Abstract
The aim of this study was to examine whether the circulating CXC chemokine ligand-12 (CXCL12) level can predict a 6-month outcome in Chinese patients with acute ischemic stroke (AIS). In a prospective study, CXCL12 levels were measured on admission in the serum of 304 consecutive patients with AIS. The prognostic value of CXCL12 to predict the functional outcome and mortality within 1 year was compared with the National Institutes of Health Stroke Scale score and with other known outcome predictors. A receiver operating characteristic (ROC) curve was used to evaluate the accuracy of serum CXCL12 in predicting functional outcome and mortality. Patients with an unfavorable outcome and non-survivors had significantly increased CXCL12 levels on admission (P < 0.0001 and P < 0.0001). Multivariate logistic regression analysis adjusted for common risk factors showed that CXCL12 (≥12.4 ng/mL; third quartile) was an independent predictor of functional outcome (odds ratio [OR] = 8.81; 95 % confidence interval [CI] 4.92–24.79) and mortality (OR = 10.15; 95 %CI 2.44–27.98). The area under the receiver operating characteristic curve of CXCL12 was 0.84 (95 % CI 0.76–0.92) for functional outcome and 0.87 (95 % CI 0.80–0.93) for mortality. Circulating CXCL12 serum levels at admission is a useful and complementary biomarker to predict functional outcome and mortality 6 months after acute ischemic stroke.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Stroke is the second commonest cause of death and leading cause of adult disability in China [1]. Approximately 15 to 30 % of stroke survivors will be permanently disabled. China has 2.5 million new stroke cases each year and 7.5 million stroke survivors, and it causes a tremendous burden on health resources in China [2]. Prediction of outcome at stroke onset based on clinical deficits only is difficult; therefore, rapid measurement of blood biomarkers predicting functional outcome and mortality could prove useful [3].
Chemokines are small chemoattractant cytokines that play key roles in the accumulation of inflammatory cells at the site of inflammation [4]. The CXC chemokine ligand-12 (CXCL12) is a member of the CXC chemokine subfamily that is constitutively expressed in the brain endothelium [5]. CXCL12 was isolated from murine stromal cell lines and first characterized as a growth-stimulating factor for a B cell precursor clone [6]. Previous studies have suggested that CXCL12 was associated with osteoarthritis [7], hyperlipidemia [8], rheumatoid arthritis [9], cancer [10], leukemia [11], hepatic injury [12], asthma [13], neurodegenerative diseases [14], and cardiovascular diseases [15].
Some studies had reported that CXCL12 played a significant role in acute stroke in animal models [16, 17]. However, the role of CXCL12 in patients with stroke had controversies [5, 18]. Schutt et al. [5] found that plasma CXCL12 levels was a predictor of future stroke, but Wurster et al. [18] reported that single-biomarker evaluation of platelet CXCL12 surface expression is not helpful to predict ischemic stroke. In addition, Ruscher et al. [19] concluded that immoderate excessive activation of the CXCL12 pathway after stroke contributes to depression of neurologic function after stroke and that CXC chemokine receptor type 4 (CXCR4) antagonism is beneficial for the recovery after stroke. Could serum levels of CXCL12 at admission predict short-term outcomes in Chinese patients with acute ischemic stroke (AIS)? Thus, the aim of this study was to examine whether the circulating CXCL12 level can predict a 6-month outcome in Chinese patients with AIS.
Subjects and Methods
Patients and Study Design
We conducted a prospective cohort study at the emergency department of our hospital. From December 2012 to September 2014, all patients with first-ever AIS were included. All patients were Chinese. All patients were admitted within 48 h of experiencing a new focal or global neurological event. Acute ischemic stroke was defined according to the World Health Organization criteria [20]. We excluded patients with malignant tumor, transient ischemic attack, epileptic seizures, intracerebral hemorrhage, and a history of recent surgery or trauma during the preceding 2 months; renal insufficiency, febrile disorders, and systemic infections (assessed by clinical symptom assessment and laboratory tests) at study enrollment; and autoimmune diseases with or without immunosuppressive therapy. The study was approved by the ethics committee of the First Affiliated Hospital of Zhengzhou University. The patients or their relatives gave written informed consent prior to entering the study.
