Abstract
Previous studies had shown that CXC chemokine ligand-12 (CXCL12) plays a significant role in animal models of ischemic stroke, but its role in human stroke is unclear. The aim of this study was to test the relationship between elevated serum circulating CXCL12 levels and the 1-year stroke recurrence in Chinese patients with acute ischemic stroke (AIS). All consecutive patients with first-ever acute ischemic stroke from January 2011 to September 2013 were recruited to participate in the study. Serum levels of CXCL12 and National Institute of Health Stroke Scale (NIHSS) were measured at the time of admission. Logistic regression analysis was used to evaluate the stroke recurrence according to serum CXCL12 levels. Receiver operating characteristic (ROC) curve was used to evaluate the accuracy of serum CXCL12 in predicting stroke recurrence. Clinical follow-up was performed at 1 year. In our study, 248 patients finished the 1-year follow-up. At 1-year follow-up, 31 patients had a recurrence ischemic stroke. The median CXCL12 levels were significantly higher in those who sustained a recurrence ischemic stroke compared with those who did not [24.2 ng/mL (IQR 15.4–33.7) vs 6.5 ng/mL (IQR 3.4–10.2); Z = 8.258, P < 0.0001]. In multivariate analysis, there was an increased risk of stroke recurrence associated with serum CXCL12 levels ≥12.15 ng/mL (OR 9.122, 95 % CI 6.103–15.104) after adjusting for above possible confounders. The time to recurrence stroke distribution between patients with baseline CXCL12 levels ≥12.15 ng/mL and those with baseline CXCL12 levels <12.15 ng/mL were significantly different (P < 0.0001, log-rank test). Elevated circulating CXCL12 levels at admission are strongly associated with the future recurrence of ischemic stroke in Chinese patients with AIS. Further studies are warranted to confirm this association and define the role for CXCL12 as a novel predictor biomarker for stroke recurrence.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Stroke is the second commonest cause of death and leading cause of adult disability in China [1]. China has 2.5 million new stroke cases each year and 7.5 million stroke survivors, and approximately 15 to 30 % of stroke survivors will be permanently disabled [2]. Stroke risk is determined by using traditional risk factors and risk assessment tools [3]. In order to improve stroke risk assessment, researchers have studied the predictive value of lipoprotein (a) [4], thioredoxin [5], high-sensitivity C-reactive protein [6], and insulin-like growth factor I [7] as biomarkers. Early detection and control of risk factors is thought to be crucial in reducing the risk of stroke and providing effective care [8]. Discovery of novel biomarkers that identify subjects at risk for stroke could significantly improve stroke prevention.
The CXC chemokine ligand-12 (CXCL12) which is highly expressed in vascular and hematopoietic progenitor cells is a chemokine that regulates leukocyte trafficking in homeostatic and inflammatory processes [9]. However, recent studies show that CXCL12 may also influence stem and progenitor cell migration, homing, and proliferation [10]. Although previous studies had shown that CXCL12 played a significant role in acute stroke in animal models [11], its role in acute stroke in humans is unclear [12, 13]. Schutt et al. [14] reported that serum CXCL12 levels may represent a novel biomarker of future ischemic stroke in patients undergoing elective coronary angiography. The prognostic value of CXCL12 levels as a predictor of future stroke in patients with stroke has not been tested. The aim of this study was to test the hypothesis that elevated serum CXCL12 levels are associated with stroke recurrence in Chinese patients with acute ischemic stroke (AIS).
Subjects and Methods
Patients and Study Design
All consecutive patients with first-ever acute ischemic stroke from the People’s Hospital of Laiwu City, China, from January 2011 to September 2013 were recruited to participate in the study. Patients were eligible for inclusion if they were admitted to the emergency department with an AIS defined according to the World Health Organization ICD-9 criteria [15] and with symptom onset within 24 h. Exclusion criteria were malignant tumor, intracerebral hemorrhage, renal insufficiency (creatinine >1.5 mg/dL), febrile disorders, acute or chronic inflammatory disease at study enrollment, autoimmune diseases, as well as those with a history of valvular heart disease. The present study has been approved by the ethics committee of the People’s Hospital of Laiwu City. All participants or their relatives were informed of the study protocol, and their written informed consents were obtained.
