Abstract
Inflammation is decisive in zinc (Zn)-induced nigrostriatal dopaminergic neurodegeneration; however, the contribution of cyclooxygenase-2 (COX-2) is not yet known. The present study aimed to explore the role of COX-2 in Zn-induced Parkinsonism and its association with the microglial activation. Male Wistar rats were treated intraperitoneally (i.p.) with Zn as zinc sulphate (20 mg/kg) along with respective controls for 2–12 weeks. In a few sets, animals were also treated with/without celecoxcib (CXB, 20 mg/kg, i.p.), a selective COX-2 inhibitor. Indexes of the nigrostriatal neurodegeneration, oxidative stress, inflammation and apoptosis were measured in the animals/nigrostriatal tissue. Zn induced time-dependent increase in the expression of COX-2 while COX-1 expression was unaltered. Zn reduced the neurobehavioral activities, striatal dopamine content, tyrosine hydroxylase (TH) expression and number of dopaminergic neurons. While oxidative stress; microglial activation; expression of microglial cell surface marker-CD11b; cytochrome c release; caspase-9/3 activation; level of pro-inflammatory cytokines, such as TNF-α, IL-1β and IL-6 and Bcl-2-associated protein x (Bax) translocation from the cytosol to mitochondria were induced in the Zn-treated group, expression of B-cell lymphoma-2 (Bcl-2) was found to be reduced. CXB significantly attenuated Zn-induced increase in COX-2 expression and restored TH-expression, dopamine content, level of inflammatory cytokines and neurobehavioral indexes towards normalcy. Moreover, CXB also attenuated Zn-induced increase in microglial activation, oxidative stress and apoptotic markers towards normal levels. Results of the study thus demonstrate that COX-2 induces microglial activation that provokes the release of inflammatory mediators, which in turn augments oxidative stress and intrinsic apoptosis leading to dopaminergic neurodegeneration in Zn-induced Parkinsonism.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Parkinson’s disease (PD) is a mysterious, chronic and progressive neurodegenerative disorder of the nigrostriatal dopaminergic pathway leading to motor disability and characterized by anatomical hallmarks like striatal dopamine depletion and Lewy body formation [1–3]. Despite extensive strategies adopted to explore the molecular explanations of the disease, aetiology remains elusive and ageing, genetic predisposition and environmental factors have been projected as the major perils [4]. Exposure to pesticides and heavy metals has been found to exhibit considerable correlation with high disease risk [5–7]. Presence of elevated zinc (Zn) content in the substantia nigra of PD patients [8] and occurrence of selective nigrostriatal dopaminergic neurodegeneration leading to PD phenotype in the experimental rodents following systemic Zn exposure have shown the magnitude of excessive Zn exposure as a probable risk factor [9–13].
The dynamic contribution of inflammation is recognized owing to the ability of non-steroidal anti-inflammatory drugs (NSAIDs) to halt/protect disease progression [14–18]. Appearance of activated microglial cells in close proximity to the selectively dying dopaminergic neurons in the nigrostriatal pathway of patients reveals the key roles of inflammation and microgliosis in PD pathogenesis [19, 20]. Moreover, microglial activation consequently augments the expression of pro-inflammatory cytokines, such as tumour necrosis factor-α (TNF-α) and interleukin-1β (IL-1β), in the cerebrospinal fluid and substantia nigra of patients substantiating the role of inflammation in PD pathogenesis [21–23]. Pro-inflammatory cytokines in turn activate the expression of other inflammation markers, such as nuclear factor-kappa B (NF-κB) and cyclooxygenase (COX)-2, which could facilitate neurodegeneration in a straight line or indirectly [24, 25]. Besides, anti-inflammatory agents are not only found to protect from inflammation but also from the microglial activation in sporadic and toxin-induced PD validating the role of inflammation in PD pathogenesis [12–14, 26].
Both constitutive and inducible forms of COX are known to catalyse prostanoid biosynthesis from the arachidonic acid. While constitutive form of COX (COX-1) is expressed virtually in all cell types and plays an imperative role in typical physiological processes, the inducible form of COX (COX-2) largely contributes to acute and chronic inflammation that makes it a key target in inflammation-mediated neurodegeneration [27]. Several studies performed employing rodent models and PD patients have suggested a vital role of COX-2 in PD pathogenesis. An increased expression of COX-2 is observed during disease progression while reduced expression and lesser neurodegeneration are detected when selective COX-2 inhibitors are administered in the patients or rodent models [25, 27–31]. Additionally, COX-2-deficient mice are also shown to be resistant against MPTP-induced dopaminergic neuronal death [25, 32]. While oxidative stress, microglial activation and inflammatory cytokines are found to participate [11, 12], the role of COX-2 in Zn-induced nigrostriatal dopaminergic neurodegeneration is not yet investigated. Therefore, the present study aimed to explore the role of COX-2 and its subsequent link with microglial activation in Zn-induced nigrostriatal dopaminergic neurodegeneration.
