Abstract
Methylenetetrahydrofolate reductase (MTHFR) is key enzyme of folate/homocysteine pathway. Case control association studies on MTHFR C677T polymorphism and Alzheimer’s disease (AD) have been repeatedly performed over the last two decades, but the results are inconclusive. The aim of the present study was to assess the risk of MTHFR C677T polymorphism for AD. Forty-one studies were identified by a search of PubMed, Google Scholar, Elsevier, and Springer Link databases, up to January 2015. Odds ratios (ORs) with corresponding 95 % confidence interval (CI) were calculated using fixed effect model or random effect model. The subgroup analyses based on ethnicity were performed. MTHFR C677T polymorphism had a significant association with susceptibility to AD in all genetic models (for T vs C OR = 1.29, 95 % CI = 1.07–1.56, p = 0.003; for TT + CT vs CC OR = 1.29, 95 % CI = 1.19–1.40, p = 0.0004; for TT vs CC OR = 1.31, 95 % CI = 1.16–1.48, p = 0.001; for CT vs CC OR = 1.24, 95 % CI = 1.13–1.35, p < 0.004; and for TT vs CT + CC OR = 1.13, 95 % CI = 1.00–1.28, p = 0.02). Results of present meta-analysis supported that the MTHFR C677T polymorphism was associated with an increased risk of AD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Alzheimer’s disease (AD) is a neurodegenerative disorder, contributing to about two thirds of all dementias in the elderly population [1]. It causes progressive memory loss in mid-to-late adult life. Some individuals inherit this form of dementia before the age of 65 (known as early-onset or familial AD); but most often, AD occurs late in life [2]. It is a heterogeneous disorder with both familial (about 1 % of cases) and sporadic forms, results from selective damage of specific neuronal circuits in the neocortex, hippocampus, and basal forebrain cholinergic system. Affected regions show senile plaques, comprised of neurites displayed around extracellular deposits of beta-amyloid peptides, and many neurons develop neurofibrillary tangles, which reflect the local accumulation of abnormal intracytoplasmic filaments, composed of hyperphosphorylated isoforms of the tau protein [3]. AD is a multifactorial pathology resulting of the interaction of both genetics and environmental factors. Over 100 rare, highly penetrant mutations have been described in three genes (amyloid beta precursor protein, presnilin 1, and presnilin 2) for early-onset familial AD [4], and for late-onset AD, only the association with the apolipoprotein E (APOE) gene has been convincingly replicated. Several clinical and epidemiological studies show a relation between vascular disorders and late-onset AD [1, 5]. Vascular risk factors such as diabetes, hyperlipidemia, hypertension, heart diseases, and high serum homocysteine are also reported risk factors for AD [6, 7].
Elevated levels of homocysteine (Hcy) have been linked to AD [5, 8]. Folate is a cofactor in one-carbon metabolism, during which it promotes the remethylation of homocysteine. Numerous epidemiological and experimental studies have linked folate deficiency and resultant increased homocysteine levels with AD [5]. Methylenetetrahydrofolate reductase (MTHFR) is an important enzyme involved in the folate-dependent metabolism of homocysteine.
MTHFR enzyme mediates the irreversible conversion of 5,10-methylenetetrahydrofolate (5,10-MTHF) to 5-methyltetrahydrofolate (5-MTHF) [9, 10]. Several polymorphisms in the MTHFR gene have been identified, out of which, the most studied and clinically important polymorphism is C677T in exon 4, resulted in substitution of alanine amino acid by valine at position 222 in MTHFR protein [11]. MTHFR functions in dimeric form and flavin adenine dinucleotide (FAD) work as a co-factor, but variant MTHFR (222 valine) dissociates into monomers and its enzymatic activity reduces [12]. This substitution makes enzyme thermolabile with reduced enzymatic activity. The mean activity of TT variant enzyme was 40–50 % that of the CC variant enzyme at 37 °C [13].
The frequency of MTHFR C677T mutation varies among racial and ethnic groups of the world. T allele frequency ranges from 0.20 to 0.55 in Europeans, 0.11 to 0.35 in Americans, 0.063 to 0.094 in Africans, from 0.04 to 0.38 in Asian population, and 0.10 to 0.47 in Australians [14–18]. Several studies showed that the mutant T allele increases homocysteine levels particularly in folate deficiency state [11, 13, 19, 20]. Impact of MTHFR C677T polymorphism on development and pathogenesis of AD have been conflicting and inconclusive [21–28]. Hence, meta-analyses of all published case control studies investigating C677T polymorphism as risk factor for AD were carried out to shed some lights on conclusive role of MTHFR C677T polymorphism in AD.
Methods
Meta-analyses of published case control articles were carried out according to MOOSE guidelines [29].
Literature and Search Strategy
Electronic databases Pubmed, Springer Link, Elsevier, and Google Scholar were searched up to January 2015 for suitable articles using keywords “MTHFR and Alzheimer’s disease,” “folate metabolism and Alzheimers disease,” and “MTHFR C677T polymorphism and Alzheimers disease.” The references from the eligible articles were also reviewed to find other potential articles. If more than one study by the same author using the same case series was published, either the studies with the largest sample size or the most recently published study was included.
