), suggesting a possible relationship between TNF-α and the increase in neutrophils. However, further studies are needed to determine the mechanism of neutrophilia and monocytosis elicited by Hcy.
In summary, in the present study, we demonstrated that acute Hcy administration induces immune activation by increasing cytokines, chemokine and nitrite levels in the hippocampus, cerebral cortex and serum of rats, in addition to increasing the relative number of neutrophils and monocytes in the blood. Our findings provide insights into the role of Hcy in the pathogenesis of human vascular inflammation, cerebrovascular and neurodegenerative disease and facilitate the identification of new therapeutic approaches in the treatment of homocystinuric patients. However, many questions and cellular mechanisms by which hyperhomocysteinemia exert these effects remain to be answered.
References
Argenbright LW, Barton RW (1992) Interaction of leukocyte integrins with intercellular adhesion molecule I in the production of inflammatory vascular injury in vivo: the Schwartzmann reaction revisited. J Clin Invest 89:259–272
Au-Yeung KKW, Woo CWH, Sung FL, Yip JCW, Siow YL, Karmin O (2003) Hyperhomocysteinemia activates nuclear factor-κB in endothelial cells via oxidative stress. Circ Res 94:28–36
Dalal S, Parkin SM, Homer-Vanniasinkam S, Nicolaou A (2003) Effect of homocysteine on cytokine production by human endothelial cells and monocytes. Ann Clin Biochem 40:534–541
de Jong SC, Stehouwer CD, van der Berg M, Vischer UM, Rauwerda JA, Emeis JJ (1997) Endothelial marker proteins in hyperhomocysteinemia. Thromb Haemost 78:1332–1337
Dudman NPB, Temple SE, Guo XW, Fu W, Perry MA (1999) Homocysteine enhances neutrophil-endothelial interactions in both cultured human cells and rats in vivo. Circ Res 84:409–416
Frode-Saleh TS, Calixto JB, Medeiros YS (1999) Analysis of the inflammatory response induced by substance P in the mouse pleural cavity. Peptides 20:259–265
Ghirnikar RS, Lee YL, Eng LF (1998) Inflammation in traumatic brain injury: role of cytokines and chemokines. Neurochem Res 23:329–340
Giulian D, Chen J, Ingeman JE, George JK, Noponen M (1989) The role of mononuclear phagocytes in wound healing after traumatic injury to the adult mammalian brain. J Neurosci 9:4416–4429
Gori AM, Corsi AM, Fedi S, Gazzini A, Sofi F, Bartali B, Bandinelli S, Gensini GF, Abbate R, Ferrucci L (2005) A proinflammatory state is associated with hyperhomocysteinemia in the elderly. Am J Clin Nutr 82:335–341
Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR (1982) Analysis of nitrate, nitrite and [15N]nitrate in biological fluids. Anal Biochem 126:131–138
Halliwell B, Gutteridge JMC (2007) Free radicals in biology and medicine. Oxford University Press, Oxford, pp 218–220
Jatakanon A, Lalloo UG, Lim S, Chung KF, Barnes PJ (1999) Increased neutrophils and cytokines, TNF-a and IL-8, in induced sputum of non-asthmatic patients with chronic dry cough. Thorax 54:234–237
Keane MP, Strieter RM (2000) Chemokine signaling in inflammation. Crit Care Med 28(4 Suppl):N13–N26
Lerouet D, Beray-Berthat V, Palmier B, Plotkine M, Margaill I (2002) Changes in oxidative stress, iNOS activity and neutrophil infiltration in severe transient focal cerebral ischemia in rats. Brain Res 958:166–175
Lindmark E, Diderholm E, Wallentin L, Siegbahn A (2001) Relationship between interleukin 6 and mortality in patients with unstable coronary artery disease: effects of an early invasive or noninvasive strategy. JAMA 286:2107–2113
Lipton SA, Kim WK, Choi YB, Kumar S, D’Emilia DM, Rayudu PV, Arnelle DR, Stamler JS (1997) Neurotoxicity associated with dual actions of homocyteine at the N-methyl-D-aspartate receptor. Proc Natl Acad Sci 94:5923–5928