Materials and Methods
Materials
Ethanol, Folin Ciocalteau reagent, nitric acid, hydrogen peroxide, methanol, n-butanol, potassium dichromate, sodium chloride, sodium hydroxide and sucrose were supplied by Merck (Darmstadt, Germany). Agarose, acrylamide, bisacrylamide, mouse monoclonal anti-TNF-α antibody, biotinylated anti-mouse secondary antibody, bovine serum albumin (BSA), bromophenol blue, β-mercaptoethanol, magnesium chloride, dithiothreitol, ethylene diamine tetraacetic acid, ethylene glycol tetraacetic acid, ethidium bromide (EtBr), 2-hydroxyethyl-1-piperazine ethane sulfonic acid (HEPES), paraformaldehyde, phenyl methyl sulfonyl fluoride, protease inhibitor cocktail, potassium hydroxide, sodium deoxycholate, sodium dodecyl sulphate, 3,3′-diaminobenzidine tetrahydrochloride (DAB) system, sodium orthovanadate, sodium pyrophosphate, thiobarbituric acid (TBA), Tris-base, triton X-100, Tween-20, xylene cyanol and zinc sulphate (ZnSO4) were obtained from Sigma-Aldrich (St. Louis, MO, USA). Acetic acid, cytochrome c (cyt c; oxidized), disodium hydrogen phosphate, dibutyl phthalate xylene, heptane sulfonic acid, nicotinamide adenine dinucleotide reduced form (NADH), nitroblue tetrazolium (NBT), phenazine methosulfate, potassium chloride, potassium dihydrogen phosphate, sodium dihydrogen phosphate, sodium fluoride and xylene were purchased from Sisco Research Laboratories (SRL, Mumbai, India). cDNA synthesis kit, dNTPs, Taq buffer and Taq DNA polymerase were procured from MBI Fermentas (Amherst, NY, USA). Gene-specific primers were obtained from Integrated DNA Technologies Ltd., Singapore. While Neg-50 was purchased from Richard Allen Scientific (Kalamazoo, MI), perchloric acid was supplied by Ranbaxy Private Limited (New Delhi, India). Santa Cruz Biotechnology (Santa Cruz, CA, USA) supplied the mouse monoclonal anti-β-actin, anti-Bax, anti-Bcl-2, anti-caspase 3, anti-COX-2, anti-TH, anti-CD11b, anti-cyt c, goat polyclonal anti-IL-1β, anti-IL-6 and anti-Tim 44 and rabbit polyclonal anti-caspase 9 primary antibodies along with goat anti-mouse, rabbit anti-goat and bovine anti-rabbit alkaline phosphatase (AP)-conjugated secondary antibodies. 5-Bromo-4-chloro-3′-indolylphosphate/nitroblue tetrazolium salt (BCIP/NBT), normal goat serum and streptavidin peroxidase were procured from Bangalore Genei India Pvt. Ltd. (Bangalore, India). While polyvinylidene difluoride (PVDF) membrane and mouse monoclonal anti-NeuN primary antibody were purchased from Millipore Corporation (MA, USA), the remaining required chemicals were procured locally.
Animal Treatment
The study was performed in male Wistar rats and was initiated after clearance from the Institutional Animal Ethics Committee. Rats (150–180 g) were kept under the standard conditions (temperature 22 ± 2 °C; humidity 45–55%; light intensity 300–400 lx; light/dark cycle 12 h/12 h) in the animal house of the institute and provided the food and water ad libitum. Zinc sulphate (ZnSO4/Zn) was administered to animals through intraperitoneal (20 mg/kg) route, twice a week for 2–12 weeks along with respective vehicles/controls [13]. In a few subsets, a COX-2 inhibitor, celecoxib (CXB, 20 mg/kg)/respective vehicle was also administered daily through the same route 1 h prior to vehicle/Zn-treatment [33].
Neurobehavioral Tests
Spontaneous locomotor activity (SLA) (OptoVarimax-Mini A; Columbus Instruments, Columbus, OH) and rotarod performance (Omnitech Electronics Inc., Columbus, OH, USA) tests were done in control and Zn-treated animals in the presence or absence of CXB to assess the effect on motor activity and coordination as described previously [9]. The results are expressed in terms of percent change from control.
Isolation of the Brain Tissues
Animals were sacrificed by the cervical dislocation and decapitated to collect the brains. Brain was dissected in ice-cold conditions to isolate the striatum and substantia nigra as described previously [10]. The nigrostriatal tissue (striatum and substantia nigra) was used for all experiments except for monoamine estimation and immunohistochemical (IHC) observations in which striatum and frozen brain sections, respectively, were used. A minimum of 4 animals per group was used for biochemical, expression and IHC studies.