Inclusion and Exclusion Criteria
The following inclusion criteria were set for the meta-analysis: (i) each study should be an independent case-control study, (ii) the purpose of all the studies and statistical methods should be similar, (iii) studies should reported enough information to calculate the odds ratio with 95 % confidence interval (CI), and (iv) inclusion of the patients should be done according to the standard diagnosis parameter (Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) and Mini Mental State Examination (MMSE)). The exclusion criteria were: (i) only case studied; (ii) review papers, editorial, and letter to editor; (iii) containing overlapping data; and (iv) study not providing enough information to estimate odds ratio (OR) with 95 % CI (incomplete raw data) and not well described.
Data Extraction
The following information was extracted from each study: the first author’s family name, year of publication, country, race, sample size, outcome, characteristics of controls, case and control diagnostic criteria, genotyping method, and genotype distribution in cases and controls. Detailed information, wherever not available, was collected by contacting authors.
Statistical Analysis
The strength of association between the MTHFR C677T polymorphisms and AD risk was evaluated by OR and 95 % CI according to allele contrast (T vs C), homozygote (TT vs CC), heterozygote/co-dominant (CT vs CC), recessive (TT vs TC + CC), and dominant (TT + CT vs CC) models. A chi square-based “Q” test defined by Cochran was used to assess the heterogeneity (between study variability) in the meta-analysis [30]. Since the Q statistics is only useful for testing the existence of heterogeneity qualitatively but not quantitatively, another index “I 2,” calculated as the percentage of the total variability in a set of effect sizes due to true heterogeneity, was used to quantify the degree of heterogeneity [31]. A tentative classification of I 2 values proposed by Higgins and Thompson has been used to interpret the magnitude, viz., 25, 50, and 75 %, which corresponds to low, medium, and high heterogeneities, respectively [32]. In the absence of significant heterogeneity determined by the results of Q test, the Mantel-Haenszel fixed effect model (Peto method) was used for the combination of data, while in the presence of significant heterogeneity, the Dersimonian-Laird random effect model (DL method) was used for combining the data [33, 34]. High-resolution plots (forest plots) were generated to estimate the pooled odds ratio corresponding to 95 % CI and the p value. Stratified analyses were performed by ethnicity. Sensitivity analysis was performed to evaluate the stability of the results by removing the studies not in Hardy-Weinberg equilibrium (HWE), studies with very high p value, and studies with small sample size. Cumulative meta-analysis was also done to observe the effect of subsequent addition of each study. Hardy-Weinberg equilibrium and chi-squared test methods were used to test the distribution of genotypes in the control group of each study. For this purpose, data was analyzed using calculator available at http://ihg.gsf.de/cgi-bin/hw/hwa1.pl.
Publication Bias
Publication bias was investigated by using the funnel plots, viz., funnel plot of standard error by log odds ratio and funnel plot of precision by log odds ratio. Different statistical tests such as Begg and Mazumdar rank correlation [35] and Egger’s regression intercept [36] were adopted to assess and quantify the publication bias and its impact on the analysis. P < 0.05 was considered to indicate statistical significance, and all the p values were two sided. All statistical analyses, forest, and funnel plots were performed by the computer program MIX version 1.7 [37].
Result
The preliminary search resulted in 119 publications from Pubmed, Google Scholar, Elsevier, and Springer Link. Out of which, 51 were irrelevant for the present meta-analysis, which includes reviews, book chapters, case reports, editorials, and articles mentioning other genes. After initial exclusion, a total of 68 article publications were identified. Out of which, 16 articles were irrelevant for the present meta-analysis, and in 11 articles only, cases were discussed. Thus, a total of 41 articles were included in present meta-analysis. The search workflow was shown in Fig. 1.
Study characteristics were summarized in Table 1. Forty-one articles that investigated the association between C677T polymorphism and AD were found suitable for the inclusion in the present meta-analysis [3, 21–28, 38–68]. One author [25] studied two different population; their data were included as two independent studies. A total of 42 studies were found suitable for the inclusion in present meta-analysis.
The studies were published between 1998 and 2014. All these 42 studies were performed in different countries—Brazil [49, 51], China [27, 44, 45, 47, 48, 50, 56, 57, 59, 60, 62, 65], Egypt [68], Germany [26], India [66, 67, 69], Iran [52], Ireland [41], Israel [21, 39], Italy [22, 23, 25, 40, 43, 63, 64], Japan [38, 42, 46], Korea [55], Poland [24, 53, 54, 58], Sweden [3, 61], Tunisia [70], and USA [25].
Summary Statistics
In all 42 studies, total cases were 4888 with CC (1676), CT (2290), and TT (922), and controls were 6142 with CC (2389), CT (2719), and TT (1034) genotypes. In control genotypes, percentages of CC, CT, and TT were 38.90, 44.27, and 16.80 %, respectively. In total cases, genotype percentages of CC, CT, and TT were 34.29, 46.85, and 18.86 %, respectively. Frequency of CC genotype was highest in both cases and controls (Table 2). In cases and controls, the allele C was the most common. Except two studies [44, 62], the genotype distributions among the controls of all 39 studies followed Hardy-Weinberg equilibrium. Out of 42 studies, ORs of 12 studies [3, 22, 23, 26, 39, 43–45, 50, 53, 56, 57] were below one; i.e., these studies did not show any association between MTHFR C677T polymorphism and AD.