Clinical Variables
At baseline, the following demographical and clinical data were taken: gender, age, leukocyte count, duration of diabetes, daily insulin dose, and history of conventional vascular risk factors (hypertension, atrial fibrillation, hyperlipoproteinemia, smoking habit, and alcohol abuse). Routine blood and biochemical tests, electrocardiogram, and a baseline brain computer tomography (CT) or magnetic resonance imaging (MRI) scan were performed in all patients at admission. All patients received treatment according to current guidelines. The National Institutes of Health Stroke Scale (NIHSS) score (scores range from 0 to 42, with greater scores indicating increasing severity) was assessed by a stroke neurologist certified in the use of this scale on admission (within 24 h) [21]. Stroke subtype was classified according to TOAST (Trial of Org 10172 in Acute Stroke Treatment) criteria [22], which distinguish large-artery arteriosclerosis, cardioembolism, small-artery occlusion, other causative factors, and undetermined causative factors. The clinical stroke syndrome was determined by applying the criteria of the Oxfordshire Community Stroke Project: total anterior circulation syndrome (TACS), partial anterior circulation syndrome (PACS), lacunar syndrome (LACS), and posterior circulation syndrome (POCS) [23].
CXCL12 Measurement
All blood samples were collected on the first day of admission (within 0–6 [n = 89], 6–12 [n = 102], 12–24 [n = 50], and 24–48 [n = 63] h from symptom onset) before any acute stroke treatment, and serum levels of high-density lipoproteins (HDL), low-density lipoproteins (LDL), triglycerides (TC), total cholesterol (TC), high-sensitivity C-reactive protein (Hs-CRP), homocysteine (HCY), and glucose analyses were also measured in accordance with standard detection methods in the hospital biochemistry department of this hospital. Serum CXCL12 levels of patients were blindly assessed simultaneously with a quantitative sandwich enzyme-linked immunosorbent assay (ELISA) kit (Quantikine; R&D Systems, Minneapolis, MN, USA). The inter-assay and intra-assay coefficients of variation for CXCL12 were shown to be 5.5–9.0 and 6.0–10.5 %.
Neuroimaging
Brain imaging (either CT or MRI) was performed routinely within 24 to 48 h after admission. Diagnosis of stroke was based on the results of strict neurological examination (CT, MRI, or both) according to the International Classification of Diseases, ninth revision. CCT was performed in all patients on admission mainly to exclude intracranial hemorrhage. Thereafter, MRI was performed using a stroke protocol, including T1-, T2-, and diffusion-weighted imaging (DWI) sequences and a magnetic resonance angiography. MRI with DWI was available in 221 stroke patients (72.7 %). In those patients, DWI lesion volumes were determined by one experienced neurologist (Cheng X) unaware of the clinical and laboratory results. The infarct volume was calculated by using the formula 0.5 × a × b × c (where a is the maximal longitudinal diameter, b is the maximal transverse diameter perpendicular to a, and c is the number of 10-mm slices containing infarct) [24].
End Points and Follow-up
Functional outcome was obtained on month 6 according to the modified Rankin Scale (mRS) [25], with the evaluator blinded to CXCL12 levels. The primary end point of this study was favorable functional outcome of stroke patients after 6 months from baseline, defined as a mRS score of 0 to 2 points. A secondary end point in stroke patients was death or withdrawal from any cause within a 6-month follow-up. Outcome assessment was performed by one trained medical staff blinded to CXCL12 levels with a structured follow-up telephone interview with the patient or, if not possible, with the relative.
