Abstract
Extracellular vesicles (EVs), including exosomes, microvesicles and apoptotic bodies, participate in intercellular communication, and particularly, in paracrine and endocrine signalling. The EVs and their specific contents have been considered hallmarks of different diseases. It has been recently discovered that EVs can co-transport nucleic acids such as DNAs, ribosomal RNAs, circular RNAs (circRNAs), long noncoding RNAs (lnRNAs) and microRNAs (miRNAs). miRNAs are important regulators of gene expression at the post-transcriptional level, although they may also play other roles. Recent evidence supports the hypothesis that miRNAs can activate Toll-like receptors (TLRs) under certain circumstances. TLRs belong to a multigene family of immune system receptors and have been recently described in the nervous system. In the immune system, TLRs are important for the recognition of the invading microorganisms, whereas in the nervous system, they recognise endogenous ligands released by undifferentiated or necrotic/injured cells. In the neuronal disease field, TLRs activity has been associated with amyotrophic lateral sclerosis (ALS), stroke, Alzheimer’s and Parkinson’s disease. Herein, we reviewed the current knowledge of the relationship between miRNA release by EVs and the inflammation signalling triggered by TLRs in neighbouring cells or during long-distance cell-to-cell communication. We highlight novel aspects of this communication mechanism, offering a valuable insight into such pathways in health and disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
To defend themselves against injuries or diseases, organisms provide ordered responses. For maintaining homeostasis, cells should be in constant communication. Three different ways of cellular communication are widely used in the nervous system. The best-known method is signal transmission via chemical synapses, initiated by the release of neurotransmitters. The second mechanism, attracting increasing attention in the recent years, is the cell coupling provided by gap junction channels [1–4]. The third form of communication is paracrine signalling, which encompasses several distinct mechanisms [5, 6]. Recent evidence suggests that the extracellular vesicles (EVs), including exosomes, microvesicles and apoptotic bodies, could be the fourth form of communication, ensuring short- and long-range exchange of information [7–10].
EVs and Transport of miRNAs
EVs are small lipid-membrane microvesicles (30–100 nm in diameter), found in prokaryotic and eukaryotic cells [11]. These vesicles originate from different cellular compartments such as membranes or endosomes, and are secreted into the extracellular medium [10, 12, 13]. The endosomes containing EVs move along microtubules to fuse with the plasma membrane and then release their microvesicles [14].
In the central nervous system (CNS), neurons, microglia, astrocytes and oligodendrocytes secrete microvesicles into the extracellular environment. Exosomes have been isolated from primary cultured neurons in vitro [15, 16].
EVs with different sizes, contents and from different sources can freely move through extracellular medium and are frequently found in diverse corporal fluids. EVs have been detected in the blood [17], urine [18], sweat [19], interstitial liquid, lung fluid [20], semen [21], colostrum [22] and saliva [23]. Notably, EV contents in the blood of cancer patients have been used as an indicator of metastasis [7]. The encapsulation of molecules in EVs enhances the protection against degradation and dilution in the extracellular space, allowing long-distance delivery through the bloodstream or interstitial fluid [17].
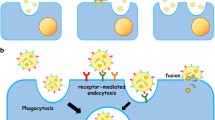
Interaction of EVs with target cells under physiological conditions is not well understood. Most of the empirical evidence has arisen from in vitro studies. According to recent data, EV functions may be executed in three distinct modes of action: (i) internalisation by target cells and cargo retrieval, (ii) binding to the cell surface and triggering second messenger pathways and (iii) releasing the components into the extracellular matrix [24].
Neuronal EVs are predominantly distributed within the somatodendritic compartment, where they are 50 times more abundant than in the axons [25]. It is well known that EVs can transport proteins and lipids [26]. It has been recently discovered that EVs could shuttle noncoding nucleic acids such as genomic DNAs [27], ribosomal RNAs (rRNAs), circular RNAs (circRNAs), long noncoding RNAs (lnRNAs) and microRNAs (miRNAs) [28].