Allele Contrast Meta-Analysis
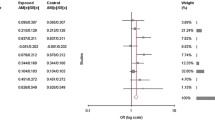
The main results of meta-analysis and the heterogeneity test were shown in Table 3. Allele contrast meta-analysis showed significant association with fixed effect model (allele contrast model ORT vs C = 1.20, 95 % CI 1.13–1.27, p < 0.0001, I 2 = 71.58 %, P heterogeneity < 0.0001, P Pb = 0.25) and random effect model (ORT vs C = 1.29, 95 % CI = 1.07–1.56, p = 0.0003) (Table 3 and Fig. 2). High significant heterogeneity was found, so random effect model was adopted. In cumulative meta-analysis using random effect model, the association of T allele with AD turned statistically significant with the addition of study of Liao et al. [47] and remained significant thereafter.
Genotype Contrast Meta-Analysis
Table 3 summarizes the ORs with corresponding 95 % CIs for association between MTHFR C677T polymorphism and risk of AD in dominant, recessive, homozygote, and co-dominant models. There was evidence of association of the MTHFR TT genotype with the risk of AD relative to the MTHFR CC genotype. Since there was no significant heterogeneity (p = 0.29, I 2 = 10.02 %) between the studies, the fixed effect pooled OR was considered (homozygote model ORTT vs CC = 1.31, 95 % CI = 1.16–1.48, p = 0.0002) (Fig. 3). Significant association was observed between C677T polymorphism and AD using co-dominant model (ORCT vs CC = 1.24, 95 % CI = 1.13–1.35, p < 0.0001). The overall analysis of the recessive model for T allele showed insignificant heterogeneity (p = 0.21, I 2 = 14.56 %) and showed significant association (ORTT vs CT + CC = 1.13, 95 % CI = 1.00–1.28, p = 0.04). However, the dominant model for the effect of T allele produced significant association overall (ORTT + CT vs CC = 1.29, 95 % CI = 1.19–1.40, p < 0.0001) (Fig. 4).
Subgroup Analysis
Subgroup analysis based on ethnicity was performed. In all eligible studies, 21 studies were conducted in Asians, 18 studies were conducted in Caucasians, and 2 studies were conducted in other population. In Asian population (number of studies = 21; 2588 cases/3630 controls), allele contrast meta-analysis showed significant association adopting both fixed (ORT vs C = 1.19, 95 % CI = 1.10–1.28, p = <0.0001, I 2 = 34.02 %, p value of heterogeneity (P hetero) = 0.06, p value of Eggers test (P Pb) = 0.46), and random (ORT vs C = 1.21, 95 % CI = 1.1–1.34, p = 0.0003) effect models. Combined mutant genotypes (dominant model) also showed significant association with fixed (ORTT + CT vs CC = 1.32, 95 % CI = 1.17–1.48, p < 0.0001) and random (ORTT + CT vs CC = 1.33, 95 % CI = 1.17–1.51, p < 0.0001) effect models. In this subgroup, heterogeneity between studies (I 2 = 11.54 %, P hetero = 0.03) was low and publication bias (P Pb = 0.16) was absent (Table 3 and Fig. 5).
In European population (number of studies = 18; 2227/2433 cases/controls), allele contrast meta-analysis showed significant association with both fixed (ORT vs C = 1.26, 95 % CI = 1.15–1.37, p < 0.0001) and random (ORT vs C = 1.34, 95 % CI = 1.01–1.68, p = 0.01) effect models with significant heterogeneity (I 2 = 83.19 %, P heterogeneity = 0.43). The combined mutant genotype (dominant model) showed statistically significant association with fixed effect model (ORTT + CT vs CC = 1.18, 95 % CI = 1.04–1.33, p = 0.008) with no heterogeneity and no publication bias (P Pb = 0.28) (Table 3 and Fig. 6).
Sensitivity Analysis
Sensitivity analyses were conducted to determine whether modification of the inclusion criteria of the meta-analysis affected the final results. In allele contrast meta-analysis, sensitivity analysis performed by exclusion of the studies in which control population was not in Hardy Weinberg equilibrium, studies with small sample size and studies with high p values. Control population of only two studies [44, 62] were not in HW equilibrium, and heterogeneity did not decreased after exclusion of these two studies (P hetero, I 2 = 72.59 %). Exclusion of ten studies involving small sample size, less than 50 (Chapman et al. [21], n = 49; Nishiyama et al. [38], n = 24; Zuliani et al. [23], n = 40; Bi et al. [44], n = 42; Fernandez et al. [49], n = 30; da Silva et al. [51], n = 43; Dorszewska et al. [54], n = 38; Yuan et al. [56], n = 30; Zhang et al. [59], n = 43; Elhaway et al. [68], n = 43), also did not decreased heterogeneity (P hetero < 0.0001, I 2 = 77.27 %).
Publication Bias
Begg’s funnel plot and the Egger’s test were conducted to estimate the publication bias of articles. Both the results of Begg’s and Egger’s test showed evidence of publication bias in allele contrast and co-dominant models, and absence of publication bias was observed in homozygote, dominant, and recessive genetic models (T vs C Begg’s test p = 0.02, Egger’s test p = 0.24; CT vs CC Begg’s test p = 0.02, Egger’s test p = 0.03; TT vs CC Begg’s test p = 0.18, Egger’s test p = 0.3; dominant model TT + CT vs CC Begg’s test p = 0.06, Egger’s test p = 0.06; and recessive model TT vs CT + CC Begg’s test p = 0.47, Egger’s test p = 0.61) (Fig. 7 and Table 3).