Statistical Analysis
Results are expressed as percentages for categorical variables and as medians (interquartile ranges, IQRs) for continuous variables. Univariate data on demographic and clinical features were compared by Mann–Whitney U test or chi-square test as appropriate. Correlations among continuous variables were assessed by the Spearman rank correlation coefficient. The relationship between CXCL12 levels and other clinical parameters was also analyzed by stepwise multiple regression analysis. To investigate whether CXCL12 allows predicting of both functional outcome and mortality in stroke, different statistical methods were used. First, the relation of CXCL12 with the two points was investigated with the use of logistic regression models. We used crude models and multivariate models adjusted for all significant predictors and report odds ratios (ORs). For multivariate analysis, we included confounders, known risk factors, and other predictors as assessed in univariate analysis. Second, a receiver operating characteristic curve (ROC) was used to test the overall predicted accuracy of CXCL12 and other markers, and results were reported as area under the curve (AUC). All statistical analysis was performed with SPSS for Windows, version 20.0 (SPSS Inc., Chicago, IL, USA) and the ROCR package (version 1.0–2), which is available from CRAN repository (http://cran.r-project.org/). Statistical significance was defined as P < 0.05.
Results
Patient Characteristics and Clinical Variables
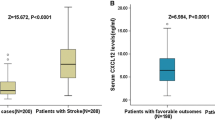
From 553 screened patients, acute ischemic stroke was diagnosed in 356 patients (60 with transient ischemic attack, 45 with onset of symptoms >48 h, 42 with hemorrhagic stroke, 18 without informed consent, 11 with epileptic seizures, 9 with systemic infections, 8 with malignant tumor, and 4 with renal insufficiency were not analyzed) and 304 completed 6-month follow-up (32 lost to follow-up and 20 withdrew). The median age of patients included in this study was 65 (IQR, 55–72) years, and 53.3 % were men. The median NIHSS score on admission was 10 points (IQR, 6–15). The median time from stroke onset to inclusion in the study was 5.9 (IQR, 2.8–11.2) h. An unfavorable functional outcome was found in 97 patients (31.9 %) with a median mRS score of 4 (IQR, 3–6). Forty-eight patients died; thus, the mortality rate was 15.8 %. In addition, the number of patients who received tissue plasminogen activator treatment was 92 (30.3 %). The baseline characteristics of the 304 patients presenting with acute ischemic stroke are described in Table 1. The median serum CXCL12 level in stroke patients was 9.5 (IQR, 5.4–14.2) ng/mL.
In patients with stroke, serum levels of CXCL12 increased with increasing severity of stroke as defined by the NIHSS score. There was a modest correlation between levels of serum CXCL12 and NIHSS score (r = 0.336, P < 0.001; Fig. 1a). In the subgroup of patients (n = 212) in whom MRI was available, CXCL12 levels paralleled lesion size. There was a positive correlation between levels of CXCL12 and the infarct volume (r = 0.355, P < 0.0001; Fig. 1b). In these patients, the multiple regression analysis found a significant positive association of NIHSS score and infarct volume with serum CXCL12 levels (all P < 0.05). In addition, there was a significant, albeit weak, positive correlation between CXCL12 levels and Hs-CRP (r = 1.663, P = 0.009). Statistical analysis here revealed no influence of sex, age, HCY, LDL, HDL, TG, TC, systolic and diastolic BP, current smoking, BMI, and vascular risk factors on CXCL12 levels in stroke patients (P > 0.05, respectively).
CXCL12 and 6-Month Functional Outcome
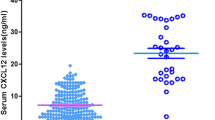
In the 97 patients with an unfavorable functional outcome, serum CXCL12 levels were higher compared with those in patients with a favorable outcome (14.7 [IQR, 9.7–19.3] ng/mL vs. 7.0 [IQR, 4.8–12.3] ng/mL; P < 0.0001; Fig. 2). In univariate logistic regression analysis, we calculated the OR of CXCL12 levels as compared with the NIHSS score and other risk factors as presented in Table 2. With an unadjusted OR of 15.27 (95 % confidence interval [CI], 6.81–44.32), CXCL12 (≥12.4 ng/mL; third quartile) had a strong association with unfavorable functional outcome. After adjusting for all other significant outcome predictors, CXCL12 (≥12.4 ng/mL) remained an independent outcome predictor with an adjusted OR of 8.81 (95 % CI, 4.92–24.79; P < 0.001). In the subgroup of patients (n = 221) in whom MRI evaluations were performed, CXCL12 (≥12.4 ng/mL) was an independent unfavorable outcome predictor with an OR of 9.64 (95 % CI, 3.34–26.34; P < 0.001) after adjustment for both lesion size and the NIHSS score. In addition, age, the NIHSS score, and laboratory findings, such as HCY level and Hs-CRP, remained significant outcome predictors (Table 2).