Several research groups have shown a close relationship between apoptosis process and the release of exosomes-containing miRNA [29, 30]. Studies about adipose tissue-derived from MSCs characterised the mRNA and miRNA cargo of EVs. Factors involved in functions associated with alternative splicing, apoptosis, and chromosome organization were found in released EVs. Furthermore, four miRNAs that target transcription factors, as well as genes that participate in several cellular pathways, including apoptosis and proteolysis were also described [31].
It was recently proposed that some of the miRNAs are expressed at higher levels in the exosomes than in the cells. In fact, around 30 % of released miRNAs do not reflect the pool of miRNAs in the source cell, suggesting that miRNA is not distributed randomly and particular sequences are selected to occupy a specific cellular microenvironment [32, 33].
Control of miRNA Specificity: New Players on the Block
miRNAs are small noncoding RNAs of approximately 18–21 nucleotides. They are important post-transcriptional regulators of gene expression, acting at the level of mRNA, usually promoting its destabilization or decreasing the translation rate [34–36]. These short oligonucleotides are evolutionarily well conserved and are involved in many aspects of the biology of metazoans, from viral infection and replication [37] to cell proliferation, differentiation [38] and apoptosis [39]. The number of miRNAs encoded in the genomes varies from a few to around a thousand in mammals [40, 41]. Computational predictions and genome-wide identification of miRNA targets estimate that each miRNA regulates hundreds of different mRNAs, suggesting that approximately 50 % of the human transcriptome is subject to miRNA regulation [42, 43]. Most miRNAs are processed from longer hairpin transcripts by the consecutive actions of the RNase III-like enzymes Drosha and Dicer [44]. One strand of the hairpin duplex is loaded into an Argonaute-family protein to form the core of miRNA-induced silencing complexes (RISCs). RISCs silence the expression of target genes, predominantly at the post-transcriptional level [43–45].
The specificity of miRNAs towards mRNAs depends on the concentrations of both molecule types [46]. The copy number of a particular miRNA depends not only on the biosynthesis level, but also on the balance of stability and degradation. Some recent studies have described the participation of an atypical RNA polymerase PAPD4 and exoribonuclease XRN2 [47–50] in miRNA stability and degradation, respectively.
It has been suggested that miRNAs move between cells of the same organism via gap junction channels [51–53], exosomes [32, 54], apoptotic bodies [55] and in the synaptic cleft, coupled to the enzyme Argonaute 2 [56]. Migrating miRNAs are apparently stable and retain their activity in the target cells [57]. Figure 1 reviews the general mechanism of miRNA formation, maturation and uptake into exosomes.
microRNA (miRNA) biogenesis pathway and exosome uptake. a miRNAs are generated when primary miRNAs (pri-miRNA) are transcribed by RNA polymerase II and cleaved by microprocessor (blue arrows), a multi-protein complex formed by Drosha and Pasha/DGCR8. This process generates a hairpin structure with approximately 70 nucleotides, known as pre-miRNA. Within neuronal nuclei, pri- and pre-miRNA may be stabilized by 3′-terminal adenylation performed by PAPD4. Exportin 5 transports both pri- and pre-miRNAs to the cytoplasm. In the cytoplasm of the neuronal soma, pre-miRNA is cleaved by Dicer, producing an RNA duplex whose strands are separated, and one of them is incorporated into the RNA-induced silencing complex (RISC, green arrows). b Alternatively, pri-miRNAs and miRNA processing proteins, such as Drosha and DGCR8/Pasha, may be assembled with proteins of RNA transport granules. These molecules are then transported to specific neuronal compartments, where mature or precursor miRNAs are enveloped in vesicles or exosomes to be released elsewhere
Defective biogenesis or function of miRNAs have been identified under various physiological and pathological conditions, e.g., in neurodegeneration and autoimmunity disorders [58]. Several miRNAs are considered to belong to a newly defined class of mediators of inflammation [59, 60]. A correlation between miRNA-146a levels and the regulation of Toll-like and interleukin-1 receptor signalling and the consequent impact on immunity has been reported; it supports this hypothesis [61, 62].