Clinical Variables and Imaging Test
Demographic data (age and gender), stroke etiology, blood pressure, presence of risk factors such as hypertension, diabetes mellitus, hyperlipoproteinemia, heart disease, alcohol consumption, and smoking habit, positive family history for myocardial infarction, stroke, or transient ischemic attack were recorded at admission. Routine laboratory testing was always done. Patients were evaluated the National Institute of Health Stroke Scale (NIHSS, scores range from 0 to 42, with greater scores indicating increasing severity) [16] score at their admission, performed by a stroke neurologist certified in the use of this scale. Stroke etiology was determined according to the criteria of the Trial of Org 10172 in Acute Stroke Treatment (TOAST) classification [17]. The clinical stroke syndrome was determined applying the criteria of the Oxfordshire Community Stroke Project (OCSP), that is, total anterior circulation syndrome (TACS), partial anterior circulation syndrome (PACS), lacunar syndrome (LACS), and posterior circulation syndrome (POCS) [18]. The OCSP and TOAST classifications were verified by the brain imaging. Brain imaging (either CT or MRI) was done routinely within 24 h after admission. MRI with diffusion-weighted imaging (DWI) was available for some patients. In those patients, DWI lesion volumes were determined by an experienced neurologist (Liu L) who was unaware of the clinical and laboratory results. The infarct volume was calculated by using the formula 0.5 × a × b × c (where a is the maximal longitudinal diameter, b is the maximal transverse diameter perpendicular to a, and c is the number of 10-mm slices containing infarct) [19].
End Points and Follow-Up
The endpoint was structured follow-up telephone interview at year 1, if the patients discharged. The follow-up was conducted base on a standardized interview protocol. The interviewers were centrally trained with the interview protocol. Patients were called on three separate occasions at 1-year time point. If there was no response, a letter was sent to the patient’s home asking them to contact the investigators to provide clinical follow-up information. In patients who had a recurrence stroke, medical records from the stroke admission were reviewed by the investigators. If the patients were all-cause death within 1-year, they would be excluded from our study to avoid disturbing the families of the deceased.
Blood Collection and Quantification
Fasting venous blood was collected from all participants in vacutainer tubes and quickly centrifuged to avoid glycolysis. Serum samples were kept at −80 °C until assay. Serum CXCL12 levels of patients were blindly assessed by multiplex immunoassay using the manufacturer’s instruction (Luminex, Bio-Rad, Bio-plex 200 system, Hercules, CA; Procarta Cytokine Assay kit, Panomics, Inc., Fremont, CA). The inter-assay and intra-assay coefficients of variation (CV) for CXCL12 were shown to be 4.2–6.3 and 4.8–7.5 %. The lower detection limit was 0.5 ng/mL. Other biomarkers, such as glucose, high-sensitivity C-reactive protein (Hs-CRP) and homocysteine (HCY) were also tested by standard laboratory method. For all measurements, levels that were not detectable were considered to have a value equal to the lower limit of detection of the assay.
Statistical Analysis
The results were expressed as percentages for categorical variables and as medians (interquartile ranges (IQRs)) for continuous variables. The Mann-Whitney U test and chi-squared test were used to compare the two groups. Spearman’s rank correlation was used for bivariate correlations. The relation of CXCL12 with the endpoint was investigated with the use of logistic regression models in multivariate adjustment with possible confounders, i.e., age, gender, infarct volume, NIHSS score, time from onset to admission, time from onset to blood collection, stroke syndrome, stroke etiology, vascular risk factors, and serum levels of Hs-CRP, HCY, and glucose. We used crude models and multivariate models adjusted for all significant predictors and reported odds ratios (ORs). Further, receiver operating characteristic curves (ROC) was used to test the overall prognostic accuracy of the NIHSS and serum biomarkers, and the results were reported as area under the curve (AUC). All statistical analyses were performed with SPSS for Windows, version 20.0 (SPSS Inc., Chicago, IL, USA) and the ROCR package (version 1.0–2), which is available from CRAN repository (http://cran.r-project.org/). Statistical significance was defined as P < 0.05.
