Abstract
The activation of the complement system may be involved in the pathology of stroke and type 2 diabetes (T2DM). We therefore evaluated the long-term prognostic value of early measurement of serum mannose-binding lectin (MBL) levels, an activator of the complement system, in Chinese T2DM with acute ischemic stroke (AIS). Serum MBL levels were determined in T2DM patients with AIS (N = 188). The adjudicated end points were 1-year functional outcomes and mortality. The prognostic value of MBL was compared with the National Institutes of Health Stroke Scale score and with other known outcome predictors. Patients with an unfavorable outcomes and nonsurvivors had significantly increased MBL levels on admission (P < 0.0001 and P < 0.0001). Multivariate logistic regression analysis adjusted for common risk factors showed that MBL was an independent predictor of functional outcome (odds ratio (OR) = 8.99, 95 % CI 2.21–30.12) and mortality (OR = 13.22, 95 % CI 2.05–41.21). The area under the receiver operating characteristic curve of MBL was 0.75 (95 % CI 0.68–0.83) for functional outcome and 0.85 (95 % CI 0.80–0.90) for mortality. In type 2 diabetic patients with stroke, high levels of MBL predict future functional outcomes and mortality. This indicated that the elevated MBL levels may play a significant role in the pathology of the subsequent damage and that the pathways leading to complement activation warrant further exploration as potential therapeutic targets to improve the prognosis for these patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
China has a high burden of type 2 diabetes (T2DM) and stroke. Diabetes has become a major public health problem in China. In 2009, the age-standardized prevalence of total diabetes and prediabetes was 9.7 and 15.5 %, respectively, accounting for 92.4 million adults with diabetes and 148.2 million adults with prediabetes [1]. Stroke is the second most common cause of death and leading cause of adult disability in China. China has 2.5 million new stroke cases each year and 7.5 million stroke survivors [2]. T2DM is a significant cause of premature mortality and morbidity especially related to cardiovascular disease (i.e., stroke), while the severity of ischemic heart disease is markedly enhanced in T2DM.
Mannose-binding lectin (MBL) is a major component of the innate immune system. It belongs to the collectin family of proteins in which lectin (carbohydrate recognition) domains are found in association with collagenous structures [3]. MBL recognizes and binds repeating carbohydrate moieties on the surfaces of microorganisms, which results in complement activation and opsonization of pathogens [4]. There is evidence that the protein has at least four distinct functions: (1) the activation of complement; (2) the promotion of (complement-independent) opsonophagocytosis; (3) the modulation of inflammation; and (4) the promotion of apoptosis [2]. Research over the past decade indicated that MBL provides a distinct third pathway of complement activation, and phylogenetic studies suggested that it may have been the first such pathway to have evolved [5]. There is increasing evidence from studies of MBL–disease associations which point to the protein having a major role in the modulation of inflammation.
The MBL2 gene codes for the active MBL protein and has three known mutations. These mutations lead to structural abnormalities and cause MBL deficiency [3]. MBL deficiency has been associated to infectious diseases [6], coronary artery disease (CAD), and atherosclerosis in different clinical situations [7]. Several recent studies have suggested that not only low, but also high MBL levels are associated with atherosclerosis [8–10]. Pesonen et al. [11] found that MBL might have a dual role both decreasing susceptibility to infections and increasing the risk of acute coronary syndromes. Elevated levels of MBL were associated with an increased risk of stroke in Chinese population [12], while MBL‐associated serine protease (MASPs) levels were associated with cardiovascular risk factors including dyslipidaemia, obesity, and hypertension [13]. Zhang et al. [14] found that the MBL genes were associated with T2DM in the Chinese population living in the northern areas of China. Elawa et al. [15] reported that elevated serum MBL in T2DM patients indicated a possible poor diabetic control and bad progression of the disease with possibility of the presence, while in another study, log MBL levels were not associated with the occurrence of cardiovascular events in type 2 diabetic South Asians [16].
A number of publications have now appeared which suggest that MBL is also able to modulate disease severity in both infectious and autoimmune disease [3]. Investigations in Denmark and Hong Kong had suggested that MBL variant alleles are associated with both severity and early onset of disease in patients with rheumatoid arthritis [17, 18]. Currently, no data were available on the role of MBL in the progression of stroke in patients with T2DM. In this study, we therefore evaluated the long-term prognostic value of early measurement of serum MBL levels in Chinese T2DM patients with acute ischemic stroke (AIS).