Liu B, Hong JS (2003) Role of microglia in inflammation-mediated neurodegenerative disease: mechanisms and strategies for therapeutic intervention. J Pharmacol Exp Ther 304:1–7
Liu X, Luo F, Li J, Wu W, Li L, Chen H (2008) Homocysteine induces connective tissue growth factor expression in vascular smooth muscle cells. J Thromb Haemost 6:184–192
Lowry OH, Rosebrough NJ, Farr AL, Randal RJ (1951) Protein measurement with the folin phenol reagent. J Biol Chem 193:265–267
Lucas SM, Rothwell NJ, Gibson RM (2006) The role of inflammation in CNS injury and disease. Br J Pharmacol 147(Suppl 1):S232–S240
Mattson MP, Shea TB (2003) Folate and homocysteine metabolism in neural plasticity and neurodegenerative disorders. Trends Neurosci 26:137–146
Matté C, Monteiro SC, Calcagnotto T, Bavaresco CS, Netto CA, Wyse AT (2004) In vivo and in vitro effects of homocysteine on Na+, K+-ATPase activity in parietal, prefrontal and cingulated cortex of young rats. Int J Dev Neurosci 22:185–190
Matté C, Scherer EBS, Stefanello FM, Barschak AG, Vargas CR, Netto CA, Wyse ATS (2007) Concurrent folate treatment prevents Na+K+-ATPase activity inhibition and memory impairments caused by chronic hyperhomocysteinemia during rat development. Int J Dev Neurosci 25:545–552
Matté C, Mackedanz V, Stefanello FM, Scherer EBS, Andreazza AC, Zanotto C, Moro AM, Garcia SC, Gonçalves CA, Erdtmann B, Salvador M, Wyse AT (2009) Chronic hyperhomocysteinemia alters antioxidant defenses and increases DNA damage in brain and blood of rats: protective effect of folic acid. Neurochem Int 54:7–13
Mudd SH, Levy HL, Skovby F (2001) Disorders of transsulfuration. In: Scriver CR, Beaudet AL, Sly WS, Valle D (eds) The metabolic and molecular basis of inherited disease, vol 2. McGraw-Hill, New York, pp 1279–1327
Napoli C, de Nigris F, Palinski W (2001) Multiple role of reactive oxygen species in the arterial wall. J Cell Biochem 82:674–682
Patti G, Ambrosio A, Dobrina A, Dicuonzo G, Giansante C, Fiotti N, Abbate A, Guarnieri G, Di Sciascio G (2002) Interleukin-1 receptor antagonist: a sensitive markers of instability in patients with coronary artery disease. J Thomb Thrombolysis 14:139–143
Poddar R, Sivasubramanian N, DiBello PM, Robinson K, Jacobsen DW (2001) Homocysteine induces expression and secretion of monocyte chemoattractant protein-1 and interleukin-8 in human aortic endothelial cells: implications for vascular disease. Circulation 103:2717–2723
Pollmacher T, Haack M, Schuld A, Reichenberg A, Yirmiya R (2002) Low levels of circulating inflammatory cytokines—do they affect human brain functions? Brain Behav Immun 16:525–532
Ridker PM, Rifai N, Stampfer MJ, Hennekens CH (2000a) Plasma concentration of interleukin-6 and the risk of future myocardial infarction among apparently healthy men. Circulation 101:1767–1772
Ridker PM, Hennekens CH, Buring JE, Rifai N (2000b) C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med 342:836–843
Rothwell NJ, Luheshi GN (2000) Interleukin 1 in the brain: biology, pathology and therapeutic target. Trends Neurosci 23:618–625
Streck EL, Matté C, Vieira PS, Rombaldi F, Wannmacher CM, Wajner M, Wyse AT (2002) Reduction of Na+, K+-ATPase activity in hippocampus of rats subjected to chemically induced hyperhomocysteinemia. Neurochem Res 27:1593–1598
Streck EL, Vieira PS, Wannmacher CM, Dutra-Filho CS, Wajner M, Wyse AT (2003) In vitro effect of homocysteine on some parameters of oxidative stress in rat hippocampus. Metab Brain Dis 18:147–154