New Insights Into TLR Pathways and Their Activation
Several roles of TLRs have recently been postulated. These receptors are classified as type I membrane-glycoproteins, mediating adaptive immune responses in the defence against pathogens [63–65]. The Toll gene was first described in Drosophila melanogaster [66]. Since then, 13 members of the TLR family have been described in mice and 11 in humans [67, 68]. As illustrated in Fig. 2, TLRs1-2, TLRs4-6 and TLRs11-13 proteins are localized on the cell surface, whereas TLR3 and TLRs 7–9 accumulate in the endosome or lysosome compartments and in the endoplasmic reticulum (ER) as shown in Fig. 3 [69]. Several cell types related to the immune system express TLRs, such as B-lymphocytes [70], mast cells [71], natural killer cells [72], T-lymphocytes [73], macrophages, monocytes, neutrophils [74], basophils and epithelial [75] and endothelial cells [76].
Neural cell types and their Toll-like receptor (TLR) expression. a Different nervous system resident cells express Toll-like receptors. Protein profile for TLR 3, 4, 7 and 9 has been documented in different neural phenotypes from humans, whereas protein profile for TLRs 2–4, 6–8 and 11–13 has been reported for murine neurons. In human oligodendrocytes, only TLR2 protein is detected; however, TLRs 2–4 are found in murine oligodendrocytes. Human astrocytes show TLRs 3–4 protein accumulation, whereas TLRs 2–5 and 9 are detected in murine astrocytes. Human microglia contains TLR1–4, whereas murine microglia has a specific TLR2, TLR4 and TLR9 protein profile. b In the cellular membrane, TLR1/TLR2 and TLR2/TLR6 form oligomers and are associated with adapter proteins containing Toll-interleukin-1 receptor (TIR) domain. TLRs activate protein adapters such as TIR-domain-containing adapter protein (TIRAP), myeloid-differentiation primary response gene 88 (MyD88) and, consequently, interleukin-1 receptor-associated kinase (IRAK). TRLs also activate TNF receptor-associated factor (TRAF)-6 adapters, leading to the activation of TRAF-family-member-associated nuclear factor-ΚB (NF-κβ) activator (TANK)-binding kinase-1 (TBK-1) and Iκ-B kinase (IKK), ending with the activation of NF-κβ and release of cytokines. TLR4 forms oligomers with another TLR4 and is associated with TIRAP, MyD88 and IRAK proteins or translocating chain-associated membrane protein (TRAM), TIR-domain-containing adapter-inducing interferon-β (TRIF) and TRAF6, to activate the NF-κβ pathway or the map kinase (MAPK) pathway via p38 and c-Jun N-terminal kinase (JNK), leading to activation of neuroprotective transcription factors (AP-1). TLRs 5, 11, 12, and 13 form homo-oligomers. Their specific signalling pathways have not been determined
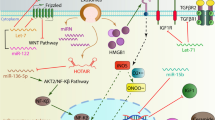
Long-distance cell–cell communication: microRNAs (miRNAs) and Toll-like receptors (TLRs). a Neurons and glial cells can release exosomes to the extracellular space. These exosomes could shuttle proteins and miRNAs for long distances via the blood vessels or act in the neighbouring cells. b In both types of cells, miRNAs are previously enveloped in exosomes or vesicles in order to be released. c When the vesicles fuse with the cell membrane, their content binds to the endosome TLRs. TLR3, TLR7, TLR8 and TLR9 oligomerise with the same receptors. TLRs 7–9 couple with myeloid-differentiation primary response gene 88 (MyD88), which activates interleukin-1 receptor-associated kinase (IRAK) and TNF receptor-associated factor (TRAF6). These processes culminate in the activation of the nuclear factor-κβ (NF-κβ). The TLR3 is coupled with TIR-domain-containing adapter-inducing interferon-β (TRIF), which activates TRAF3 and receptor-interacting serine-threonine kinase RIP1 protein, leading to apoptosis. Our hypothesis is that these TLRs could recognise mature miRNAs, triggering inflammatory signalling under various conditions, including neurogenesis and diseases
During the last decade, these receptors were found in different neural cells. Protein profiles for TLRs 3, 4, 7 and 9 were documented in human neuronal cells [77, 78], whereas TLRs 2–4, 6–8 and 11–13 were detected in murine neurons [78–81]. TLR2 protein has been detected only in human oligodendrocytes [82]; however, expression of TLRs 2–4 has been reported in murine oligodendrocytes [83–85]. Human astrocytes show TLR3- and TLR4-specific protein expression [86, 87], whereas TLRs2–5 and TLR9 have been detected in murine astrocytes [85, 88, 89]. Human microglia expresses TLR1–4 proteins [86, 87, 90], and murine microglia expresses TLR2, 4 and TLR9 proteins [83, 91, 92].