Subjects and Methods
Patients and Study Design
We conducted a prospective cohort study at the emergency department of our hospital. From 2011 to 2012, all T2DM mellitus patients with first-ever AIS were included. All patients were Chinese. All patients were admitted within 48 h of experiencing a new focal or global neurological event. T2DM mellitus and AIS were defined according to the World Health Organization criteria. We excluded patients with malignant tumor, intracerebral hemorrhage, and a history of recent surgery or trauma during the preceding 2 months, renal insufficiency, febrile disorders, and systemic infections at study enrollment, autoimmune diseases with or without immunosuppressive therapy.
In our study, 100 age- and gender-matched healthy volunteers were assigned to the normal group. The median age of normal included in this study was 68 years (interquartile range (IQR), 57–79) and 44 % were women. The study was approved by the ethics committee of Linyi People’s Hospital. The patients or their relatives gave written informed consent prior to entering the study.
Clinical Variables
At baseline, the following demographic and clinical data were taken: gender, age, leukocyte count, duration of diabetes, daily insulin dose, and history of conventional vascular risk factors (hypertension, atrial fibrillation, hyperlipoproteinemia, smoking habit, and alcohol abuse). Routine blood and biochemical tests, electrocardiogram, and a baseline brain computer tomography (CT) or magnetic resonance imaging (MRI) scan were performed in all patients at admission. All patients received treatment according to current guidelines. Stroke severity was assessed on admission using the National Institutes of Health Stroke Scale (NIHSS) by a neurologist [19]. Stroke subtype was classified according to Trial of Org 10172 in Acute Stroke Treatment (TOAST) criteria [20]. The clinical stroke syndrome was determined by applying the criteria of the Oxfordshire Community Stroke Project: total anterior circulation syndrome (TACS); partial anterior circulation syndrome (PACS); lacunar syndrome (LACS); and posterior circulation syndrome (POCS) [21].
Neuroimaging
Brain imaging (either CT or MRI) was performed routinely within 24 to 48 h after admission. Diagnosis of stroke was based on the results of strict neurological examination (CT, MRI, or both) according to the International Classification of Diseases, ninth revision. CCT was performed in all patients on admission mainly to exclude intracranial hemorrhage. Thereafter, MRI was performed using a stroke protocol, including T1-, T2-, and diffusion-weighted imaging (DWI) sequences, and a magnetic resonance angiography. In those patients, DWI lesion volumes were determined by one experienced neurologist unaware of the clinical and laboratory results. The infarct volume was calculated by using the formula 0.5×a×b×c (where a is the maximal longitudinal diameter, b is the maximal transverse diameter perpendicular to a, and c is the number of 10-mm slices containing infarct) [22].
End Points and Follow-up
Functional outcome was obtained on year 1 according to the modified Rankin Scale (mRS) [23] blinded to MBL levels. The primary end point of this study was favorable functional outcome of stroke patients after 1 year from baseline, defined as an mRS score of 0 to 2 points. Secondary end point in stroke patients was death or withdrawn from any cause within a 1-year follow-up. Outcome assessment was performed by one trained medical staff blinded to MBL levels with a structured follow-up telephone interview with the patient or, if not possible, with the relative.
MBL Analyses
For the purpose of this study, blood samples of patients who were admitted to hospital were drawn from the antecubital vein at the first morning after admission. After centrifugation, serum of the samples was immediately stored at −80 °C before assay. MBL was measured by time-resolved immunofluorometric assay on serum samples. Microwells coated with anti-MBL antibody were incubated with dilutions of patient serum, were developed with europium-labeled anti-MBL antibody, and europium was quantified with time-resolved fluorometric assay (Baoman Biological Technology Co., Ltd, Shanghai, China). The detection limit was 1.5 μg/L. The standard concentrations in these kits range from 1.5 to 100 μg/L, providing a range of 150–10,000 μg/L at 1/100 dilution. The coefficients of variation (CV) for the intra- and inter-assay reproducibility were 4.2–5.0 and7.8–9.0 %, respectively. The median value of morning serum MBL level in our normal cases was 913 μg/L. The median in healthy individuals using this modification was in the range of the reported by Hansen and colleagues [24] (800–1,000 ng/mL in healthy Caucasiansis), while was lower than another study finished in Chinese sample [25]. For all measurements, levels that were not detectable were considered to have a value equal to the lower limit of detection of the assay.