Results
Baseline Characteristics of the Study Population
From 359 screened patients, a total of 302 patients with first-ever AIS were included in this study (24 with transient ischemic attack, 10 with hemorrhagic stroke, 8 with onset of symptoms >24 h, 6 without informed consent, 7 with systemic infections, and 2 with malignant tumor were not analyzed), and 248 finished the 1-year follow-up (39 patients were died and 15 lost flow-up). At 1-year follow-up, 31 patients had a recurrence ischemic stroke, thus, the rate of recurrence rate was 12.5 %. In the study population, 144 (58.1 %) were male and the median age was 64 years (IQR 55–72). The median time from symptom recognition to admission to hospital was 7.2 h (IQR 3.3–15.2), and 155 patients (62.5 %) were admitted within 12 h of symptom recognition. The median time from symptom recognition to blood collection was 13.2 h (IQR 8.5–23.7). The median NIHSS score on admission was 7 points (IQR 4–11). In addition, the number of tissue plasminogen activator-treated patients was 72 (29.0 %). The baseline characteristics of the 248 patients presenting with and without recurrence stroke are described in Table 1.
Main Results
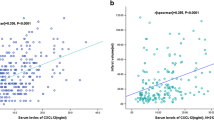
Serum CXCL12 levels increased with increasing severity of stroke as defined by the NIHSS score. There was a positive correlation between levels of CXCL12 and NIHSS score (r = 0.301, P < 0.0001). We also found that there a positive trend between serum CXCL12 levels and Hs-CRP (r = 0.202, P = 0.006), age (r = 0.193, P = 0.012). Statistical analysis here also revealed no influence of sex, time from symptom onset to include, stroke syndrome, stroke etiology risk factors of stroke, and HCY on CXCL12 in AIS patients (P > 0.05, respectively). In the subgroup of patients (n = 186) in whom MRI was available, the median infarct volume was 25 mL (IQR 10–48). The serum CXCL12 levels paralleled with the size of lesions. There was a significant positive association between serum CXCL12 levels and infarct volume (r = 0.313, P < 0.0001).
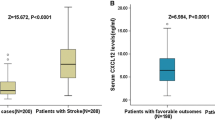
Serum CXCL12 levels were significantly higher in patients who had a recurrence ischemic stroke at follow-up compared with the nonrecurrence stroke cohort [24.2 ng/mL (IQR 15.4–33.7) vs 6.5 ng/mL (IQR 3.4–10.2); Z = 8.258, P < 0.0001; Fig. 1.]. Based on the ROC curve, the optimal cutoff value of serum CXCL12 levels as an indicator for predicting recurrence ischemic stroke within 1 year was projected to be 12.15 ng/mL, which yielded a sensitivity of 83.9 % and a specificity of 82.7 %, with the area under the curve at 0.893 (95 % CI 0.823–0.964). With an AUC of 0.893, CXCL12 showed a significantly greater discriminatory ability as compared with Hs-CRP (AUC 0.759; 95 % CI 0.678–0.841; P < 0.001), HCY (AUC 0.708; 95 % CI 0.628–0.787, P < 0.001) and NIHSS score (AUC 0.679; 95 % CI 0.577–0.780; P < 0.0001; Fig. 2). Interestingly, combined model (CXCL12 and NIHSS) improved those markers alone (AUC of the combined model 0.913; 95 % CI 0.843–0.979; P < 0.01). This improvement was stable in an internal fivefold cross validation that resulted in an average AUC (standard error) of 0.68 (0.045) for the NIHSS and 0.91 (0.022) for the combined model, corresponding to a difference of 0.23 (0.023).
In univariate logistic regression analysis, CXCL12 as a continuous variable was associated with an increased risk of recurrence ischemic stroke with an unadjusted OR of 2.390 (95 % CI 1.432–3.867; P < 0.0001). After adjusting for all other possible covariates, such as sex, age, family history for stroke, clinical and laboratory findings, CXCL12 remained can be seen as an independent risk of recurrence ischemic stroke with an adjusted OR of 1.587 (95 % CI 1.244–2.208; P < 0.0001). This relationship was confirmed in the dose-response model. Further, in our study, we found that an increased risk of recurrence ischemic stroke was associated with CXCL12 serum level ≥12.15 ng/mL (unadjusted OR 19.117, 95 % CI 7.620–47.963). In multivariate analysis, there was an increased risk of recurrence ischemic stroke associated with serum CXCL12 levels ≥12.15 ng/mL (OR 9.122, 95 % CI 6.103–15.104) after adjusting for above possible confounders. In the subgroup of patients (n = 186) in whom MRI evaluations were performed, serum CXCL12 levels ≥12.15 ng/mL was an independent predictor with an OR of 11.641 (95 % CI 3.302–22.564; P < 0.001) after adjustment for both lesion size and the NIHSS score. In addition, the NIHSS score and laboratory findings, such as HCY and Hs-CRP remained significant predictors (Table 2).