Estimation of Monoamine Neurotransmitters
Monoamines viz., dopamine and its metabolites (3,4-dihydroxy phenyl acetic acid/DOPAC and homovanillic acid/HVA) and serotonin, were measured in the striatal tissue homogenate using high-performance liquid chromatography employing electrochemical detector as described previously [10]. The values were calculated using respective standards and results are expressed as percent of control.
IHC Studies
IHC staining of TH/NeuN-positive neurons was performed to analyse the number of dopaminergic neurons in controls and treated groups as described previously [13]. Similarly, the IHC staining for microglial cells was also conducted in frozen brain sections using anti-integrin α-M primary antibody as described earlier [3]. Results are expressed as the percent change from the controls.
Protein Estimation
Protein content was determined in mg/ml in all fractions by Lowry’s method using BSA as a standard [34].
LPO, SOD and Catalase
Lipid peroxidation (LPO) was determined by TBA-based method as described previously [9]. The absorbance was recorded at 532 nm and results are expressed in percent change from the controls.
Superoxide dismutase (SOD) activity was estimated by NBT-based procedure [11]. The absorbance of chromogen was recorded at 560 nm against butanol blank. The values are expressed in terms of % change of controls.
Catalase activity was determined by estimating the conversion of hydrogen peroxide to water [11]. The absorbance was read at 570 nm against the control, and results are expressed as percent change from the controls.
Gene Expression
Total RNA was isolated from the nigrostriatal tissue using Trizol reagent [10]. The c-DNA was synthesized using total RNA by RT-Mul M reverse transcriptase kit as per the manufacturer’s protocol. Amplification of COX-1, COX-2 and β-actin was carried out using gene-specific primers designed through DNA star software. The sequences of primers used were as follows: COX-1: forward 5′-TGCTCCCGGGTCTGATGCTCTT-3′ and reverse 5′-ATGGCGATGCGGTTGCGATAC-3′; COX-2: forward 5′-CCGGATCCCCAAGGCACAA-3′ and reverse 5′-CCCGGCACCAGACCAAAGACT-3′ and β-actin: forward 5′-CTGGGACGATATGGAGAAGATTTG-3′ and reverse 5′-CAT GGCTGGGGTGTTGAAGG-3′. Amplicons were visualized by agarose gel electrophoresis using EtBr. Densitometry was performed employing computerized software (Alpha Imager, Alpha Innotech Corporation, South Africa). β-Actin was used as a reference in data analysis and presentation.
Western Blotting
Cytosolic, mitochondrial and microsomal fractions were separated using standard procedures [13]. The level of COX-2, TH, TNF-α, IL-1β, IL-6, pro-caspase-9 and pro-caspase-3 proteins was measured in the cytosolic fraction, Bcl-2 in the mitochondrial fraction and CD11b in the microsomal fraction of the nigrostriatal tissue homogenate. Translocation of Bax and cyt c release was measured in the cytosolic and mitochondrial fractions. Denatured proteins were resolved on SDS-polyacrylamide gel and electroblotted onto PVDF membrane. Blots were blocked for non-specific binding with Tris-buffered saline [0.05% Tween-20 (TBS-T) and 5% non fat dry milk] and incubated with primary antibody against TH, COX-2, CD11b, pro-caspase-9, pro-caspase-3, TNF-α, IL-1β, IL-6, Bax, Bcl-2, cyt c, β-actin or Tim-44 for 3 h followed by incubation with the respective secondary antibody. Blots were visualized by a combination of NBT and BCIP substrate. Relative band density was calculated using β-actin as a reference for the cytosolic and microsomal fractions while Tim-44 was used as a reference for the mitochondrial fraction. The band density ratio is expressed in mean ± standard error of mean (SEM).
Statistical Analysis
Statistical analysis was performed by using one/two-way analysis of variance (ANOVA). Newman-Keuls post-test was used in case of one-way ANOVA while the Bonferroni post-test was used in case of two-way ANOVA for comparison between the groups. Results are expressed as mean ± SEM. The differences were considered statistically significant when p value was less than 0.05.
Results
Expression of COX-1 and COX-2
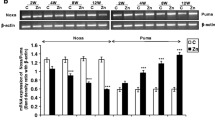
While Zn augmented COX-2 expression in a time of exposure-dependent manner (Fig. 1a), COX-1 expression remained unchanged (Fig. 1b).