Statistical Analysis
Results are expressed as percentages for categorical variables and as medians (IQRs) for the continuous variables. Univariate data on demographic and clinical features were compared by Mann–Whitney U test or Chi-Square test as appropriate. Correlations among continuous variables were assessed by the Spearman rank correlation coefficient. In addition, associations between MBL and NIHSS score and infarct volume were also assessed using ordered logistic regression models in multivariate adjustment with possible confounders.
To investigate whether MBL allows predicting of both functional outcome and death after 1 year in T2DM with stroke, different statistical methods were used. First, the relation of MBL with the two end points was investigated with the use of logistic regression models. Therefore, common logarithmic transformation (i.e., ln) was performed to obtain normal distribution for skewed variables (i.e., MBL concentrations). We used crude models and multivariate models adjusted for all significant outcome predictors and report odds ratios (ORs). For multivariate analysis, we included confounders, known risk factors, and other outcome predictors as assessed in univariate analysis. Note that the OR corresponds to a one-unit increase in the explanatory variable; for the ln-transformed MBL values, this corresponds to an e-fold increase.
Second, we compared different prognostic risk scores from different predictive models by calculating receiver operating characteristic curve (ROC) analysis. ROC was used to test the overall prognostic accuracy of MBL; the NIHSS and other serum biomarkers and results were reported as area under the curve (AUC). To test whether the MBL levels improve score performance, we considered the nested models with NIHSS and MBL as compared with NIHSS only.
Third, new reclassification metrics have been shown to provide information about the usefulness of the serum MBL. We calculated reclassification tables to further investigate the benefit of MBL levels as compared with the NIHSS score alone, and results are reported as net reclassification improvement for outcome and mortality risk categories, as proposed previously [26]. Finally, in order to study the ability of MBL for mortality prediction, we calculated Kaplan–Meier survival curves and stratified patients by MBL quartiles. All statistical analysis was performed with SPSS for Windows, version 20.0 (SPSS Inc., Chicago, IL, USA), and STATA 9.2 (Stata Corp, College Station, TX), R version 2.8.1. Statistical significance was defined as P < 0.05.
Results
Patient Characteristics
In our study, 188 AIS patients with T2DM completed 1-year follow-up and were included in the analysis. The median age of patients included in this study was 68 years (IQR, 57–79) and 56.4 % were men. In those patients, the median duration of diabetes on admission was 10 years (IQR, 4–15). The median NIHSS score on admission was 8 points (IQR, 5–13). The median time from stroke onset to inclusion in the study was 5.5 h (IQR, 2.6–10.8). An unfavorable functional outcome was found in 67 patients (35.6 %) with a median mRS score of 4 (IQR, 3–6). Thirty-two patients died; thus, the mortality rate was 17.0 %. In addition, the number of tissue plasminogen activator-treated patients was 82 (43.6 %), while 87 patients received intensive glucose treatment. Basal characteristics of patients with AIS are provided in Table 1 and supplementary Table 1.
Main Results
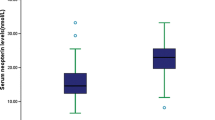
The results indicated that the serum MBL levels were significantly (P < 0.0001) higher in stroke patients with T2DM as compared to normal cases [2,992 (IQR, 2,414–3,515 μg/L); 913 (IQR, 690–1,100 μg/L), respectively] (Fig. 1). Serum MBL levels increased with increasing severity of stroke as defined by the NIHSS score (Fig. 2a). There was a modest correlation between levels of MBL and NIHSS score (r = 0.716, P < 0.0001). There was still a significant positive correction between MBL serum levels and NIHSS score, using ordered logistic regression after multivariate adjustment for possible confounders (P = 0.009). In addition, serum MBL levels were also correlated with Hs-CRP (Fig. 2b), whereas there was a significant, albeit weak, positive correlation between MBL levels and blood glucose in T2DM patients with stroke(r = 0.15, P = 0.003). There was no correlation between serum levels of MBL and others factors, such as sex, age, triglyceride, cholesterol, LDL and HDL, homocysteine, duration of diabetes, and daily insulin dose (P > 0.05, respectively). In patients for whom MRI data were available (n = 120), there was a positive correlation between levels of MBL and the infarct volume (r = 0.404, P < 0.0001; Fig. 2c.)