The time to recurrence stroke distribution between patients with baseline CXCL12 levels ≥12.15 ng/mL and those with baseline CXCL12 levels <12.15 ng/mL were significantly different (P < 0.0001, log-rank test; Fig. 3). The weighted Cox proportional hazard model demonstrated that baseline CXCL12 levels ≥12.15 ng/mL were significantly associated with stroke at follow-up (hazards ratio 19.12; 95 % CI 7.62–47.96; P < 0.0001).
Discussion
CXCL12 plays a pivotal role in angiogenesis and the regeneration of ischemic tissue through the regulation of hematopoietic progenitor cells and is upregulated at the sites of vascular injury and platelet activation [20]. Thus, CXCL12 has recently been discussed as a predictor in acute ischemic stroke. After ischemic stroke, CXCL12 mediates the inflammatory response by recruitment of neural progenitor cells and the mobilization of bone marrow-derived progenitor cells for tissue regeneration and neovascularization [21]. We firstly found that, in our cohort, elevated CXCL12 levels at admission were strongly associated with recurrence ischemic stroke at 1-year follow-up even after adjusting for traditional risk factors in Chinese patients with AIS. Thus, CXCL12 may be an important biomarker for stroke recurrence and individuals in whom more aggressive risk factor modification, diagnostic evaluation, or even intervention is warranted.
A few reports described the behavior of CXCL12 in patients with acute ischemic stroke, but its role in humans with stroke had controversies. Kim et al. [11] found that serum CXCL12 levels were higher in acute-stage stroke patients compared with the normal control group (P = 0.011), but Wurster et al. [20] reported that there was no significant difference in circulating CXCL12 levels between patients with stroke and normal control subjects. Schutt et al. [14] suggested that serum CXCL12 levels may represent a novel biomarker of future ischemic stroke in patients undergoing elective coronary angiography, which were supported by our results. Our study added to the literature regarding CXCL12 and stroke in humans and was unique because we were able to use CXCL12 levels measured at baseline to predict a future stroke occurrence in patients with AIS.
In a previous study, Wurster et al. [20] reported that CXCL12 expression showed a trend with the severity of stroke according to NIHSS (r = 0.125; P = 0.085), but significantly correlated with the peak levels of C-reactive protein (r = 0.218; P = 0.002). Interestingly, in our study, we found that there were positive correlation between levels of CXCL12 and NIHSS score (r = 0.301, P < 0.0001) and Hs-CRP (r = 0.202, P = 0.006). In addition, our findings of a positive linear association between serum CXCL12 and infarct volume (r = 0.313, P < 0.0001) stand in contrast to a previous report showing a moderately inversely (r = −0.49) correlated with baseline diffusion-weighted imaging lesion volumes (P < 0.04) [12]. This study included only 17 patients and may have lacked the power to support its conclusions.
Whether higher circulating CXCL12 level is an accelerator or only be a marker of stroke recurrence remains uncertain. It is important to discuss whether CXCL12 in patients with stroke recurrence have pathological roles or just was as indicator. CXCL12 play important roles in multiple processes after ischemic stroke, which include inflammatory response, focal angiogenesis, and the recruitment of bone marrow-derived cells (BMCs) and neural progenitor cell (NPC) to injury [22]. Investigators have demonstrated increased CXCL12 expression in atherosclerotic plaques obtained from human carotid endarterectomy specimens [23]. Hill et al. [24] suggested CXCL12 was important in the homing of bone marrow-derived cells, especially monocytes, to areas of ischemic injury, while Schönemeier et al. [25] reported that CXCl12 expression increased strongly in the peri-infarct and infarct region, which was accompanied by the appearance of numerous CXCR4-expressing but not CXCR7-expressing cells in the rat. More works should be done to draw conclusions about the connection between CXCl12 and stroke risk.