The serum levels of CXCL12 between stroke patients with favorable outcomes and unfavorable outcomes. The horizontal lines indicate median levels and interquartile ranges (IQR). P values refer to Mann–Whitney U tests for differences between groups. A favorable functional outcome was defined as a mRS score of 0 to 2 points, while unfavorable outcome was defined as 3–6 points. CXCL12 CXC chemokine ligand-12
With an AUC of 0.84 (95 % CI, 0.76–0.91), CXCL12 showed a significantly greater discriminatory ability as compared with Hs-CRP (AUC, 0.69; 95 % CI, 0.60–0.78; P < 0.001), age (AUC, 0.60; 95 % CI, 0.54–0.67; P < 0.0001), and HCY (AUC, 0.64; 95 % CI, 0.58–0.73; P < 0.0001), while it was in the range of the NIHSS score (AUC, 0.81; 95 % CI, 0.74–0.89; P = 0.042). Interestingly, CXCL12 improved the NIHSS score (AUC of the combined model, 0.90; 95 % CI, 0.84–0.95; P < 0.01).
CXCL12 and 6-Month Mortality
At 6 months, 48 patients (15.8 %) had died. Non-survivors had significantly higher CXCL12 levels than survivors (18.3 [IQR, 14.3–25.0] vs. 8.0 [IQR, 5.3–13.0] ng/mL; P < 0.0001; Fig. 3). In univariate logistic regression analysis, we calculated the OR of CXCL12 levels as compared with the NIHSS score and other risk factors as presented in Table 2. With an unadjusted OR of 24.15 (95 % CI, 5.04–63.13), CXCL12 (≥12.4 ng/mL; third quartile) had a strong association with mortality. After adjusting for all other significant mortality predictors, CXCL12 (≥12.4 ng/mL) remained an independent mortality predictor with an adjusted OR of 10.15 (95 % CI, 2.44–27.98). In the subgroup of patients (N = 221) in whom MRI evaluations were performed, CXCL12 (≥12.4 ng/mL) was an independent mortality predictor with an OR of 12.22 (95 % CI, 2.29–33.45; P < 0.001) after adjustment for both lesion size and the NIHSS score. In addition, age, the NIHSS score, and laboratory findings, such as HCY level and Hs-CRP, remained significant outcome predictors (Table 2).
Similarly, with an AUC of 0.87 (95 % CI, 0.80–0.93), CXCL12 showed a significantly greater discriminatory ability as compared with the NIHSS score (AUC, 0.79; 95 % CI, 0.68–0.87; P < 0.001), Hs-CRP (AUC, 0.72; 95 % CI, 0.65–0.78; P < 0.0001), and age (AUC, 0.66; 95 % CI, 0.59–0.73; P < 0.0001). Interestingly, CXCL12 also improved the NIHSS score (AUC of the combined model, 0.94; 95 % CI, 0.89–0.98; P < 0.001).
The time to death was analyzed by Kaplan–Meier survival curves based on serum CXCL12 quartiles. Patients in the upper two quartiles had a higher risk of death compared to patients with CXCL12 levels in the lower two quartiles (Fig. 4).