MBL and 1-Year Functional Outcome
In the 67 patients with an unfavorable functional outcome, serum MBL levels were higher compared with those in patients with a favorable outcome [3,230 μg/L (IQR, 3,030–3,950) vs 2,650 μg/L (IQR, 2,183–3,300); P < 0.0001) (Fig. 3a). In univariate logistic regression analysis, we calculated the OR of ln-transformed MBL levels as compared with the NIHSS score and other risk factors as presented in Table 2. With an unadjusted OR of 28.15 (95 % CI, 7.07–112.00), MBL had a strong association with unfavorable functional outcome. After adjusting for all other significant outcome predictors, MBL remained an independent outcome predictor with an adjusted OR of 8.99 (95 % CI, 2.21–30.12). In the subgroup of patients (n = 120) in whom MRI evaluations were performed, MBL was an independent unfavorable outcome predictor with an OR of 10.64 (95 % CI, 1.98–36.56; P < 0.001) after adjustment for both lesion size and the NIHSS score. In addition, age, the NIHSS score, and laboratory findings, such as glucose level and Hs-CRP, remained significant outcome predictors (Table 2).
Serum MBL levels in different groups. a Serum levels of MBL in patients with favorable outcomes and unfavorable outcomes; b serum levels of MBL in survivor and nonsurvivor. A favorable functional outcome was defined as an mRS score of 0 to 2 points, while unfavorable outcome was defined as 3–6 points
With an AUC of 0.75 (95 % CI, 0.68–0.83), MBL showed a significantly greater discriminatory ability as compared with Hs-CRP (AUC, 0.65; 95 % CI, 0.57–0.73; P < 0.001) and age (AUC, 0.56; 95 % CI, 0.50–0.63; P < 0.0001), while was in the range of NIHSS score (AUC, 0.73; 95 % CI, 0.65–0.80; P = 0.046). Interestingly, MBL improved the NIHSS score (AUC of the combined model, 0.83; 95 % CI, 0.76–0.89; P < 0.001). This improvement was stable in an internal 5-fold cross-validation that resulted in an average AUC (standard error) of 0.73 (0.037) for the NIHSS and 0.83 (0.027) for the combined model, corresponding to a difference of 0.10(0.010). In addition, a model containing known risk factors plus MBL compared with a model containing known risk factors without MBL showed a greater discriminatory ability (Table 3).
MBL and 1-Year Mortality
At 1 year, 32 patients (17.0 %) had died. Nonsurvivors had significantly higher MBL levels than survivors (3,910 [IQR, 3,360–4,120] vs 2,765 μg/L [IQR, 2,320–3,300]; P < 0.0001; see Fig. 3b). In univariate logistic regression analysis, we calculated the OR of ln-transformed MBL levels as compared with the NIHSS score and other risk factors as presented in Table 2. With an unadjusted OR of 34.11 (95 % CI, 6.32–126.12), MBL had a strong association with mortality. After adjusting for all other significant outcome predictors, MBL remained an independent outcome predictor with an adjusted OR of 13.22 (95 % CI, 2.05–41.21). In the subgroup of patients (n = 120) in whom MRI evaluations were performed, MBL was an independent mortality predictor with an OR of 14.87 (95 % CI, 1.55–51.11; P < 0.001) after adjustment for both lesion size and the NIHSS score. In addition, age, the NIHSS score, and laboratory findings, such as glucose level and Hs-CRP, remained significant outcome predictors (Table 2).
Similarly, with an AUC of 0.85 (95 % CI, 0.80–0.90), MBL showed a significantly greater discriminatory ability as compared with NIHSS score (AUC, 0.77; 95 % CI, 0.67–0.87; P < 0.001), Hs-CRP (AUC, 0.69; 95 % CI, 0.63–0.75; P < 0.0001), and age (AUC, 0.65; 95 % CI, 0.58–0.71; P < 0.0001). Interestingly, MBL also improved the NIHSS score (AUC of the combined model, 0.90; 95 % CI, 0.82–0.96; P < 0.0001). Again, this improvement was stable in an internal 5-fold cross-validation that resulted in an average AUC (standard error) of 0.77 (0.041) for the NIHSS and 0.90 (0.024) for the combined model, corresponding to a difference of 0.13 (0.017). In addition, a model containing known risk factors plus MBL compared with a model containing known risk factors without MBL showed a greater discriminatory ability (Table 3).