The extracellular domain of TLRs contains leucine-rich repeat motif that recognises conserved pathogen-associated molecular patterns (PAMPs) of a broad spectrum of infectious agents such as bacteria, viruses, yeasts, fungi and parasites [63]. TLR1 and TLR6 form heterodimers with TLR2, which can discriminate between triacylated and diacylated lipoproteins. TLR2 and TLR4 also form oligomers which interact with microbial motifs like peptidoglycan (PGN), lipoproteins and lipopolysaccharide (LPS) [93]. TLR5 is known for sensing flagella of motile bacterial species. TLRs 3 and 7–9 recognise intracellular pathogen-derived nucleic acid motifs, double-stranded RNA (dsRNA), single-stranded RNA (ssRNA) and DNA delivered to the intracellular compartments after the uptake of viruses, other pathogens or infected cells [94]. TLR9 recognises non-methylated CpG motifs of bacterial and viral DNA; TLR11 respond to pathogenic bacteria such as uropathogenic E. coli, as well as a profilin-like protein from the parasite T. gondii. However, respective PAMPs for TLR10, 12 and 13 are still unknown [95–97].
Apart from PAMP detection, recently reported evidence has disclosed that another class of molecules may trigger TLRs. TLRs in the CNS are activated by endogenous ligands released by necrotic cells in injured or stressed tissues [98, 99]. Some of these released molecules act as pro-inflammatory factors, and are also known as damage-associated molecular patterns (DAMPs). β-defensin 2, heat shock protein (HSP) 60, HSP70, high-mobility group protein B1 (HMGB1), oxygen radicals and urate crystals are considered DAMPs for associated TLR1/2 and/or TLR2/6, the TLR proteins that form oligomers [100]. The ssRNA acts as DAMP for TLR3 [101]. Similarly, β-defensin 2, HSP60, HSP70, HSP72, HMGB1, fibrinogen/fibrin, surfactant protein, minimally modified LDL (cholesterol) and pancreatic elastase activate TLR4. An RNA-immune complex was identified as DAMP for TLR7 and 8, whereas CpG chromatin-IgG complexes, the DNA immune complexes, are possible ligands for TLR9 [101–104]. The association of TLRs with their specific PAMPs or DAMPs leads to receptor activation and initiation of the cascade of intracellular signalling, culminating with NF-κβ activation and changes in gene expression.
Several adapter proteins containing Toll-interleukin-1 (TIR) domain associate with TLRs when activated. Most of the TLRs are coupled with myeloid-differentiation factor 88 (MyD88), similar to MyD88 adapter. TLR3 is an exception; it is the only TLR coupled with an adapter-inducing IFNβ of the TIR domain (TRIF) [105, 106]. The binding of these proteins triggers the signalling cascade that leads to activation of nuclear factor kappaβ (with NF-κβ). As a result, genes encoding pro-inflammatory tumour cytokines, such as tumour necrosis factor (TNF), interleukin 1 (IL-1), IL-6, IL-8, IL-12 and chemokines, are overexpressed. Although cytokine production is critical for host defence, it can also lead to irreversible tissue damage [107].
Some new data suggest that miRNAs regulate the TLR-signalling pathway at several steps, including the regulation of TLR mRNA expression, direct activation of the receptor, binding to TLR or TLR-specific signalling pathway components and TLR-induced transcription factors and functional cytokines [97, 101–104, 108].