Effect of Zn on mRNA expression of COX-2 (a) and COX-1 (b) in rats following 2, 4, 8 and 12 weeks of exposure. The upper panel of each figure shows the representative gel image, and the lower panel shows the densitometric analysis of the same. Data are expressed as mean ± SEM (n = 4). [***p < 0.001 and *p < 0.05 as compared with controls]
CXB Alleviated Zn-Induced Neurobehavioral Anomalies
Zn is found to attenuate SLA and rotarod performance in the animals. Pre-treatment with CXB significantly prevented Zn-induced changes. CXB per se did not alter the motor activities (Fig. 2a).
a Effect of Zn on SLA and rotarod performance in rats following 12 weeks of exposure in the presence and absence of CXB. b Effect of CXB on Zn-induced alterations in the level of striatal dopamine and its metabolites, i.e. DOPAC and HVA along with serotonin in rats following 12 weeks of exposure. Data are expressed as mean ± SEM (n = 4) (***p < 0.001 as compared with control; ##p < 0.01 and ###p < 0.001 as compared to Zn-treated groups)
CXB Prevented Zn-Mediated Alterations in Monoamine Neurotransmitters
Zn depleted the striatal dopamine, DOPAC and HVA after 12 weeks of exposure. CXB noticeably protected from Zn-induced reductions in monoamines (Fig. 2b). No significant change was observed in the dopamine or its metabolites in CXB alone-treated groups. Striatal serotonin was not considerably changed in any of the groups (Fig. 2b).
CXB Protected Against Zn-Induced Dopaminergic Neuronal Loss
IHC analysis exhibited a significant decrease in the number of TH-positive cells in Zn-exposed groups, which was discernibly prevented by CXB pre-treatment. CXB alone did not produce any marked change in the number of TH-positive neurons (Fig. 3a).
a The immunohistochemical analysis of the number of TH-positive dopaminergic neurons in the SNpc region of rat brain following 12 weeks of exposure in presence and absence of CXB. b Western blot analysis of TH and COX-2 protein expression in the nigrostriatal tissues of control and treated rats with β-actin as the reference. The upper panel shows the representative western blot, and the lower panel shows the densitometric analysis of the same. Data are expressed as mean ± SEM (n = 4) (**p < 0.01 and ***p < 0.001 as compared with control; ###p < 0.001 as compared to Zn-treated group)
CXB Mitigated Zn-Induced Alterations in COX-2 and TH Protein Expression
Zn elevated COX-2 protein while CXB attenuated Zn-induced alteration in COX-2 expression. CXB alone did not alter the expression of COX-2 protein (Fig. 3b).
A marked reduction in TH protein in Zn-exposed groups was seen. Pre-treatment with CXB mitigated Zn-induced change in TH protein. TH expression was unaltered in CXB alone-treated animals (Fig. 3b).
Effect of CXB on Zn-Induced Microglial Activation and CD11b Protein Expression
Zn-induced microglial activation was averted by CXB pre-treatment. CXB alone did not affect the integrin α-M immunoreactivity (Fig. 4a). Zn treatment elevated CD11b protein, which was abated by CXB. No change in the expression of CD11b protein was observed in CXB alone-treated animals (Fig. 4b).
a Effect of CXB on Zn-induced alterations in the number of integrin-αM-positive microglial cells in the SNpc region of rat brain. b The figure shows protein expression of microglial cell surface marker CD11b in the nigrostriatal tissues of rats following 12 weeks of Zn exposure in the presence and absence of CXB. The upper panel shows representative western blot picture, and the lower panel depicts the densitometric analysis of the same. Data are expressed as mean ± SEM (n = 4) (***p < 0.001 as compared with control and ###p < 0.001 as compared to Zn-treated group)
CXB Ameliorated Zn-Induced Changes in Oxidative Stress Indexes
Zn elevated SOD activity and LPO content while reduction was observed in catalase activity. CXB pre-treatment attenuated Zn-induced changes in the aforementioned indices. No alterations were seen in the oxidative stress indexes in CXB alone-treated animals (Fig. 5).
Effect of CXB on Zn-induced alterations in the lipid peroxidation and activities of SOD and catalase in the nigrostriatal tissues of rats following 12 weeks of exposure. Data are expressed as mean ± SEM (n = 4) (***p < 0.001 and **p < 0.01 as compared with control; ###p < 0.001 as compared to Zn-treated group)
Effect of CXB on Zn-Mediated Modulations in Expression of TNF-α, IL-1β and IL-6
Elevated level of pro-inflammatory mediators, i.e. TNF-α, IL-1β and IL-6, was observed in the nigrostriatal tissues of Zn-exposed animals. CXB exhibited significant amelioration in Zn-induced increase in pro-inflammatory cytokines. No change was observed in CXB per se treated animals (Fig. 6a).