Several limitations of this study must be acknowledged. Firstly, the relatively small sample size may limit the generalization of the results of this study. Before broad implementation, additional studies are needed for external validation. In addition, some patients with recurrence stroke were died and excluded in our follow-up. This will cause a selection bias. Another potential limitation of this approach is the fact that none of the biomarker is disease specific and may be elevated in the setting of medical comorbidities. Thirdly, we only tested the serum levels of CXCL12 at admission. Without serial measurement of the circulating CXCl12 levels, this study yielded no data regarding when and how long biomarkers were elevated in these patients. Through measurement and analysis of this marker, we might know who will respond to a particular therapy, and then is serves a greater purpose. Fourthly, functional outcome data are lacking. Further studies are needed to determine whether serum CXCL12 levels predict outcomes after a stroke in our population. Interestingly, in another study, Kim et al. [11] found that CXCL12 was an independent predictor of functional outcome after stroke. Fifthly, infarct volume based on the formula for hematoma volumetry (0.5 × a × b × c) in our study protocol was suboptimal. Besides, number and location of the infarct was not evaluated. Future studies on location of the infarct and white matter changes will be needed to further disentangle the effect of these factors on stroke recurrence. Finally, we measured CXCL12 in serum, not in cerebral spinal fluid (CSF). It is still uncertain whether peripheral CXCL12 levels reflect similar changes in the central nervous system (CNS).
Conclusions
Elevated circulating CXCL12 levels at admission are strongly associated with the future recurrence of ischemic stroke in Chinese patients with AIS. Further studies are warranted to confirm this association and define the role for CXCL12 as a novel predictor biomarker for stroke.
Abbreviations
- CXCL12:
-
CXC chemokine ligand-12
- AIS:
-
Acute ischemic stroke
- NIHSS:
-
National Institutes of Health Stroke Scale
- TOAST:
-
Trial of Org 10172 in Acute Stroke Treatment
- OCSP:
-
Oxfordshire Community Stroke Project
- TACS:
-
Total anterior circulation syndrome
- PACS:
-
Partial anterior circulation syndrome
- LACS:
-
Lacunar syndrome
- POCS:
-
Posterior circulation syndrome
- MRI:
-
Magnetic resonance imaging
- DWI:
-
Diffusion-weighted imaging
- Hs-CRP:
-
High-sensitivity C-reactive protein
- HCY:
-
Homocysteine
- IQR:
-
Interquartile range
- CV:
-
Coefficients of variation
- ORs:
-
Odds ratios
- ROC:
-
Receiver operating characteristic
- BMCs:
-
Marrow-derived cells
- NPC:
-
Neural progenitor cell
- CSF:
-
Cerebral spinal fluid
- CNS:
-
Central nervous system
References
Zhang ZG, Wang C, Wang J et al (2015) Prognostic value of mannose-binding lectin: 90-day outcome in patients with acute ischemic stroke. Mol Neurobiol 51(1):230–239
Bonita R, Mendis S, Truelsen T, Bogousslavsky J, Toole J, Yatsu F (2004) The global stroke initiative. Lancet Neurol 3:391–393
Wolf PA, D’Agostino RB, Belanger AJ, Kannel WB (1991) Probability of stroke: a risk profile from the Framingham Study. Stroke 22:312–318
Li S, Gao Y, Ma W et al (2014) The relationship between serum lipoprotein (a) levels and ischemic stroke risk: a cohort study in the Chinese population. Inflammation 37:686–693
Wu MH, Song FY, Wei LP et al (2014) Serum levels of thioredoxin are associated with stroke risk, severity, and lesion volumes. Mol Neurobiol. doi:10.1007/s12035-014-9016-y
Ballantyne CM, Hoogeveen RC, Bang H et al (2005) Lipoprotein-associated phospholipase A2, high-sensitivity C-reactive protein, and risk for incident ischemic stroke in middle-aged men and women in the Atherosclerosis Risk in Communities (ARIC) study. Arch Intern Med 165:2479–2484