Since miRNAs are short single-stranded RNA molecules, they can mimic viral RNA, and consequently, bind directly to TLRs. It has been reported that in the immune system, the natural killer cells (NK) can detect miRNAs via TLR1 activation [109, 110]. Specific miRNA sequences in miR-122 and miR-15b have been identified as ligands of TLR1 that can activate the transcription factor NF-κβ. The adapter proteins interleukin-1 receptor-associated kinase 1 (IRAK1) and TNF receptor-associated factor 6 (TRAF6) are important components of the myeloid-differentiation primary response gene (MYD88)-dependent pathway. MYD88 is an adapter protein used by almost all TLRs (except TLR3) to activate NF-κβ. IRAK1 and TRAF6 are also targets of miR-146. Taganov et al. have suggested that miR-146 downregulates the signalling pathway MyD88/NF-κβ after microbial infection [61, 111]. miR-155 controls the expression of inhibitor of NF-κβ kinase subunits beta (IKKβ) and epsilon (IKKƐ), reducing NF-κβ activity [112].
However, it has been recently discovered that TLRs 7–9 recognise specific miRNAs as agonists in the CNS. For example, miRNA let-7 is an abundant regulator of gene expression, highly expressed in microglia cells and in neurons, which interacts with TLRs [29]. miRNA-21 and 29a have been also described as agonists of TLRs 7–8 in rat and human macrophages. The binding of these miRNAs to TLRs induces the secretion of TNF-α and IL-6, leading to the activation of NF-κβ signalling and secretion of pro-inflammatory cytokines [113]. Besides secretion of cytokines, the regulation of TLR signalling by miRNAs occurs at different levels. Various molecules involved in the TLR pathway are targeted, such as TLR-signalling molecules, TLR-induced transcription factors, regulators of the TLR-signalling pathway and the expression of TLRs themselves [97, 114].
Considering the role of TLRs and assuming that exosomes carry miRNAs, we can hypothesise that miRNAs are signalling molecules with important functions in NS diseases (Fig. 3) [115].
miRNAs Activating TLRs in Neurological Diseases
Neurological diseases, including Alzheimer’s disease (AD), Parkinson’s disease (PD), amyotrophic lateral sclerosis (ALS), are characterised by neuronal cell loss. These diseases are expected to become more common due to extended life expectancy. Despite significant research efforts, the primary causes of neurodegeneration remain largely unknown. It has been recognised that these disorders emerge as a result of different genetic programming and environmental influences [116].
miRNAs have been associated with pathological alterations during the course of many neurological diseases, including AD, PD, ALS and stroke, suggesting that miRNAs may be a contributing factor in neurodegeneration [116]. It has been recently reported that miRNA levels are altered in the blood of AD, PD, ALS and stroke patients. These small RNAs may be used as biomarkers to enable an early diagnosis and identify new therapeutic targets [117].
It is not clear whether inflammation in the CNS contributes to the progress of neurological diseases. However, increasing evidence highlights the participation of TLR-dependent pathways in neuronal diseases [118]. Neuroinflammation is observed as consequences of trauma, infections, tumours and neurodegenerative diseases and involves microglia, pericytes and reactive astrocytes as well as T-lymphocytes, macrophages and dendritic cells crossing the brain-blood barrier, which is damaged in the inflamed brain (reviewed in [119]). Innate immunity providing an onset of the inflammatory response involves the actions of TLRs and the liberation of pro-inflammatory cytokines. Short neuroinflammatory responses are considered to be neuroprotective and may contribute neuronal development; however, when persisting they result in neurodegeneration [120]. In this regard, crucial functions may be attributed to endogenous miRNAs as ligands of TLR-promotion of neuroinflammation, as these are responsible for fine-tuning activity levels of TLRs and subsequent kinetics of innate immune response.
In agreement with the hypothesis of a chronic neuroinflammatory process, the involvement of TLR activation has been documented in AD [29, 121], PD [121, 122], ALS [123] and stroke [124]. The analysis of EVs is now an increasingly popular topic in the field of neurodegeneration; these vesicles may transport pathogenic proteins such as alpha-synuclein (α-syn) and amyloid precursor protein (APP) that are involved in PD and AD, respectively.
Alzheimer’s Disease
AD is the most common cause of dementia in the modern world [125]. The main characteristics of the disease are the accumulation of extracellular senile plaques (composed of amyloid-β peptide, Aβ), intracellular neurofibrillary tangles (NFTs) containing hyperphosphorylated tau protein, activated microglia, astrocytes and degenerating neurons [126].