The time to death was analyzed by Kaplan–Meier survival curves based on serum MBL quartiles. Patients in the upper two quartiles had a higher risk of death compared to patients with MBL levels in the lower two quartile (Fig. 4).
Kaplan–Meier survival based on MBL quartiles. Time to death was analyzed by Kaplan–Meier curves based on MBL quartiles. Patients in the lower two quartile (MBL <2,414 μg/l and MBL between 2,414 and 2,992μg/l) had a minor risk of death compared to patients with MBL levels in the higher two quartile (MBL >3,515 μg/l and MBL between 2,992 and 3,515 μg/l, P < 0.0001)
Reclassification
In-sample reclassification behavior was further calculated. Eighteen patients with poor outcome were classified in higher-risk categories using the combined model with the MBL and the NIHSS, while seven patients with poor outcome were classified in lower risk categories using the combined model as compared with the NHISS as the only predictor variable. Thus, the estimated net reclassification improvement for functional outcome was 23.74 % (P < 0.001). Similarly, six patients who survived were classified in higher-risk categories, and one patient who died was classified in lower risk categories using the combined model as compared with the NHISS as the only predictor variable (net reclassification improvement 27.15 %, P < 0.001).
Discussion
The complement system has evolved as a defense against pathogens, but it may act as a double-edged sword and cause self-damage following adverse activation. High levels of MBL are known to protect against invading microorganisms and, in other situations, may mediate detrimental inflammation through exaggerated complement activation [27]. In this study, we first assessed the serum MBL levels with regard to their accuracy to predict functional outcome and mortality in T2DM patients with AIS within 1 year in Chinese sample. There have been several papers in the literature linking MBL and T2DM, fewer linking such deficiencies to stroke patients with T2DM and, as far as I could find, none in an ethnic Chinese sample. As such, the manuscript adds significantly to the literature, especially as Asian diabetic patients account for more than 60 % of the world’s diabetes population [28].
In our study, we reported that serum MBL levels at admission were significantly higher in stroke patients with T2DM as compared to normal cases (P < 0.0001). The difference was not explained by genetic differences, because we, in line with a recent Japanese study [29], found identical frequencies of the different genotypes in patients and healthy control subjects. Importantly, these preliminary results confirmed an interesting conclusion: Elevated MBL was correlated with long-term outcome and mortality, suggesting that this biomarker disturbance was prognostically unfavorable. MBL could be seen as one independent long-term prognostic marker of functional outcome and mortality even after correcting for possible confounding factors. Similarly, Hansen et al. [30] found that in patients with T2DM, measurements of MBL alone or in combination with CRP can provide prognostic information on mortality and the development of albuminuria. Mellbin et al. [31] reported that in type 2 diabetic patients with myocardial infarction, high levels of sC5b-9 predict future cardiovascular events. In addition, in patients with type 1 diabetes, higher levels of MBL have been found to be associated with microvascular and macrovascular complications [8], while Hovind et al. [27] suggested that elevated MBL can be seen as a predictor of microalbuminuria.
MBL is a slower reacting and much weaker acute-phase reactant than CRP [24]. Previous studies in type 1 diabetic patients have found no correlation between MBL and CRP [8, 32]. We did indeed observe significant correlations between Hs-CRP and MBL levels. Even though CRP and MBL are closely interrelated in inflammation and CRP may inhibit MBL activity [33], prognostic value remained statistically significant after correction for differences in Hs-CRP, which indicates that CRP and MBL may carry different types of information as markers of inflammation. Similarly, another study demonstrated that concentrations of both MBL and Hs-CRP were associated with the progression of renal disease in type 1 diabetes [34]. In addition, there was a significant correlation between MBL levels and blood glucose in our sample (r = 0.15, P = 0.003). Likewise, Hansen et al. [8] found that there was a significant positive correlation between MBL concentrations and HbA1c in another study group (r = 0.17, P = 0.001).