Funnel plots of total studies a precision versus OR for T vs C model, b standard error versus OR for T vs C model, c precision versus OR for TT vs CC model, d standard error versus OR for TT vs CC model, e precision versus OR for TT + CT vs CC model, and f standard error versus OR for TT + CT vs CC model
Discussion
Results of present meta-analysis showed strong association between MTHFR C677T polymorphism and Alzheimer’s disease. Several epidemiological and experimental evidences have linked derangements of one-carbon metabolism to vascular, neurodegenerative, and neuropsychiatric diseases, including strokes [71]. Folate deficiency and thermolabile MTHFR enzyme (TT genotype) with low activity increase concentration of homocysteine. The deficiency of MTHFR causes an accumulation of 5,10-methylenetetrahydrofolate as well as the inhibition of 5-methyltetrahydrofolate synthesis. Reduced synthesis of 5-methyltetrahydrofolate will cause decreased homocysteine remethylation. Hyperhomocysteinemia, hypomethioninemia, and reduced S-adenosylmethionine occur frequently in severe MTHFR deficiency [72]. There is several evidences that MTHFR mutant TT genotype is in accordance with elevated homocysteine level, which in itself is an independent risk factor for vascular diseases and cognitive impairment [73]. Folic acid supplementation has a protective effect on homocysteine-induced oxidative stress by reducing intracellular superoxide levels and, to a lesser extent, quenching hydrogen peroxide [74].
Higher concentration of homocysteine could affect adult brain and cause degeneration in adult brain by several mechanisms like (i) it altered DNA methylation pattern [71] and affects gene expression; (ii) it impaired DNA repair [71]; (iii) it increased oxidative stress [74], generated free oxygen radicals, accelerated DNA damage, and eventually lead to neuron apoptosis [74–77]; (iv) it reduced antioxidant reserves of the cell; (v) it enhanced beta-amayloid peptide generation [78]; (vi) it sensitized neurons to amyloid toxicity [75]; (vii) it released inflammatory mediators such as nuclear factor (NF)-kappa B, interleukin (IL)-1beta, IL-6, and IL-8 [66, 79]; (viii) it modulated IL-6 genes [80], which is a proinflammatory cytokine and promoted neuronal expression of neurofilaments, tau protein, and beta-amyloid precursor protein; all are involved in pathogenesis of AD [66, 81]; and (ix) Hcy impaired vascular endothelial function [82] (Stuhlinger et al. 2003), stimulated vascular smooth muscle proliferation [83], breaks balance between coagulation and bleeding pathway [84], and mediated thrombosis. Ultimately, these adverse effects reduce blood supply to the brain and accelerated the neuron apoptosis [77]. Meta-analysis is the statistical analysis of a large collection of analysis results for the purpose of integrating the findings, and it is a powerful tool for systematic review of a focused topic in the literature that provides a quantitative estimate for the effect of a gene, treatment intervention, or exposure [85]. Numerous meta-analyses were published considering MTHFR C677T as risk for several diseases and defects like neural tube defects [10], Down syndrome [86], congenital heart defects [87], schizophrenia [88], bipolar [89], anxiety [89], depression [90], and cancer [91]. One meta-analysis was identified during literature search on the same topic [77]. Zhang et al. [77] pooled 19 studies and reported significant association between AD and MTHFR 677 T polymorphism (OR = 1.15, 95 % CI = 1.08–1.39) using allele contrast genetic model. There are several published studies available but not included in the previous meta-analysis. So, author conducted a comprehensive meta-analysis with the largest number of studies to date to investigate the possible relationship between maternal MTHFR C677T polymorphism and the risk of AD.
The results of this meta-analysis should be interpreted with some caution because there were few limitations in present analysis like (i) results were based on unadjusted OR values that lack the original data from the eligible studies, which could lead to relatively weak power to estimate the real relationship; (ii) sample size in few included studies was relatively small to investigate the association between MTHFR C677T polymorphism and AD risk; (iii) significant between-study heterogeneity was detected and may be distorting the meta-analysis; and (iv) present meta-analysis was based on single-factor estimates, which overlooked the interactions of gene-gene and gene-environment in the development of AD. Present meta-analysis had several strengths also. First, substantial number of cases and controls were pooled from different studies, which significantly increased the statistical power of the analysis. Second, no publication biases were detected, indicating that the whole pooled results may be unbiased.
In conclusion, present comprehensive meta-analysis indicates that there is a conclusive association between the MTHFR C677T polymorphism and the risk of AD, whereas significant heterogeneity was evident across the individual studies. The subgroup analysis also indicated that there is a significant association between MTHFR C677T gene polymorphic variation and AD patients in Asian and Caucasian population.