Effect of CXB on Zn-induced alterations in the expression of TNF-α, IL-1β and IL-6 (a), Bcl-2 (b) and translocation of Bax (c) in the nigrostriatal tissues of rats after 12 weeks of exposure. The upper panel of each figure shows representative western blot picture, and the lower panel of each figure depicts the densitometric analysis of the same. Data are expressed as mean ± SEM (n = 4) (***p < 0.001 as compared with control and ###p < 0.001 as compared to Zn-treated group)
Protein Expression of Bcl-2
Western blot analysis of Bcl-2 revealed a marked decline in Bcl-2 expression in the nigrostriatal tissues of Zn-treated animals. CXB pre-exposure mitigated Zn-induced reduction in Bcl-2 expression. Bcl-2 was unaffected in animals exposed to CXB alone as compared with controls (Fig. 6b).
Translocation of Bax
Increased translocation of Bax from the cytosol to the mitochondria was observed in Zn-exposed animals, which was evident by the reduced level of Bax in the cytosolic fraction with a concomitant increase in the mitochondrial fraction. Pre-treatment with CXB reduced the Zn-induced Bax translocation. CXB per se did not affect Bax translocation (Fig. 6c).
Cyt c Release and Caspase Cascade Activation
Zn induced cyt c release while CXB pre-treatment prevented Zn-induced cyt c release. CXB per se did not affect the cyt c level (Fig. 7a). Reduced expression of pro-caspase 3 and pro-caspase 9 was observed in Zn-treated animals. Pre-treatment with CXB significantly mitigated Zn-induced activation of pro-caspase 3 and pro-caspase 9. No change was observed in pro-caspase 3 and pro-caspase 9 expressions in CXB alone-treated animals (Figs. 7b).
Western blot analysis of mitochondrial cyt c release (a) and pro-caspase 3/9 activation (b) in the nigrostriatal tissues of control and Zn-exposed groups in the presence and absence of CXB. The upper panel of each figure shows representative western blot, and the lower panel of each figure depicts the densitometric analysis of the same. Data are expressed as mean ± SEM (n = 4) (***p < 0.001 as compared with control and ###p < 0.001 as compared to Zn-treated group)
Discussion
Systemic Zn exposure is found to induce the progressive and selective degeneration of the nigrostriatal dopaminergic neurons [10–12]. Increased COX-2 and unaltered COX-1 contents in Zn-exposed animals suggested the role of COX-2 in Zn-mediated neurotoxicity. It is also supported by the previous reports illustrating an increased COX-2 expression in the brain of PD patients and toxin-induced models [14, 25, 27]. Moreover, increased level of inflammatory markers in PD and rodents models showed the role of inflammation in PD pathogenesis [14, 16, 18, 35, 36]. Elevated COX-2 content in the brain of patients and protection offered by COX-2 inhibitors also strengthen the notion [25, 28, 37, 38].
In order to establish the involvement of COX-2 in Zn-induced Parkinsonism, effect of COX-2 inhibitor- celecoxib (CXB) was measured. Reduced motor activity and coordination observed in Zn-exposed animals are in concurrence with the earlier reports demonstrating that the systemic Zn exposure causes motor dysfunction in rodents [10, 11]. Alleviation in Zn-induced neurobehavioral anomalies in CXB pre-exposed animals suggested the protective effect of CXB against Zn-mediated neurotoxicity [27, 37, 39]. Motor dysfunction was supported by decline in the striatal dopamine and its metabolites in Zn-exposed rats [12, 13]. Mitigation of Zn-induced decrease in neurotransmitters by CXB pre-exposure showed a key role of COX-2 in Zn-mediated neurodegenerative changes [25, 40]. It is further supported by the histochemical analysis exhibiting selective loss of TH-positive dopaminergic neurons along with reduced expression of TH in the substantia nigra of Zn-exposed animals [11, 13]. Protection afforded against Zn-induced dopaminergic neuronal cell loss and alleviation of Zn-induced reduction in TH-protein by CXB reaffirmed the contribution of COX-2 in Zn-induced neurotoxicity [37, 41, 42].
COX-2 contributes to neurodegeneration through the PGE2-mediated inflammatory pathway or microglial activation [27, 43, 44]. The amelioration of Zn-induced microglial activation and elevated protein level of CD11b in the animals pre-exposed to CXB implied that COX-2 contributes to Zn-induced microglial activation. Besides, simultaneous amelioration by CXB in Zn-induced increase in pro-inflammatory cytokines, such as TNF-α, IL-1β and IL-6, suggested that COX-2-mediated microglial activation could be responsible for inflammation-mediated dopaminergic neuronal death. Results are in accordance with the earlier studies, which have reported that selective inhibition of COX-2 protects from dopaminergic neuronal death by the inhibition of microglial cell-mediated inflammation [37, 39, 45].