Several appraisals of AD pathogenesis have revealed that the catabolism of APP occurs in the endosome; the pathogenic proteins, such as Aβ and tau, are secreted from the exosomes into the extracellular space [127–129]. TLR2, 4 and 9 are overexpressed in an animal model of AD [130]. These receptors could be activated by Aβ as they mediate the microglial inflammatory response and are associated with Aβ-plaque clearance from the brain [131–133].
Studies using blood samples from AD patients have identified 60 miRNAs differentially expressed in these patients in comparison with healthy individuals [134, 135]. miR-191 has a regulatory role in cellular processes such as cell proliferation, differentiation, apoptosis and migration; it targets important transcription factors, chromatin remodellers and cell cycle-associated genes [136]. It is likely that this miRNA is a key player in the initiation and progression of several diseases.
Type III RNase Dicer enzyme is responsible for the maturation of miRNA. Aberrant expression or malfunction of this regulator in adult forebrain impairs the expression of several miRNAs, ultimately causing pathological hyperphosphorylation of NFT-forming tau protein, leading to neuronal death [137].
The levels of miRNA let-7 are enhanced in AD patients. It has been suggested that let-7 activates the RNA-sensing TLR7, and thus, induces neurodegeneration in these patients [29]. The results of experiments with TLR7-KO mice have shown that these mice are resistant to neurodegenerative factors [29]. It is not clear how the let-7 miRNA reaches the endosome TLR7 receptor in the CNS. However, studies of the metastatic gastric cancer have revealed that let-7 miRNA is secreted into the extracellular environment via exosomal transport [133].
Inflammation has been held responsible for many neurological diseases as it increases cell damage and causes neuronal death. Further studies of the receptors associated with these processes and molecules triggering the inflammation are necessary to understand these serious disorders.
Parkinson’s Disease
PD is characterised by a selective degeneration of dopaminergic neurons in the substantia nigra pars compacta (SNpc) with various symptoms affecting the motor system such as tremor, stiffness, bradykinesia and postural instability [138]. The cellular hallmark of PD is the accumulation of proteinaceous intracellular inclusions termed Lewy bodies (LB), primarily composed of fibrillar alpha-synuclein (α-syn) and ubiquitinated proteins, in the surviving neurons [139].
The aggregation of α-syn activates microglia, increasing dopaminergic neurotoxicity [140, 141]. However, the precise molecular mechanism of the process is still unclear. Increased secretion of exosomes is one mechanism for α-syn action. These activated exosomes express a high level of major histocompatibility complex (MHC) II and TNF-α, which then promote apoptosis in the recipient cells [142]. α-syn can also be encapsulated in exosomes released by neuroblastoma and cause neuronal cell death [129].
Some cancer studies report that protein-transporting exosomes can also transport miRNAs [117]. The levels of miR-205, miR-184 and let-7 are correlated with the expression of α-syn and leucine-rich repeat kinase2 (LRRK2), coded by the two main genes associated with PD [143]. A recent report has also indicated that let-7 represses the expression of α-syn and is downregulated in PD models [144]. Increasing evidence suggests the existence of a close relationship between PD and TLRs. It has been recently shown that extracellular α-syn increases the expression of TLR1, TLR2, TLR3 and TLR7 [145, 146].
Recent studies have described TLR2 as an endogenous receptor for α-syn that is released from damaged neurons, responsible for microglial activation observed in PD [121]. However, TLR4-KO mice are less vulnerable to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) intoxication than wild-type mice. After MPTP administration, these TLR4-KO animals also have fewer ionised calcium-binding adaptor molecules 1 (Iba1)+ and MHC II+ activated microglial cells and lower levels of microglia/macrophage-specific calcium-binding protein. These results suggest that the TLR4 pathway is involved in PD [108].
The available experimental evidence points to a close relationship between EVs release, miRNA and TLR signalling in PD. However, further studies in this area should be conducted to clarify the specific roles of these molecules in this disease.