References
Luchsinger J, Mayeux R (2004) Cardiovascular risk factors and Alzheimer’s diseases. Curr Atheroscler Rep 6:261–266
Wingo TS, Lah JJ, Levey AI, Cutler DJ (2012) Autosomal recessive causes likely in early-onset Alzheimer disease. Arch Neurol 69:59–64
Prince DL, Sisodia SS, Borchett DR (1998) Alzheimer disease: when or why? Nat Genet 19:314–316
Sherrington R, Froelich S, Sorbi S, Campion D, Chi H, Rogaeva EA, Levesque G, Rogaev EI et al (1996) Fifteen-year longitudinal study of blood pressure and dementia. Lancet 347:1141–1145
Seshadri S, Beiser A, Selhub J, Jacques PF, Rosenberg IH, D’Agostino RB, Wilson PW, Wolf PA (2002) Plasma homocysteine as a risk factor for dementia and Alzheimer’s disease. N Engl J Med 346:476–483
Ott A, Stolk RP, van Harskamp F et al (1999) Diabetes mellitus and the risk of dementia: the Rotterdam study. Neurology 53:1937–1942
Jick H, Zornberg GL, Jick SS et al (2000) Statins and the risk of dementia. Lancet 356:1627–1631
Quadri P, Fragiacomo C, Pezzati R, Zanda E, Forloni G, Tettamanti M, Lucca U (2004) Homocysteine, folate, and vitamin B-12 in mild cognitive impairment, Alzheimer disease, and vascular dementia. Am J Clin Nutr 80:114–122
Choi SW, Mason JB (2000) Folate and carcinogenesis: an integrated scheme. J Nutr 130:129–132
Yadav U, Kumar P, Yadav SK, Mishra OP, Rai V (2015) Polymorphisms in folate metabolism genes as maternal risk factor for neural tube defects: an updated meta-analysis. Metab Brain Dis 9:25005003
Frosst P, Blom HJ, Milos R, Goyette P, Sheppard CA, Matthews RG, Boers GJ, den Heijer M et al (1995) A candidate genetic risk factor for vascular disease: a common mutation in methylenetetrahydrofolate reductase. Nat Genet 10:111–113
Yamada K, Zhoutao C, Rima R, Mathews RG (2001) Effects of common polymorphisms on the properties of recombinant human methylenetetrahydrofolate reductase. Proc Natl Acad Sci U S A 98:14853–14858
Rozen R (1997) Genetic predisposition to hyperhomocysteinemia: deficiency of methelenetetrahydrofolate reductase (MTHFR). Thromb Haemost 78:523–526
Pepe G, Venegas OC, Giusti B, Brunelli T, Marcucci R, Attanasio M et al (1998) Heterogeneity in world distribution of thermolabile C677T mutation in 5, 10-methylenetetrahydrofolate reductase. Am J Hum Genet 63:917–920
Schneider JA, Rees DC, Liu YT, Clegg JB (1998) World distribution of a common methylenetetrahydrofalate reductase mutation. Am J Hum Gene 62:1258–1260
Wilcken B, Bamforth F, Li Z, Zhu H, Ritvanen A, Renlund M et al (2003) Geographical and ethnic variation of the 677C > T allele of 5,10-methylenetetrahydrofolate reductase (MTHFR): findings from over 7000 newborns from 16 areas worldwide. J Med Genet 40:619–625
Spiridonova MG, Stepanov VA, Maksimova NR, Puzyrev VP (2004) Population study of frequency of methylenetetrahydrofolate reductase C677T gene polymorphism in Yakutia. Genetika 40:704–708
Rai V, Yadav U, Kumar P (2012) Prevalence of methylenetetrahydrofolate reductase C677T polymorphism in eastern Uttar Pradesh. Indian J Human Genetics 18:43–46
Kluijtmans LA, Young IS, Boreham CA, Murray L, McMaster D, McNulty H et al (2003) Genetic and nutritional factors contributing to hyperhomocysteinemia in young adults. Blood 101:2483–2488
Devlin AM, Clarke R, Birks J, Evans JG, Halsted CH (2006) Interactions among polymorphisms in folate-metabolizing genes and serum total homocysteine concentrations in a healthy elderly population. Am J Clin Nutr 83:708–713
Chapman J, Wang N, Treves A, Korczyn AD, Bornstein NM (1998) ACE, MTHFR, factor V Leiden, and APOE polymorphisms in patients with vascular and Alzheimer’s dementia. Stroke 29:1401–1404
Brunelli T, Bagnoli S, Giusti B, Nacmias B, Pepe G, Sorbi S, Abbate R (2001) The C677T methylenetetrahydrofolate reductase mutation is not associated with Alzheimer’s disease. Neurosci Lett 315:103–105
Zuliani G, Ble A, Zanca R, Munari MR, Zurlo A, Vavalle C, Atti AR, Fellin R (2001) Genetic polymorphisms in older subjects with vascular or Alzheimer’s dementia. Acta Neurol Scand 103:304–308
Religa D, Styczynska M, Peplonska B, Gabryelewicz T, Pfeffer A, Chodakowska M, Luczywek E, Wasiak B et al (2003) Homocysteine, apolipoproteine E and methylenetetrahydrofolate reductase in Alzheimer’s disease and mild cognitive impairment. Dement Geriatr Cogn Disord 16:64–70
Seripa D, Forno GD, Matera MG, Gravina C, Margaglione M, Palermo MT, Wekstein DR, Antuono P et al (2003) Methylenetetrahydrofolate reductase and angiotensin converting enzyme gene polymorphisms in two genetically and diagnostically distinct cohort of Alzheimer patients. Neurobiol Aging 24:933–939