The mechanism of increased serum concentrations of MBL with poor outcome and mortality following an acute stroke in patients with T2DM is not yet established. MBL is more likely to be a contributing factor to the functional outcomes rather than a mere marker and may involve multiple mechanisms. MBL may aggravate local and systemic inflammation through complement activation [35, 36] and modulation of proinflammatory cytokine production [37], and it has been documented that inhibition of MBL reduces postischemic reperfusion injury in a rat model of acute myocardial infarction (MI) [38]. In a recent study, downstream inhibition of the complement system with a C5 inhibitor significantly reduced mortality after percutaneous coronary intervention in patients with MI [39], and it could be hypothesized that increased levels of MBL may contribute to this difference through increased activation of the complement cascade. We can speculate that high levels of MBL and subsequent complement activation will result in a net proinflammatory state, potentiating allograft damage, leading downstream to chronic allograft dysfunction. In addition, ischemia–reperfusion injury has been shown in both animal models and after human renal transplantation to result in complement being activated by means of the MBL pathway. In myocardial ischemia–reperfusion (I/R) injury as well as in other settings of I/R injury, experimental data pointed toward a favorable effect of low serum MBL [40–42]. Last, oxidative stress leading to changes in cell surface glycosylations may activate the complement system via MBL [35], and MBL binding to fructoselysine and the ensuing complement activation may provide a physiopathological link between enhanced glycation and complement activation in diabetes [43]. In addition, one study indicated that MBL/MASP complexes, and specifically MASP-1, play a key role in thrombus formation in vitro and in vivo [44]. Future studies are required to further elucidate these mechanisms.
Several limitations of this study should be considered. First, the relatively small sample size may limit the generalization of the results of this study. Before broad implementation, additional studies (multicenter, large sample) are needed for external validation. Second, without serial measurement of the circulating MBL, this study yielded no data regarding when and how long biomarkers were elevated in these patients. Additionally, it should be investigated whether serial MBL testing further improves the risk stratification of stroke patients. Third, infarct volume based on the formula for hematoma volumetry (0.5×A×B×C) in our study protocol was suboptimal. Besides, number and location of the infarct were not evaluated. Future studies on location of the infarct and white matter changes will be needed to further disentangle the effect of these factors on outcomes. Fourth, this study measured MBL in serum, not in cerebral spinal fluid (CSF). It is still uncertain whether peripheral MBL levels reflect similar changes in the central nervous system (CNS). Further study is needed to confirm the correction between MBL serum levels and CSF. Last, further studies should investigate whether MBL can help physicians tailor the therapy in view of the relative risk and allocate resources accordingly and whether this strategy might affect outcome.
Conclusions
Our study suggested that MBL levels may reliably predict long-term stroke prognosis at its onset in Chinese patients with T2DM. We recommend that further studies should be carried out with respect to what is the cause of the increased MBL levels and the role in the pathology of the stroke outcomes. If it is possible to elucidate this, more intensive efforts could be directed toward the cause, thus hopefully improve the prognosis of these patients.
References
Yang W, Lu J, Weng J et al (2010) Prevalence of diabetes among men and women in China. N Engl J Med 362:1090–1101
Cheng SY, Zhao YD, Li J et al (2014) Plasma levels of glutamate during stroke is associated with development of post-stroke depression. Psychoneuroendocrinology 47:126–135
Turner MW (2003) The role of mannose-binding lectin in health and disease. Mol Immunol 40:423–429
Fuchs A, Lin TY, Beasley DW et al (2010) Direct complement restriction of flavivirus infection requires glycan recognition by mannose-binding lectin. Cell Host Microbe 8:186–195