Amyotrophic Lateral Sclerosis
ALS is a chronic neurodegenerative disease, characterised by progressive loss of motor neurons, leading to muscle atrophy, paralysis and death usually within 3 to 5 years after diagnosis [147]. Several studies have demonstrated the involvement of non-neuronal cells in ALS pathogenesis, including microglia and astrocytes, increasing the release of superoxide dismutase 1 (SOD1), nitrate and nitrite [148].
SOD1 is secreted via exosomes from mouse motor neuron-like (NSC-34) cells overexpressing the wild-type and a mutant enzyme, used as in vitro model for ALS [149]. It has been demonstrated that exosomes cargo may include several different classes of molecules [32]. As we have previously mentioned, in addition to SOD1, exosomes may transport miRNAs. Several miRNAs such as miR-146b, miR-29b, let-7a/b, miR-27b, miR-21, miR-210 and miR-155 have their expression upregulated in ALS [150, 151]. Furthermore, the levels of miR-9 are enhanced in this disease in the ventral horn of the spinal cord, the locus of neurodegeneration [152]. Among those miRNAs, miR-155, miR-146b and miR-125b are typical components of the innate immune system, and most of them converge in NFΚB-mediated immune cell response [151].
The aetiology and pathogenesis of ALS still remain unclear, although available evidence suggests that inflammation plays a critical role in this process [153]. Studies of high expression of SOD1 in mice have shown elevated levels of TLR1, 2, 7 and 9 [123]. TLR2 and TLR4 gene expression levels are upregulated in ALS patients. TLR2 is predominantly detected in the microglia, whereas the TLR4 is strongly expressed in astrocytes. The activation of TLRs may contribute to the progression of inflammation and can explain the resultant motor neuron injury in ALS [154]. A study using combined inhibitory antibodies against TLR2 and TLR4 has shown significant microglial suppression [155].
An effective therapy for this disease is still undiscovered. However, the results showing that in ALS patients both, neuronal and non-neuronal cells, release EVs concomitantly with the activation of TLRs add to our knowledge of ALS and immune responses.
Stroke
Stroke is one of the most common causes of adult disability, and its prevalence augments with ageing population, despite the advances in prevention and acute interventions [156]. Stroke injury mechanisms include the excitotoxicity, mitochondrial dysfunction, oxidative stress [157] and inflammation [158].
Molecular chaperones and some members of the Bcl-2 family (apoptosis regulatory proteins) that protect mitochondrial function have been suggested as miRNA targets [157]. miRNA expression following stroke and other types of hypoxia-ischemia/reperfusion injuries varies regionally and temporally. The regional distribution of miR-181 and miR-121 differs depending on the distribution of blood flow [157].
Altered expression of several miRNAs (miR-140, miR-145 and miR-331) has been reported 3 days after ischemia/reperfusion; a progressive increase in the levels of miRNAs has been observed 3 h following reperfusion [159]. miR-200b, miR200c and miR-429 are elevated after 3 h of reperfusion in a model of ischemic preconditioning [160]. In a rat model of stroke, the levels of miR-290 [161], miR-10a, miR-182, miR-200b and miR-298 [162] increase in the blood and brain 24 h after ischemia/reperfusion; increased plasma levels of miR-124 are observed 6 h after reperfusion [163]. The level of miR-210, known as the major hypoxia-inducible miRNA or hypoxamir [164], is positively correlated with improved prognosis in stroke patients [165].
miRNAs are differentially expressed in the blood of patients with acute ischemic stroke; the levels of miR-122, miR-148a, let-7i, miR-19a, miR-320d and miR-4429 decrease, whereas miR-363 and miR-487b levels increase. These miRNAs are predicted to regulate several genes in pathways previously identified by gene expression analyses, including TLR signalling and NF-κβ signalling [158]. Several of these miRNAs have a known biological function. miRNA let-7 regulates TLR signalling in monocytes and modulates the differentiation of dendritic cells [166]. miR-122 regulates the expression of peroxiredoxin 2, a DAMP involved in immune activation after stroke [167]. miR-148 fine-tunes the immune response by altering cytokine production (IL6, TNF-a, IL-12, TNFSF7) [162, 168], although their biological effects in neuronal cells are unknown.