Linnebank M, Linnebank A, Jeub M, Klockgether T, Wullne U, Kolsch H, Heun R, Koch HG et al (2004) Lack of genetic dispositions to hyperhomocysteinemia in Alzheimer disease. Am J Med Genet A 131:101–102
Wang B, Jin F, Kan R, Ji S, Zhang C, Lu Z, Zheng C, Yang Z et al (2005) Association of MTHFR gene polymorphism C677T with susceptibility to late-onset Alzheimer’s disease. J Mol Neurosci 27:23–27
Pandey P, Pradhan S, Modi DR, Mittal B (2009) MTHFR and ACE gene polymorphisms and risk of vascular and degenerative dementias in the elderly. Brain Cogn 71:295–299
Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D et al (2000) Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 283(15):2008–2012
Cochran WG (1954) The combination of estimates from different experiments. Biometrics 10:101–129
Higgins JP, Thompson SE (2002) Quantifying heterogeneity in a meta-analysis. Stat Med 21:1539–1558
Whitehead A (2002) Meta-analysis of controlled clinical trials. John Wiley & Sons Ltd, Chichester, West Sussex, England
Mantel N, Haenszel W (1959) Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst 22(4):719–748
DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7:177–188
Begg CB, Mazumdar M (1994) Operating characteristics of a rank correlation test for publication bias. Biometrics 50:1088–1101
Egger M, Dave Smith G, Schneider M, Minde C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315:629–634
Bax L, Yu ILM, Tsuruta N, Moons KG (2006) Development and validation of MIX: comprehensive free software for meta-analysis of causal research data. BMC Med Res Methodol 6:50
Nishiyama M, Kato Y, Hashimoto M, Yukawa S, Omori K (2000) Apolipoprotein E, methylenetetrahydrofolate reductase (MTHFR) mutation and the risk of senile dementia—an epidemiological study using the polymerase chain reaction (PCR) method. J Epidemiol 10:163–172
Pollak RD, Pollak A, Idelson M, Bejarano-Achache I, Doron D, Blumenfeld A (2000) The C677T mutation in the methylenetetrahydrofolate reductase (MTHFR) gene and vascular dementia. J Am Geriatr Soc 48:664–668
Postiglione A, Milan G, Ruocco A, Gallotta G, Guiotto G, Di Minno G (2001) Plasma folate, vitamin B(12), and total homocysteine and homozygosity for the C677T mutation of the 5,10-methylene tetrahydrofolate reductase gene in patients with Alzheimer’s dementia. A case-control study. Gerontology 47(6):324–329
McIlroy SP, Dynan KB, Lawson JT, Patterson CC, Passmore AP (2002) Moderately elevated plasma homocysteine, methylenetetrahydrofolate reductase genotype, and risk for stroke, vascular dementia, and Alzheimer disease in Northern Ireland. Stroke 33:2351–2356
Wakutani Y, Kowa H, Kusumi M, Yamagata K, Wada-Isoe K, Adachi Y, Takeshima T, Urakam K et al (2002) Genetic analysis of vascular factors in Alzheimer’s disease. Ann N Y Acad Sci 977:232–238
Anello G, Gueant-Rodriguez RM, Bosco P, Gueant JL, Romano A, Namour B, Spada R, Caraci F et al (2004) Homocysteine and methylenetetrahydrofolate reductase polymorphism in Alzheimer’s disease. Neuroreport 15:859–861
Bi S, Pan S, Zhang Y, Wu J (2004) Relationship between folate £-vitamin B12 plasma homocysteine levels and polymorphism of MTHFR gene in Alzheimer’s disease. Chin J Mod Med 977:15–18
Jiang K, Li F, Zhang M, Qian Y, Wang D, Zhang Y, Jiang S (2004) A study on relationship between the C677T mutation in the methylenetetrahydrofolate reductase (MTHFR) gene and Alzheimer disease. Shanghai Arch Psyc 16:196–208
Kida T, Kamino K, Yamamoto M, Kanayama D, Tanaka T, Kudo T, Takeda M (2004) C677T polymorphism of methylenetetrahydrofolate reductase gene affects plasma homocysteine level and is a genetic factor of late-onset Alzheimer’s disease. Psychogeriatrics 4:4–10
Liao W, Huang S, Chen S, Wang Y, Liu X (2004) The relationship of polymorphism of MTHFR and plasma homocysteine level with Alzheimer’s disease. Zhong Guo You Sheng Yi Yi Chuan 12:13–15
Wang L, Ye L, Wu D, Liu J, Niu J (2004) Genetic risk factors of sporadic Alzheimer’s disease among Chinese in Beijing. Chin J Geriatr 23:460–463
Fernandez LL, Scheibe RM (2005) Is MTHFR polymorphism a risk factor for Alzheimer disease like APOE? Arq Neuropsiquiatr 63:1–6
Zhang YD, Ke XY, Shen W, Liu Y (2005) Relationship of homocysteine and gene polymorphisms of its related metabolic enzymes with Alzheimer’s disease. Chin Med Sci J 20:247–251