Dong X, Chang G, Ji XF et al (2014) The relationship between serum insulin-like growth factor I levels and ischemic stroke risk. PLoS One 9:e94845
Wang C, Gao L, Zhang ZG et al (2015) Procalcitonin is a stronger predictor of long-term functional outcome and mortality than high-sensitivity C-reactive protein in patients with ischemic stroke. Mol Neurobiol. doi:10.1007/s12035-015-9112-7
Gerard C, Rollins BJ (2001) Chemokines and disease. Nat Immunol 2:108–115
Takahashi M (2010) Role of the SDF-1/CXCR4 system in myocardial infarction. Circ J 74:418–423
Kim YS, Baek W, Kim MK et al (2012) Association between serum stromal cell-derived factor-1α and long-term outcome of acute ischemic stroke. Eur Neurol 67:363–369
Bogoslovsky T, Spatz M, Chaudhry A, Maric D, Luby M, Frank J et al (2011) Stromal-derived factor-1α correlates with circulating endothelial progenitor cells and with acute lesion volume in stroke patients. Stroke 42:618–625
Paczkowska E, Kucia M, Koziarska D, Halasa M, Safranow K, Masiuk M et al (2009) Clinical evidence that very small embryonic-like stem cells are mobilized into peripheral blood in patients after stroke. Stroke 40:1237–1244
Schutt RC, Burdick MD, Strieter RM et al (2012) Serum CXCL12 levels as a predictor of future stroke. Stroke 43:3382–3386
Hatano S (1976) Experience from a multicentre stroke register: a preliminary report. Bull World Health Organ 54:541–553
Brott T, Adams HP Jr, Olinger CP, Marler JR, Barsanl WG et al (1989) Measurements of acute cerebral infarction: a clinical examination scale. Stroke 20:864–870
Adams HP Jr, Bendixen BH, Kappelle LJ et al (1993) Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke 24:35–41
Bamford J, Sandercock P, Dennis M, Burn J, Warlow C (1991) Classification and natural history of clinically identifiable subtypes of cerebral infarction. Lancet 337:1521–1526
Sims JR, Gharai LR, Schaefer PW, Vangel M, Rosenthal ES (2009) ABC/2 for rapid clinical estimate of infarct, perfusion, and mismatch volumes. Neurology 72:2104–2110
Wurster T, Stellos K, Geisler T, Seizer P, Andia ME, Schuster A et al (2012) Expression of stromal-cell-derived factor-1 (SDF-1): a predictor of ischaemic stroke? Eur J Neurol 19:395–401
Li M, Ransohoff RM (2008) Multiple roles of chemokine CXCL12 in the central nervous system: a migration from immunology to neurobiology. Prog Neurobiol 84:116–131
Wang Y, Huang J, Li Y et al (2012) Roles of chemokine CXCL12 and its receptors in ischemic stroke. Curr Drug Targets 13:166–172
Abi-Younes S, Sauty A, Mach F, Sukhova GK, Libby P, Luster AD (2000) The stromal cell-derived factor-1 chemokine is a potent platelet agonist highly expressed in atherosclerotic plaques. Circ Res 86:131–138
Hill WD, Hess DC, Martin-Studdard A et al (2004) SDF-1 (CXCL12) is upregulated in the ischemic penumbra following stroke: association with bone marrow cell homing to injury. J Neuropathol Exp Neurol 63:84–96
Schönemeier B, Schulz S, Hoellt V et al (2008) Enhanced expression of the CXCl12/SDF-1 chemokine receptor CXCR7 after cerebral ischemia in the rat brain. J Neuroimmunol 198:39–45
Acknowledgments
We also express our gratitude to all the patients who participated in this study and thereby made this work possible.
Conflict of Interest
The authors have no relevant potential conflicts of interest to declare.
Author information
Authors and Affiliations
Corresponding author
Additional information
The content has not been published or submitted for publication elsewhere.
Rights and permissions
About this article
Cite this article
Gu, XL., Liu, L., Lu, XD. et al. Serum CXCL12 Levels as a Novel Predictor of Future Stroke Recurrence in Patients with Acute Ischemic Stroke. Mol Neurobiol 53, 2807–2814 (2016). https://doi.org/10.1007/s12035-015-9151-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-015-9151-0