Augmented LPO and SOD activity and reduced catalase activity have been well-known indicators of oxidative stress in Zn-induced neurodegeneration [10–12]. Significant mitigation of Zn-induced oxidative stress by CXB with simultaneous prevention of Zn-induced microglial activation suggested that COX-2-mediated microglial activation resulted in increased oxidative stress leading to neurodegeneration. It is in concurrence with the reports, which have shown the role of microglial activation in oxidative stress-mediated neuronal damage [12, 46]. This is substantiated by the studies, which have shown that selective inhibition of COX-2 diminishes oxidative stress and provides neuroprotection against cadmium-, lipopolysaccharide- and MPTP-induced neurodegeneration [37, 47, 48].
Involvement of intrinsic apoptosis is documented in Zn-induced neurodegeneration [12, 13] that is reflected even in this study from the attenuated level of Bcl-2 protein, increased translocation of Bax from the cytosol to the mitochondria, cyt c release in the cytosol to caspase cascade activation. Mitigation of Zn-induced mitochondria-mediated apoptosis by CXB further affirmed the role of COX-2 in Zn-induced dopaminergic neurodegeneration.
Although CXB provided protection against Zn-mediated dopaminergic neurodegeneration, it was not able to completely abolish Zn-induced neurotoxicity implicating that COX-2 is not the sole factor responsible for the microglia-mediated oxidative stress and inflammation, which is in concurrence with available literature documenting that NADPH oxidase, nitric oxide synthase, depleted glutathione levels, etc. contribute in the oxidative stress and inflammation [10, 11]. Additionally, PD is progressive in nature and therapy delays the progression rather than cure the disease. It could be a reason for higher level of neurodegenerative indexes in Zn + CXB-treated animals as compared with controls. Conclusively, the protection provided by CXB against Zn-induced neuronal cell death implied that COX-2-guided dopaminergic neuronal cell death could be an outcome of the microglia-mediated oxidative stress and inflammation [37, 42, 47, 48] as depicted by the schematic representation (Fig. 8).
Conclusion
The results of the study demonstrated that Zn induces COX-2 that causes microglial-activation leading to increased pro-inflammatory cytokines and oxidative stress, which subsequently results in the demise of dopaminergic neurons through Bax-mediated apoptosis.
References
Dexter DT, Jenner P (2013) Parkinson disease: from pathology to molecular disease mechanisms. Free Radic Biol Med 62:132–144
Singhal NK, Srivastava G, Agrawal S, Jain SK, Singh MP (2012) Melatonin as a neuroprotective agent in the rodent models of Parkinson’s disease: is it all set to irrefutable clinical translation? Mol Neurobiol 45:186–199
Srivastava G, Dixit A, Yadav S, Patel DK, Prakash O, Singh MP (2012) Resveratrol potentiates cytochrome P450 2d22-mediated neuroprotection in maneb- and paraquat-induced parkinsonism in the mouse. Free Radic Biol Med 52:1294–1306
Dauer W, Przedborski S (2003) Parkinson’s disease: mechanisms and models. Neuron 39(6):889–909
Priyadarshi A, Khuder SA, Schaub EA, Priyadarshi SS (2001) Environmental risk factors and Parkinson’s disease: a metaanalysis. Environ Res 86(2):122–127
Singh C, Ahmad I, Kumar A (2007) Pesticides and metals induced Parkinson’s disease: involvement of free radicals and oxidative stress. Cell Mol Biol (Noisy-le-grand) 53(5):19–28
Jomova K, Vondrakova D, Lawson M, Valko M (2010) Metals, oxidative stress and neurodegenerative disorders. Mol Cell Biochem 345(1–2):91–104
Dexter DT, Carayon A, Javoy-Agid F, Agid Y, Wells FR, Daniel SE, Lees AJ, Jenner P et al (1991) Alterations in the levels of iron, ferritin and other trace metals in Parkinson’s disease and other neurodegenerative diseases affecting the basal ganglia. Brain 114(Pt 4):1953–1975
Kumar A, Ahmad I, Shukla S, Singh BK, Patel DK, Pandey HP, Singh C (2010) Effect of zinc and paraquat co-exposure on neurodegeneration: modulation of oxidative stress and expression of metallothioneins, toxicant responsive and transporter genes in rats. Free Radic Res 44(8):950–965