Kaplan–Meier survival based on CXCL12 quartiles. Time to death was analyzed by Kaplan–Meier curves based on CXCL12 quartiles. Patients in the lower two quartiles (CXCL12 < 5.4 ng/mL and CXCL12 between 5.4 and 9.5 ng/mL) had a minor risk of death compared to patients with CXCL12 levels in the higher two quartiles (CXCL12 > 14.2 ng/mL and CXCL12 between 9.5 and 14.2 ng/mL, P < 0.0001). CXCL12 CXC chemokine ligand-12
Discussion
There is growing evidence that chemokines are potentially important mediators of the pathogenesis of atherosclerotic disease and major atherothrombotic complications, such as stroke and myocardial infarction [26]. Gu et al. [27] reported that elevated circulating CXCL12 levels at admission are strongly associated with the future recurrence of ischemic stroke in Chinese patients with AIS. Wurster et al. [18] reported that CXCL12 plays a pivotal role in angiogenesis and the regeneration of ischemic tissue through the regulation of hematopoietic progenitor cells and is upregulated at the sites of vascular injury and platelet activation. Another study found that in chronic kidney disease (CVD), baseline plasma levels of CXCL12 were associated with known cardiovascular (CV) risk factors, prevalent CV disease, as well as incident MI/death after adjustment for traditional CV risk factors and measures of CKD, suggesting that plasma CXCL12 levels may be atherogenic [28]. In this study, we firstly assessed the serum CXCL12 levels with regard to their accuracy to predict functional outcome and mortality in patients with AIS within 6 months in the Chinese sample.
Importantly, this preliminary result confirmed an interesting conclusion: elevated CXCL12 levels at admission were correlated with 6-month outcome and mortality, suggesting that this biomarker disturbance was prognostically unfavorable. CXCL12 could be seen as one independent prognostic marker of functional outcome and mortality even after correcting for possible confounding factors in the Chinese sample. Kim et al. [29] found that there was a correlation between serum CXCL12 and long-term outcome in patients with AIS. Similarly, Kwon et al. [30] reported that lower levels of CXCL12 were related with a favorable prognosis in stroke patients, and another study finished by Duan et al. [31] demonstrated that an elevated serum CXCL12 level at admission was an independent 3-month prognostic marker in patients with AIS. It is comparable with a previous study that showed that the level of CXCL12 was positively correlated with mRS score 3 months after acute ischemic stroke [32]. In addition, in our study, we found that serum CXCL12 was positively correlated with infarct volume and stroke severity, which was supported by Liu et al. [33]. However, a previous report showed a moderately inverse (r = −0.49, P < 0.04) correlation with baseline diffusion-weighted imaging lesion volumes [34].
CXCL12 expression is increased in the ischemic penumbra zone and is involved in both focal angiogenesis and inflammatory reactions [35]. It binds to the CXCR4 receptor, and the CXCL12/CXCR4 signaling pathway promotes angiogenesis in the ischemic tissue [36]. CXCL12 has been implicated in neuroinflammation after ischemic stroke [37]. After ischemic stroke, CXCL12 mediates the inflammatory response by recruitment of neural progenitor cells and the mobilization of bone marrow-derived progenitor cells for tissue regeneration and neovascularization [38]. Similarly, we found that there a significant positive trend between serum CXCL12 levels and Hs-CRP (r = 0.1663, P = 0.009). However, the prognostic value remained statistically significant after correction for differences in Hs-CRP, which indicates that CRP and CXCL12 may carry different types of information as markers of inflammation.
Whether a higher circulating CXCL12 level is an accelerator or only a marker of AIS remains uncertain. In our study, we suggested that CXCL12 may play a role in the process of stroke. Except the role in inflammation, some other possible mechanisms should be considered. Firstly, one study indicated that CXCL12/CXCR4 controls the important contribution of neutrophils to atherogenesis in mice [39]. Another study found that macrophage migration inhibitory factor (MIF) has shown to be a more pro-inflammatory and thus pro-atherogenic chemokine; instead, CXCL12 seems to have a more protective function [40]. Interestingly, CXCL12 mRNA expression was detected in human atherosclerotic plaques [41]. Secondly, CXCL12 plays important roles in multiple processes after ischemic stroke, which include inflammatory response, focal angiogenesis, and the recruitment of bone marrow-derived cells (BMCs) and neural progenitor cells (NPC) to injury [35]. In particular, CXCL12 induced the cerebral recruitment of monocytes, protective endothelial cell progenitors, and neuroblasts [42]. Schönemeier et al. [43] reported that CXCL12 expression increased strongly in the peri-infarct and infarct regions, which was accompanied by the appearance of numerous CXCR4-expressing cells in the rat. Thirdly, Ardelt et al. [44] suggested that CXCL12 was in a position to coordinate neovascularization and neurogenesis during the repair process after cerebral ischemia–reperfusion.