Fujita T (2002) Evolution of the lectin-complement pathway and its role in innate immunity. Nat Rev Immunol 2:346–353
Koch A, Melbye M, Sørensen P et al (2001) Acute respiratory tract infections and mannose-binding lectin insufficiency during early childhood. JAMA 285:1316–1321
Ohlenschlaeger T, Garred P, Madsen HO, Jacobsen S (2004) Mannose-binding lectinvariant alleles and the risk of arterial thrombosis in systemic lupus erythematosus. N Engl J Med 351:260–267
Hansen TK, Tarnow L, Thiel S et al (2004) Association between mannose-binding lectin and vascular complications in type 1 diabetes. Diabetes 53:1570–1576
Keller TT, van Leuven SI, Meuwese MC et al (2006) Serum levels of mannose-binding lectin and the risk of future coronary artery disease in apparently healthy men and women. Arterioscler Thromb Vasc Biol 26:2345–2350
Rugonfalvi-Kiss S, Dósa E, Madsen HO et al (2005) High rate of early restenosis after carotid eversion endarterectomy in homozygous carriers of the normal mannose-binding lectin genotype. Stroke 36:944–948
Pesonen E, Hallman M, Sarna S et al (2009) Mannose-binding lectin as a risk factor for acute coronary syndromes. Ann Med 41:591–598
Wang ZY, Sun ZR, Zhang LM (2014) The relationship between serum mannose-binding lectin levels and acute ischemic stroke risk. Neurochem Res 39:248–253
Frauenknecht V, Thiel S, Storm L et al (2013) Plasma levels of mannan‐binding lectin (MBL)‐associated serine proteases (MASPs) and MBL‐associated protein in cardio‐and cerebrovascular diseases. Clin Exp Immunol 173:112–120
Zhang NN, Ma AX, Cheng P et al (2011) Association between mannose-binding-lectin gene and type 2 diabetic patients in Chinese population living in the northern areas of China. Zhonghua Liu Xing Bing Xue Za Zhi 32:930–935
Elawa G, AoudAllah AM, Hasaneen AE, El-Hammady AM (2011) The predictive value of serum mannan-binding lectin levels for diabetic control and renal complications in type 2 diabetic patients. Saudi Med J 32:784–790
Siezenga MA, Shaw PK, Daha MR, Rabelink TJ, Berger SP (2011) Low mannose-binding lectin (MBL) genotype is associated with future cardiovascular events in type 2 diabetic South Asians. A prospective cohort study. Cardiovasc Diabetol 10:60
Garred P, Madsen HO, Marquart H et al (2000) Two edged role of mannose binding lectin in rheumatoid arthritis: a cross sectional study. J Rheumatol 27:26–34
Ip WK, Lau Y-L, Chan SY et al (2000) Mannose-binding lectin and rheumatoid arthritis in southern Chinese. Arthritis Rheum 43:1679–1687
Brott T, Adams HP Jr, Olinger CP et al (1989) Measurements of acute cerebral infarction: a clinical examination scale. Stroke 20:864–870
Adams HP, Bendixen BH, Kappelle LJ et al (1993) Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke 24:35–41
Bamford J, Sandercock P, Dennis M et al (1991) Classification and natural history of clinically identifiable subtypes of cerebral infarction. Lancet 337:1521–1526
Sims JR, Gharai LR, Schaefer PW (2009) ABC/2 for rapid clinical estimate of infarct, perfusion, and mismatch volumes. Neurology 72:2104–2110
Bonita RBR (1988) Modification of Rankin Scale: recovery of motor function after stroke. Stroke 19:1497–1500
Hansen TK, Thiel S, Wouters PJ, Christiansen JS, Van den Berghe G (2003) Intensive insulin therapy exerts anti inflammatory effects in critically ill patients and counteracts the adverse effect of low mannose-binding lectin levels. J Clin Endocrinol Metab 88:1082–1088
Zhang ZG, Wang C, Wang J, Zhang Z, Yang YL, Gao L, Zhang XY, Chang T, Gao GD, Li LH (2014) Prognostic value of mannose-binding lectin: 90-day outcome in patients with acute ischemic stroke. Mol Neurobiol. doi:10.1007/s12035-014-8682-0
Pencina MJ, D’Agostino RB Sr, D’Agostino RB Jr, Vasan RS (2008) Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med 27(157–172):207–212
Hovind P, Hansen TK, Tarnow L, Thiel S, Steffensen R, Flyvbjerg A, Parving HH (2005) Mannose-binding lectin as a predictor of microalbuminuria in type 1 diabetes: an inception cohort study. Diabetes 54:1523–1527
Sone H, Tanaka S, Suzuki S et al (2013) Leisure-time physical activity is a significant predictor of stroke and total mortality in Japanese patients with type 2 diabetes: analysis from the Japan Diabetes Complications Study (JDCS). Diabetologia 56:1021–1030