Singh BK, Kumar A, Ahmad I, Kumar V, Patel DK, Jain SK, Singh C (2011) Oxidative stress in zinc-induced dopaminergic neurodegeneration: implications of superoxide dismutase and heme oxygenase-1. Free Radic Res 45(10):1207–1222
Kumar A, Singh BK, Ahmad I, Shukla S, Patel DK, Srivastava G, Kumar V, Pandey HP et al (2012) Involvement of NADPH oxidase and glutathione in zinc-induced dopaminergic neurodegeneration in rats: similarity with paraquat neurotoxicity. Brain Res 1438:48–64
Kumar V, Singh BK, Chauhan AK, Singh D, Patel DK, Singh C (2016) Minocycline rescues from zinc-induced nigrostriatal dopaminergic neurodegeneration: biochemical and molecular interventions. Mol Neurobiol 53(5):2761–2777
Chauhan AK, Mittra N, Kumar V, Patel DK, Singh C (2016) Inflammation and B-cell lymphoma-2 associated X protein regulate zinc-induced apoptotic degeneration of rat nigrostriatal dopaminergic neurons. Mol Neurobiol 53(8):5782–5795
Singh A, Tripathi P, Prakash OP, Singh MP (2016) Ibuprofen abates cypermethrin-induced expression of pro-inflammatory mediators and mitogen-activated protein kinases and averts the nigrostriatal dopaminergic neurodegeneration. Mol Neurobiol 53:6849–6858
Bartels AL, Leenders KL (2007) Neuroinflammation in the pathophysiology of Parkinson’s disease: evidence from animal models to human in vivo studies with [11C]-PK11195 PET. Mov Disord 22(13):1852–1856
Nolan YM, Sullivan AM, Toulouse A (2013) Parkinson’s disease in the nuclear age of neuroinflammation. Trends Mol Med 19(3):187–196
Chen H, Zhang SM, Hernan MA, Schwarzschild MA, Willett WC, Colditz GA, Speizer FE, Ascherio A (2003) Nonsteroidal anti-inflammatory drugs and the risk of Parkinson disease. Arch Neurol 60(8):1059–1064
Wahner AD, Bronstein JM, Bordelon YM, Ritz B (2007) Nonsteroidal anti-inflammatory drugs may protect against Parkinson’s disease. Neurology 69(19):1836–1842
McGeer PL, Itagaki S, Boyes BE, McGeer EG (1988) Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson’s and Alzheimer’s disease brains. Neurology 38(8):1285–1291
Teismann P, Schulz JB (2004) Cellular pathology of Parkinson’s disease: astrocytes, microglia and inflammation. Cell Tissue Res 318(1):149–161
Mogi M, Harada M, Kondo T, Riederer P, Inagaki H, Minami M, Nagatsu T (1994a) Interleukin-1 beta, interleukin-6, epidermal growth factor and transforming growth factor-alpha are elevated in the brain from parkinsonian patients. Neurosci Lett 180(2):147–150
Mogi M, Harada M, Riederer P, Narabayashi H, Fujita K, Nagatsu T (1994b) Tumor necrosis factor-alpha (TNF-alpha) increases both in the brain and in the cerebrospinal fluid from parkinsonian patients. Neurosci Lett 165(1–2):208–210
Nagatsu T, Sawada M (2005) Inflammatory process in Parkinson’s disease: role for cytokines. Curr Pharm Des 11(8):999–1016
Hunot S, Brugg B, Ricard D, Michel PP, Muriel MP, Ruberg M, Faucheux BA, Agid Y et al (1997) Nuclear translocation of NFkappaB is increased in dopaminergic neurons of patients with Parkinson disease. Proc Natl Acad Sci U S A 94:7531–7536
Teismann P, Vila M, Choi DK, Tieu K, Wu DC, Jackson-Lewis V, Przedborski S (2003) COX-2 and neurodegeneration in Parkinson’s disease. Ann N Y Acad Sci 991:272–277
Gupta SP, Patel S, Yadav S, Singh AK, Singh S, Singh MP (2010) Involvement of nitric oxide in maneb- and paraquat-induced Parkinson’s disease. Neurochem Res 35:1206–1213
Bartels AL, Leenders KL (2010) Cyclooxygenase and neuroinflammation in Parkinson’s disease neurodegeneration. Curr Neuropharmacol 8(1):62–68
Minghetti L (2004) Cyclooxygenase-2 (COX-2) in inflammatory and degenerative brain diseases. J Neuropathol Exp Neurol 63(9):901–910
Carrasco E, Casper D, Werner P (2005) Dopaminergic neurotoxicity by 6-OHDA and MPP+: differential requirement for neuronal cyclooxygenase activity. J Neurosci Res 81(1):121–131
Dannhardt G, Kiefer W (2001) Cyclooxygenase inhibitors—current status and future prospects. Eur J Med Chem 36(2):109–126
Rao P, Knaus EE (2008) Evolution of nonsteroidal anti-inflammatory drugs (NSAIDs): cyclooxygenase (COX) inhibition and beyond. J Pharm Pharm Sci 11(2):81s–110s