Several limitations of this study should be considered. Firstly, without serial measurement of the circulating CXCL12, this study yielded no data regarding when and how long biomarkers were elevated in these patients. The serum CXCL12 level was reported to increase shortly after acute ischemic stroke [32]. Additionally, it should be investigated whether serial CXCL12 testing further improves the risk stratification of stroke patients. In addition, our work lacks long-term clinical outcome data. In fact, long-term clinical outcome would also make this study more relevant. Further study should be considered. Secondly, CXCL12 measurements were performed after the stroke and may not accurately reflect pre-stroke exposure. Thirdly, ongoing drugs (i.e., statins, antiplatelets, anti-inflammatories, and anti-hypertensives) potentially affecting CXCL12, however, were not obtained and taken into account in this analysis. Thus, we could not determine the association of those factors with circulating CXCL12 and functional outcome. Future studies on those factors will be needed to further disentangle the effect of these factors on outcomes. Lastly, we assessed all-cause mortality because classification of death in clinical practice can sometimes be difficult and unreliable. In addition, further studies should investigate whether CXCL12 can help physicians tailor the therapy in view of the relative risk and allocate resources accordingly and whether this strategy might affect the outcome.
Conclusions
Circulating CXCL12 serum levels at admission is a useful and complementary biomarker to predict functional outcome and mortality 6 months after acute ischemic stroke. We recommend that further studies should be carried out with respect to its role in the pathophysiology of stroke. If it is possible to elucidate this, more intensive efforts could be directed toward the cause, thus hopefully improving the prognosis of these patients.
References
Bonita R, Mendis S, Truelsen T et al (2004) The global stroke initiative. Lancet Neurol 3:391–393
Song FY, Wu MH, Zhu LH et al (2015) Elevated serum mannose-binding lectin levels are associated with poor outcome after acute ischemic stroke in patients with type 2 diabetes. Mol Neurobiol 52(3):1330–1340
Zhang JL, Yin CH, Zhang Y et al (2013) Plasma copeptin and long term outcomes in acute ischemic stroke. Acta Neurol Scand 128:372–380
Iwamoto T, Okamoto H, Toyama Y et al (2008) Molecular aspects of rheumatoid arthritis: chemokines in the joints of patients. FEBS J 275:4448–4455
Schutt RC, Burdick MD, Strieter RM et al (2012) Plasma CXCL12 levels as a predictor of future stroke. Stroke 43:3382–3386
Nagasawa T (2014) CXC chemokine ligand 12 (CXCL12) and its receptor CXCR4. J Mol Med 92:433–439
Xu Q, Sun XC, Shang XP, Jiang HS (2012) Association of CXCL12 levels in synovial fluid with the radiographic severity of knee osteoarthritis. J Investig Med 60:898–901
Camnitz W, Burdick MD, Strieter RM et al (2012) Dose-dependent effect of statin therapy on circulating CXCL12 levels in patients with hyperlipidemia. Clin Transl Med 1:1–5
Karouzakis E, Rengel Y, Jüngel A et al (2011) DNA methylation regulates the expression of CXCL12 in rheumatoid arthritis synovial fibroblasts. Genes Immun 12:643–652
Domanska UM, Kruizinga RC, Nagengast WB et al (2013) A review on CXCR4/CXCL12 axis in oncology: no place to hide. Eur J Cancer 49:219–230