Tsutsumi A, Ikegami H, Takahashi R et al (2003) Mannose binding lectin gene polymorphism in patients with type I diabetes. Hum Immunol 64:621–624
Hansen TK, Gall MA, Tarnow L, Thiel S, Stehouwer CD, Schalkwijk CG, Parving HH, Flyvbjerg A (2006) Mannose-binding lectin and mortality in type 2 diabetes. Arch Intern Med 166:2007–2013
Mellbin LG, Bjerre M, Thiel S, Hansen TK (2012) Complement activation and prognosis in patients with type 2 diabetes and myocardial infarction: a report from the DIGAMI 2 trial. Diabetes Care 35:911–917
Saraheimo M, Forsblom C, Hansen TK et al (2005) On behalf of the FinnDiane study group: increased levels of mannan-bindinglectin (MBL) in type 1 diabetic patients with incipient and overt nephropathy. Diabetologia 48:198–202
Suankratay C, Mold C, Zhang Y, Potempa LA, Lint TF, Gewurz H (1998) Complement regulation in innate immunity and the acute-phase response: inhibition of mannan-binding lectin-initiated complement cytolysis by C-reactive protein (CRP). Clin Exp Immunol 113:353–359
Hansen TK, Forsblom C, Saraheimo M et al (2010) Association between mannose-binding lectin, high-sensitivity C-reactive protein and the progression of diabetic nephropathy in type 1 diabetes. Diabetologia 53:1517–1524
Collard CD, Vakeva A, Morrissey MA, Agah A, Rollins SA, Reenstra WR, Buras JA, Meri S, Stahl GL (2000) Complement activation after oxidative stress: role of the lectin complement pathway. Am J Pathol 156:1549–1556
Fiane AE, Videm V, Lingaas PS, Heggelund L, Nielsen EW, Geiran OR, Fung M, Mollnes TE (2003) Mechanism of complement activation and its role in the inflammatory response after thoracoabdominal aortic aneurysm repair. Circulation 108:849–856
Jack DL, Read RC, Tenner AJ, Frosch M, Turner MW, Klein NJ (2001) Mannose-binding lectin regulates the inflammatory response of human professional phagocytes to Neisseriameningitidis serogroup B. J Infect Dis 184:1152–1162
Jordan JE, Montalto MC, Stahl GL (2001) Inhibition of mannose-binding lectin reduces post-ischemic myocardial reperfusion injury. Circulation 104:1413–1418
Granger CB, Mahaffey KW, Weaver WD et al (2003) Pexelizumab, an anti-C5 complement antibody, as adjunctive therapy to primary percutaneous coronary intervention in acute myocardial infarction: the Complement Inhibition in Myocardial Infarction Treated with Angioplasty(COMMA) trial. Circulation 108:1184–1190
Summerfield JA, Sumiya M, Levin M, Turner MW (1997) Association of mutations in mannose binding protein gene with childhood infection in consecutive hospital series. BMJ 314:1229–1232
Hart ML, Ceonzo KA, Shaffer LA et al (2005) Gastrointestinal ischemia–reperfusion injury in lectin complement pathway dependent without involving C1q. J Immunol 174:6373–6380
Chan RK, Ibrahim SI, Takahashi K et al (2006) The differing roles of the classical and mannose-binding lectin complement pathways in the events following skeletal muscle ischemia–reperfusion. J Immunol 177:8080–8085
Fortpied J, Vertommen D, Van Schaftingen E (2010) Binding of mannose-binding lectin to fructosamines: a potential link between hyperglycaemia and complement activation in diabetes. Diabetes Metab Res Rev 26:254–260
La Bonte LR, Pavlov VI, Tan YS et al (2012) Mannose-binding lectin-associated serine protease-1 is a significant contributor to coagulation in a murine model of occlusive thrombosis. J Immunol 188:885–891
Acknowledgments
We are grateful to the Department of Neurology and Emergency; the nurses, physicians, and patients who participated in our study; and the staff of the central laboratory of the hospital. Authors also acknowledge the contribution of the editors and reviewers who have helped us to improve the manuscript.
Conflict of Interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Fang-Yu Song and Meng-Hai Wucontributed equally to this study.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 17 kb)
Rights and permissions
About this article
Cite this article
Song, FY., Wu, MH., Zhu, Lh. et al. Elevated Serum Mannose-Binding Lectin Levels Are Associated with Poor Outcome After Acute Ischemic Stroke in Patients with Type 2 Diabetes. Mol Neurobiol 52, 1330–1340 (2015). https://doi.org/10.1007/s12035-014-8941-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-014-8941-0