Suffredini AF, Fantuzzi G, Badolato R, Oppenheim JJ, O’Grady NP (1999) New insights into the biology of the acute phase response. J Clin Immunol 19:203–214
Sung FL, Slow YL, Wang G, Lynn EG, O K (2001) Homocysteine stimulates the expression of monocyte chemoattractant protein-1 in endothelial cells leading to enhanced monocyte chemotaxis. Mol Cell Biochem 216:121–128
Thomas PS, Yates DH, Barnes PJ (1995) Tumor necrosis factor-a increases airway responsiveness and sputum neutrophilia in normal human subjects. Am J Respir Crit Care Med 152:76–80
Tyagi N, Gillespie W, Vacek JC, Sen U, Tyagi SC, Lominadze D (2009) Activation of GABA-A receptor ameliorates homocysteine-induced MMP-9 by ERK pathway. J Cell Physiol 220:257–266
Upchurch GR Jr, Welch GN, Fabian AJ, Freedman JE, Johnson JL, Keaney JF Jr, Loscalzo J (1997) Homocysteine decreases bioavailable nitric oxide by a mechanism involving glutathione peroxidase. J Biol Chem 272:17012–17017
Wang G, Siow YL, O K (2000) Homocysteine stimulates nuclear factor kappaB activity and monocyte chemoattractant protein-1 expression in vascular smooth muscle cells: possible role for protein kinase C. Biochem J 352:817–826
Wang G, Siow YL, O K (2001) Homocysteine induces monocyte chemoattractant protein-1 expression by activating NF-kappaB in THP-1 macrophages. Am J Physiol Heart Circ Physiol 280:H2840–H2847
Wang G, Woo CW, Sung FL, Siow YL, O K (2002) Increased monocyte adhesion to aortic endothelium in rats with hyperhomocysteinemia: role of chemokine and adhesion molecules. Arterioscler Thromb Vasc Biol 22:1777–1783
Weiss N (2005) Mechanisms of increased vascular oxidant stress in hyperhomocysteinemia and its impact on endothelial function. Curr Drug Metab 6:27–36
Welch GN, Loscalzo J (1998) Homocysteine and atherothrombosis. N Engl J Med 338:1042–1050
Weiss N, Heydrick SJ, Postea O, Keller C, Keaney JFJ, Loscalzo J (2003) Influence of hyperhomocysteinemia on the cellular redox state: impact on homocysteine-induced endothelial dysfunction. Clin Chem Lab Med 41:1455–1461
Wyse AT, Zugno AI, Streck EI, Matté C, Calcagnotto T, Wannmacher CM, Wajner M (2002) Inhibition of Na+, K+-ATPase activity in hippocampus of rats subjected to acute administration of homocysteine is prevented by vitamins E and C treatment. Neurochem Res 27:1685–1689
Yun J, Kim JY, Kim OY, Jang Y, Chae JS, Kwak JH, Lim HH, Park HY, Lee SH, Lee JH (2009) Associations of plasma homocysteine level with brachial-ankle pulse wave velocity, LDL atherogenicity, and inflammation profile in healthy men. Nutr Metab Cardiovasc Dis 22:1–8
Zhang Q, Zeng X, Guo J, Wang X (2001) Effects of homocysteine on murine splenic B lymphocyte proliferation and its signal transduction mechanism. Cardiovasc Res 52:328–336
Zhang L, Jin M, Hu X, Zhu J (2006) Homocysteine stimulates nuclear factor kB activity and interleukin-6 expression in rat vascular smooth muscle cells. Cell Biol Int 30:592–597
Zhou Z, Connell MC, MacEwan DJ (2007) TNFR1-induced NF-kappaB, but not ERK, p38MAPK or JNK activation, mediates TNF-induced ICAM-1 and VCAM-1 expression on endothelial cells. Cell Signal 19:1238–1248
Acknowledgments
This work was supported in part by grants from Conselho Nacional de Desenvolvimento Científico Tecnológico (CNPq-Brazil) and Instituto Nacional de Ciências e Tecnologia para Excitotoxicidade e Neuroproteção (Processo n0: 573677/2008-5).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
da Cunha, A.A., Ferreira, A.G.K. & Wyse, A.T.S. Increased inflammatory markers in brain and blood of rats subjected to acute homocysteine administration. Metab Brain Dis 25, 199–206 (2010). https://doi.org/10.1007/s11011-010-9188-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11011-010-9188-8