Feng ZH, Wang TG, Li DD, Fung P, Wilson BC, Liu B, Ali SF, Langenbach R et al (2002) Cyclooxygenase-2-deficient mice are resistant to 1-methyl-4-phenyl1, 2, 3, 6-tetrahydropyridine-induced damage of dopaminergic neurons in the substantia nigra. Neurosci Lett 329(3):354–358
Chu K, Jeong SW, Jung KH, Han SY, Lee ST, Kim M, Roh JK (2004) Celecoxib induces functional recovery after intracerebral hemorrhage with reduction of brain edema and perihematomal cell death. J Cereb Blood Flow Metab 24:926–933
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Bio Chem 193:265–275
Long-Smith CM, Sullivan AM, Nolan YM (2009) The influence of microglia on the pathogenesis of Parkinson’s disease. Prog Neurobiol 89(3):277–287
Mosley RL, Benner EJ, Kadiu I, Thomas M, Boska MD, Hasan K, Laurie C, Gendelman HE (2007) Neuroinflammation, oxidative stress and the pathogenesis of Parkinson’s disease. Clin Neurosci Res 6(5):261–281
Sanchez-Pernaute R, Ferree A, Cooper O, Yu M, Brownell AL, Isacson O (2004) Selective COX-2 inhibition prevents progressive dopamine neuron degeneration in a rat model of Parkinson’s disease. J Neuroinflammation 1(1):6
Zhang X, Dong F, Mayer GE, Bruch DC, Ren J, Culver B (2007) Selective inhibition of cyclooxygenase-2 exacerbates methamphetamine-induced dopamine depletion in the striatum in rats. Neuroscience 150(4):950–958
Vijitruth R, Liu M, Choi DY, Nguyen XV, Hunter RL, Bing G (2006) Cyclooxygenase-2 mediates microglial activation and secondary dopaminergic cell death in the mouse MPTP model of Parkinson’s disease. J Neuroinflammation 3:6
Teismann P, Ferger B (2001) Inhibition of cyclooxygenase isoenzymes COX-1 and COX-2 provide neuroprotection in the MPTP-mouse model of Parkinson’s disease. Synapse 39(2):167–174
Feng Z, Li D, Fung PC, Pei Z, Ramsden DB, Ho SL (2003) COX-2 deficient mice are less prone to MPTP-neurotoxicity than wild-type kice. Neuroreport 14:1927–1929
Gupta A, Dhir A, Kumar A, Kulkarni SK (2010) Effect of preferential cyclooxygenase-2 (COX-2) inhibitor against 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced striatal lesions in rats: behavioral, biochemical and histological evidences. Indian J Exp Biol 48(6):577–585
Teismann P (2012) Cox-2 in the neurodegenerative process of Parkinson’s disease. Biofactors 38:395–397
Ricciotti E, Fitzgerald GA (2011) Prostaglandins and inflammation. Arterioscler Thromb Vasc Biol 31:986–1000
Wang T, Pei Z, Zhang W, Liu B, Langenbach R, Lee C, Wilson B, Reece JM et al (2005) MPP+-induced COX-2 activation and subsequent dopaminergic neurodegeneration. FASEB J 19(9):1134–1136
Peterson LJ, Flood PM (2012) Oxidative stress and microglial cells in Parkinson’s disease. Mediat Inflamm 2012:401264
Figueiredo-Pereira ME, Li Z, Jansen M, Rockwell P (2002) N-acetylcysteine and celecoxib lessen cadmium cytotoxicity which is associated with cyclooxygenase-2 up-regulation in mouse neuronal cells. J Biol Chem 277(28):25283–25289
Hunter RL, Dragicevic N, Seifert K, Choi DY, Liu M, Kim HC, Cass WA, Sullivan PG et al (2007) Inflammation induces mitochondrial dysfunction and dopaminergic neurodegeneration in the nigrostriatal system. J Neurochem 100(5):1375–1386
Acknowledgements
The Council of Scientific and Industrial Research (CSIR), New Delhi, and Department of Science and Technology are gratefully acknowledged for rendering doctoral scholarship to Amit Kumar Chauhan and Namrata Mittra, respectively. Financial support to Chetna Singh through the CSIR-networked programme “neurodegenerative diseases: causes and corrections” (miND; BSC0115) is sincerely acknowledged. The CSIR-IITR communication number of this article is 3437.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The study was initiated after the approval of the institutional animal ethics committee. The guidelines of the committee for the purpose of control and supervision of experiments on animals (CPCSEA) were stringently followed all the way through the study.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Chauhan, A.K., Mittra, N., Patel, D.K. et al. Cyclooxygenase-2 Directs Microglial Activation-Mediated Inflammation and Oxidative Stress Leading to Intrinsic Apoptosis in Zn-Induced Parkinsonism. Mol Neurobiol 55, 2162–2173 (2018). https://doi.org/10.1007/s12035-017-0455-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-017-0455-0