da Silva VC, Ramos FJ, Freitas EM, de Brito-Marques PR, Cavalcanti MN, D’Almeida V, Cabral-Filho JE, Muniz MT (2006) Alzheimer’s disease in Brazilian elderly has a relation with homocysteine but not with MTHFR polymorphisms. Arq Neuropsiquiatr 64:941–945
Keikhaee MR, Hashemi SB, Najmabadi H, Noroozian M (2006) C677T methylentetrahydrofolate reductase and angiotensin converting enzyme gene polymorphisms in patients with Alzheimer’s disease in Iranian population. Neurochem Res 31:1079–1083
Wehr H, Bednarska-Makaruk M, Łojkowska W, Graban A, Hoffman-Zacharska D, Kuczynska-Zardzewiały A, Mrugała J, Rodo M et al (2006) Differences in risk factors for dementia with neurodegenerative traits and for vascular dementia. Dement. Geriatr Cogn Disord 22:1–7
Dorszewska J, Florczak J, Rozycka A, Kempisty B, Jaroszewska-Kolecka J, Chojnacka K, Trzeciak WH, Kozubski W (2007) Oxidative DNA damage and level of thiols as related to polymorphisms of MTHFR, MTR, MTHFD1 in Alzheimer’s and Parkinson’s diseases. Acta Neurobiol Exp (Wars) 67:113–129
Kim JM, Stewart R, Kim SW, Yang SJ, Shin IS, Shin HY, Yoon JS (2008) Methylenetetrahydrofolate reductase gene and risk of Alzheimer’s disease in Koreans. Int J Geriatr Psychiatry 23:454–459
Yuan Y, Ye Q, Chen Y, Zhang S, Li H, Lu R, Mei G, Li Y et al (2007) Plasma homocysteine level and MTHFR gene polymorphism in old age depression and mild Alzheimer’s disease. Chin J Geriatr 26:767–769
Zhang X (2007) Relationship of Hcy and its related enzyme gene polymorphisms with Alzheimer’s disease. Chin Med Sci J 20:1–28
Styczynska M, Strosznajder JB, Religa D, Chodakowska-Zebrowska M, Pfeffer A, Gabryelewicz T, Czapsk GA, Kobrys M et al (2008) Association between genetic and environmental factors and the risk of Alzheimer’s disease. Folia Neuropathol 46:249–254
Zhang J, Dai Q, Lu W, Meng F (2008) Seruam homocysteine level and MTHFR gene polymorphism and Alzheimer’s disease (AD) risk. Chin J Postgrad Med 31:40–42
Bi XH, Zhao HL, Zhang ZX, Zhan JW (2009) Association of RFC1 A80G and MTHFR C677T polymorphisms with Alzheimer’s disease. Neurobiol Aging 30:1601–1607
Giedraitis V, Kilander L, Degerman-Gunnarsson M, Sundelof J, Axelsson T, Syvanen AC, Lannfelt L, Glaser A (2009) Genetic analysis of Alzheimer’s disease in the Uppsala longitudinal study of adult men. Dement. Geriatr Cogn Disord 27:59–68
Li K, Liu S, Yao S, Wang B, Dai D, Yao L (2009) Interaction between interleukin-8 and methylenetetrahydrofolate reductase genes modulates Alzheimer’s disease risk. Dement. Geriatr Cogn Disord 27:286–291
Ferlazzo N, Gorgone G, Caccamo D, Curro M, Condello S, Pisani F, Ernieri F, Rossini PM et al (2011) The 894G > T (Glu298Asp) variant in the endothelial NOS gene and MTHFR polymorphisms influence homocysteine levels in patients with cognitive decline. Neuromolecular Med 13:167–174
Coppede F, Tannorella P, Pezzini I, Migheli F, Ricci G, Caldarazzo lenco E, Piaceri I, Polin A et al (2012) Folate, homocysteine, vitamin B12, and polymorphisms of genes participating in one-carbon metabolism in late-onset Alzheimer’s disease patients and healthy controls. Antioxid Redox Signal 17:195–204
Deng X, Wang Y (2012) Association between methylenetetrahydrofolate reductase C677T and methionine synthase A2756G gene polymorphisms, Hcy plasma levels and Alzheimer’s disease. Shi Yong Yi Xue 28:3545–3548
Mansoori N, Tripathi M, Luthra K, Alam R, Lakshmy R, Sharma S, Arulselvi S, Parveen S et al (2012) MTHFR (677 and 1298) and IL-6-174 G/C genes in pathogenesis of Alzheimer’s and vascular dementia and their epistatic interaction. Neurobiol Aging 33(1003):e1001–e1008
Divyakolu S, Tejaswini Y, Thomas W, Thumoju S, Sreekanth VR, Vasavi M, OmSai VR, Nagaratna V et al (2013) Evaluation of C677T polymorphism of the methylenetetrahydrofolate reductase (MTHFR) gene in various neurological disorders. Neurol Disord 2(1):1000142
Elhawary NA, Hewedi D, Arab A, Teama S, Shaibah H, Tayeb MT, Bogari N (2013) The MTHFR 677T allele may influence the severity and biochemical risk factors of Alzheimer’s disease in an Egyptian population. Dis Markers 35:439–446
Chhillar N, Singh NK, Banerjee BD, Bala K, Basu M, Sharma D (2014) Intergenotypic variation of vitamin B12 and folate in AD: in North Indian population. Ann Indian Acad Neurol 17(3):308–312
Mansouri L, Fekih-Mrissa N, Klai S, Mansour M, Gritli N, Mrissa R (2013) Association of methylenetetrahydrofolate reductase polymorphisms with susceptibility to Alzheimer’s disease. Clin Neurol Neurosurg 115:1693–1696