van den Berk LCJ, van der Veer A, Willemse ME et al (2013) Disturbed CXCR4/CXCL12 axis in pediatric precursor B-cell acute lymphoblastic leukemia. Blood 122:2643–2643
Saiman Y, Jiao JJ, Fiel MI et al (2015) Inhibition of the CXCL12/CXCR4 chemokine axis with AMD3100, a CXCR4 small molecule inhibitor, worsens murine hepatic injury. Hepatol Res 45:794–803
Negrete-García MC, Velazquez JR, Popoca-Coyotl A et al (2010) Chemokine (CXC motif) ligand 12/stromal cell-derived factor-1 is associated with leukocyte recruitment in asthma. CHEST J 138:100–106
Li M, Hale JS, Rich JN et al (2012) Chemokine CXCL12 in neurodegenerative diseases: an SOS signal for stem cell-based repair. Trends Neurosci 35:619–628
Van Der Vorst E, Doering Y, Weber C (2015) MIF and CXCL12 in cardiovascular diseases: functional differences and similarities. Frontiers Immunol 6:373
Robin AM, Zhang ZG, Wang L, Zhang RL, Katakowski M, Zhang L et al (2006) Stromal cell-derived factor 1alpha mediates neural progenitor cell motility after focal cerebral ischemia. J Cereb Blood Flow Metab 26:125–134
Yu X, Chen D, Zhang Y, Wu X, Huang Z, Zhou H et al (2012) Over-expression of CXCR4 in mesenchymal stem cells promotes migration, neuroprotection and angiogenesis in a rat model of stroke. J Neurol Sci 316:141–149
Wurster T, Stellos K, Geisler T, Seizer P, Andia ME, Schuster A et al (2012) Expression of stromal-cell-derived factor-1 (SDF-1): a predictor of ischaemic stroke? Eur J Neurol 19:395–401
Ruscher K, Kuric E, Liu Y et al (2013) Inhibition of CXCL12 signaling attenuates the postischemic immune response and improves functional recovery after stroke. J Cereb Blood Flow Metab 33:1225–1234
Hatano S (1976) Experience from a multicentre stroke register: a preliminary report. Bull World Health Organ 54:541–553
Brott T, Adams HP Jr, Olinger CP et al (1989) Measurements of acute cerebral infarction: a clinical examination scale. Stroke 20:864–870
Adams HP, Bendixen BH, Kappelle LJ et al (1993) Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke 24:35–41
Bamford J, Sandercock P, Dennis M et al (1991) Classification and natural history of clinically identifiable subtypes of cerebral infarction. Lancet 337:1521–1526
Sims JR, Gharai LR, Schaefer PW, Vangel M, Rosenthal ES (2009) ABC/2 for rapid clinical estimate of infarct, perfusion, and mismatch volumes. Neurology 72:2104–2110
Zhang ZG, Wang C, Wang J, Zhang Z et al (2015) Prognostic value of mannose-binding lectin: 90-day outcome in patients with acute ischemic stroke. Mol Neurobiol 51:230–239
Guo Y, Apostalakis S, Blann AD, Lip GY (2014) Plasma CX3CL1 levels and long term outcomes of patients with atrial fibrillation: the West Birmingham Atrial Fibrillation Project. Cerebrovasc Dis 38:204–211
Gu XL, Liu L, Lu XD et al (2015) Serum CXCL12 levels as a novel predictor of future stroke recurrence in patients with acute ischemic stroke. Mol Neurobiol. doi:10.1007/s12035-015-9151-0
Mehta NN, Matthews G, Krishnamoorthy P et al (2012) Higher plasma CXCL12 levels predict incident cardiovascular disease events: findings from the Chronic Renal Insufficiency Cohort Study. Circulation 126(21 Supplement), A13352
Kim YS, Baek W, Kim K et al (2012) Association between serum stromal cell-derived factor-1α and long-term outcome of acute ischemic stroke. Eur Neurol 67:363–369
Kwon HS, Kim YS, Park HH et al (2015) Increased VEGF and decreased SDF-1α in patients with silent brain infarction are associated with better prognosis after first-ever acute lacunar stroke. J Stroke Cerebrovasc Dis 24:704–710