Kronenberg G, Colla M, Endres M (2009) Folic acid, neurodegenerative and neuropsychiatric disease. Curr Mol Med 9:315–323
Yoo JH, Choi GD, Kang SS (2000) Pathogenicity of thermolabile methylenetetrahydrofolate reductase for vascular dementia. Arterioscler Thromb Vasc Biol 20:1921–1925
Castro R, Rivera I, Blom HJ, Jakobs C, Tavares de Almeida I (2006) Homocysteine metabolism, hyperhomocysteinaemia and vascular disease: an overview. J Inherit Metab Dis 29:3–20
Sachdev PS (2005) Homocysteine and brain atrophy. Pro Neuropsychopharmacology Biol Psychiatry 29:1152–1161
Kruman II, Kumaravel TS, Lohani A, Pedersen WA, Cutler RG, Kruman Y, Haughey N, Lee J et al (2002) Folic acid deficiency and homocysteine impair DNA repair in hippocampal neurons and sensitize them to amyloid toxicity in experimental models of Alzheimer’s disease. J Neurosci 22:1752–1762
Mattson MP, Shea TB (2003) Folate and homocysteien metabolism in neural plasticity and neurodegenerative disorders. Trends Neurosci 26:137–146
Zhang MY, Miao L, Li YS, Hu GY (2010) Meta-analysis of the methylenetetrahydrofolate reductase C677T polymorphism and susceptibility to Alzheimer’s disease. Neurosci Res 68:142–150
Hasegawa T, Ukai W, Jo DG, Xu X, Mattson MP, Nakagawa M, Araki W, Saito T et al (2005) Homocysteic acid induces intraneuronal accumulation of neurotoxic Abeta42: implications for the pathogenesis of Alzheimer’s disease. J Neurosci Res 80:869–876
Ji C, Kaplowitz N (2004) Hyperhomocysteinemia, endoplasmic reticulum stress, and alcoholic liver injury. World J Gastroenterol 10:1699–1708
Sharma P, Senthilkumar RD, Brahmachari V, Sundaramoorthy E, Mahajan A, Sharma A, Sengupta S (2006) Mining literature for a comprehensive pathway analysis: a case study for retrieval of homocysteine related genes for genetic and epigenetic studies. Lipids Health Dis 5:1
Del Bo R, Angeretti N, Lucca E, De Simoni MG, Forloni G (1995) Reciprocal control of inflammatory cytokines, IL-1 and IL-6, and beta-amyloid production in cultures. Neurosci Lett 188:70–74
Stuhlinger MC, Oka RK, Graf EE, Schmolzer I, Upson BM, Kapoor O, Szuba A, Malinow MR et al (2003) Endothelial dysfunction induced by hyperhomocyst(e)inemia: role of asymmetric dimethylar. Circulation 108:933–938
Kartal ON, Taha S, Azzi A (2005) Homocysteine induces DNA synthesis and proliferation of vascular smooth muscle cells by interfering with MAPK kinase pathway. Biofactors 24:193–199
Midorikawa S, Sanada H, Hashimoto S, Watanabe T (2000) Enhancement by homocysteine of plasminogen activator inhibitor-1 gene expression and secretion from vascular endothelial and smooth muscle cells. Biochem Biophys Res Commun 272:182–185
Munafo MR, Flint J (2004) Meta-analysis of genetic association studies. Trends Genet 20:439–444
Rai V, Yadav U, Kumar P, Yadav SK, Mishra OP (2014) Maternal methylenetetrahydrofolate reductase C677T polymorphism and down syndrome risk: a meta-analysis from 34 studies. PLoS ONE 9:e108552
Wang W, Wang Y, Gong F, Zhu W, Fu S (2013) MTHFR C677T polymorphism and risk of congenital heart defects: evidence from 29 case-control and TDT studies. PLoS ONE 8:e58041
Hu CY, Qian ZZ, Gong FF, Lu SS, Feng F, Wu YL, et al. (2014) Methylenetetrahydrofolate reductase (MTHFR) polymorphism susceptibility to schizophrenia and bipolar disorder: an updated meta-analysis. J Neural Transm. 2014; [Ahead of print DOI: 10.1007/s00702-014-1261-8].
Peerbooms OL, van Os J, Drukker M, Kenis G, Hoogveld L, de Hert M et al (2011) Meta-analysis of MTHFR gene variants in schizophrenia, bipolar disorder and unipolar depressive disorder: evidence for a common genetic vulnerability? Brain Behav Immun 25:1530–1543
Wu YL, Ding XX, Sun YH, Yang HY, Chen J, Zhao X, Jiang YH, Lv XL et al (2013) Association between MTHFR C677T polymorphism and depression: an updated meta-analysis of 26 studies. Prog Neuro-Psychopharmacol Biol Psychiatry 46:78–85
Rai V (2014) The methylenetetrahydrofolate reductase C677T polymorphism and breast cancer risk in Asian populations. Asian Pac J Cancer Prev 15:5853–5860
Acknowledgments
The authoress is highly grateful to Leon Bax (Chief Scientific Officer at BiostatXL, UMC Utrecht) for his valuable suggestions, which help me in statistical analysis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rai, V. Methylenetetrahydrofolate Reductase (MTHFR) C677T Polymorphism and Alzheimer Disease Risk: a Meta-Analysis. Mol Neurobiol 54, 1173–1186 (2017). https://doi.org/10.1007/s12035-016-9722-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-016-9722-8