Duan XX, Zhang GP, Wang XB et al (2015) The diagnostic and prognostic value of serum CXCL12 levels in patients with ischemic stroke. Neurol Sci 36:2227–2234
Navarro-Sobrino M, Rosell A, Hernandez-Guillamon M et al (2011) A large screening of angiogenesis biomarkers and their association with neurological outcome after ischemic stroke. Atherosclerosis 216:205–211
Liu P, Xiang JW, Jin SX (2015) Serum CXCL12 levels are associated with stroke severity and lesion volumes in stroke patients. Neurol Res 37:853–858
Bogoslovsky T, Spatz M, Chaudhry A, Maric D, Luby M, Frank J et al (2011) Stromal-derived factor-1α correlates with circulating endothelial progenitor cells and with acute lesion volume in stroke patients. Stroke 42:618–625
Wang Y, Huang J, Li Y et al (2012) Roles of chemokine CXCL12 and its receptors in ischemic stroke. Curr Drug Targets 13:166–172
Petit I, Jin D, Rafii S (2007) The SDF-1-CXCR4 signaling pathway: a molecular hub modulating neo-angiogenesis. Trends Immunol 28:299–307
Emsley HC, Tyrrell PJ (2002) Inflammation and infection in clinical stroke. J Cereb Blood Flow Metab 22:1399–1419
Li M, Ransohoff RM (2008) Multiple roles of chemokine CXCL12 in the central nervous system: a migration from immunology to neurobiology. Prog Neurobiol 84:116–131
Zernecke A, Bot I, Djalali-Talab Y et al (2008) Protective role of CXC receptor 4/CXC ligand 12 unveils the importance of neutrophils in atherosclerosis. Circ Res 102:209–217
van der Vorst EP, Döring Y, Weber C. (2015) Chemokines and their receptors in atherosclerosis. J Mol Med (Berl); Jul 15, DOI: 10.1007/s00109-015-1317-8.
Patel JR, McCandless EE, Dorsey D et al (2010) CXCR4 promotes differentiation of oligodendrocyte progenitors and remyelination. Proc Natl Acad Sci U S A 107:11062–11067
Mirabelli-Badenier M, Braunersreuther V, Viviani GL et al (2011) CC and CXC chemokines are pivotal mediators of cerebral injury in ischaemic stroke. Thromb Haemost 105(3):409
Schönemeier B, Schulz S, Hoellt V et al (2008) Enhanced expression of the CXCl12/SDF-1 chemokine receptor CXCR7 after cerebral ischemia in the rat brain. J Neuroimmunol 198:39–45
Ardelt AA, Bhattacharyya BJ, Belmadani A et al (2013) Stromal derived growth factor-1 (CXCL12) modulates synaptic transmission to immature neurons during post-ischemic cerebral repair. Exp Neurol 248:246–253
Acknowledgments
This study was supported by the National Natural Science Foundation of China (81371438). The funding plays no role in the study. We are grateful to the department of neurology and emergency department; the nurses, physicians, and patients who participated in our study; and the staff of the central laboratory of the hospital.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no competing interests.
Statement of Human Rights
The study was approved by the ethics committee of the First Affiliated Hospital of Zhengzhou University. The patients or their relatives gave written informed consent prior to entering the study.
Funding
This study was supported by the National Natural Science Foundation of China (81371438).
Rights and permissions
About this article
Cite this article
Cheng, X., Lian, YJ., Ma, YQ. et al. Elevated Serum Levels of CXC Chemokine Ligand-12 Are Associated with Unfavorable Functional Outcome and Mortality at 6-Month Follow-up in Chinese Patients with Acute Ischemic Stroke. Mol Neurobiol 54, 895–903 (2017). https://doi.org/10.1007/s12035-015-9645-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-015-9645-9