Studies focusing on stroke therapies with multipotent mesenchymal stromal cells (MSCs) have reported that these cells can release exosomes-containing miR-133b. These exosomes are transferred to the adjacent astrocytes and neurons, where they regulate gene expression, with subsequent benefits for neurites remodelling and functional recovery after stroke [169]. However, several studies have indicated the participation of TLRs in stroke [170, 171]. TLR9 gene expression is upregulated in ischemia-neuronal damage and may play a critical role in the induction of inflammatory response and apoptosis [172, 173]. Studies using TLR7 and TLR9 preconditioned with unmethylated cytosine-phosphate-guanine rich oligonucleotide (CpG) have shown some neuroprotective effects [174].
Other reports reveal a significant increase in TLR8 gene expression 6 h post-ischemia. The levels of pro-inflammatory cytokines such as IL-6 and IL-1β also change along with TLR8 levels. Treatment with a TLR8 agonist activates pro-apoptotic c-Jun N-terminal kinases (JNK) and increases neuronal cell death after stroke [80].
TLR2 and TLR4 gene expression is also upregulated under the stress or damage conditions such as ischemia or hypoxia [172, 173]. These oligomerised receptors can detect dangerous proteins like HSP and low molecular weight hyaluronan. HMGB1 and fibrin/fibrinogen are predominantly detected by TLR4 [175]. Studies using LPS for preconditioning have found that it re-programmes the cellular response (through activation of its receptor TLR4), possibly reflecting the endogenous processes that protect the brain against additional injury [176]. Following a cerebral focal ischemia injury, TLR2- and TLR4-KO mice have smaller infarcts than wild-type animals [177, 178]. miR-19b negatively regulates inflammation in humans and activates the expression of TLR2 and TLR4, promoting the inflammatory response in ischemic stroke [24, 25]. In neonatal hypoxic-ischemic (HI) mice brain, the activation of TLR3 can increase susceptibility to injury [124]. It is now widely accepted that miRNAs activate TLRs in the immune system. However, more studies are needed to determine the mechanisms of their action in the neuronal cells. We also need to confirm the relationship between EVs and the transport of these miRNAs in stroke.
Conclusions and Future Directions
In cancer research, EVs have been considered important biomarkers for the detection of metastases. The information transfer by EVs may constitute a novel mechanism of intercellular shuttling of molecules related to apoptosis. It is possible that EVs have similar roles in different systems, especially in the nervous system. The recent discovery of the ability of exosomes-containing miRNAs to reach TLRs in the endosomes of surrounding cells offers a new insight into various regulation mechanisms employed under physiological condition and in disease.
Investigation of the possible relationships between exosomes, miRNAs and TLRs in the nervous systems is still in its infancy. However, we can hypothesise that miRNAs entering the cells via exosomes may regulate the activation of TLRs. Furthermore, TLR tolerance, a hyporesponsive state of the receptor, characterised by reprogramming of TLR-mediated signal transduction [179], may achieved by intracellular delivery of miRNA using exosomes. Positive effects based on TLR tolerance have been observed in an animal model of stroke. If this hypothesis is confirmed, it will provide a new insight into the regulation of TLRs and new therapeutic strategies for CNS inflammation-related diseases.
A recent study has demonstrated an effective delivery of functional siRNA into mouse brain by systemic injection of exosomes [180]. Systemic exosome administration could be an alternative way to deliver the active components of cell-based therapy to the CNS [181]. Further detailed investigation of cellular communications mediated by EVs holds great promise for drug delivery and interference-RNA applications.
References
Kihara AH, Santos TO, Osuna-Melo EJ, Paschon V, Vidal KS, Akamine PS, Castro LM, Resende RR, Hamassaki DE, Britto LR (2010) Connexin-mediated communication controls cell proliferation and is essential in retinal histogenesis. Int J Dev Neurosci 28(1):39–52
Paschon V, Higa GS, Resende RR, Britto LR, Kihara AH (2012) Blocking of connexin-mediated communication promotes neuroprotection during acute degeneration induced by mechanical trauma. PLoS One 7(9):e45449
Theis M, Sohl G, Eiberger J, Willecke K (2005) Emerging complexities in identity and function of glial connexins. Trends Neurosci 28(4):188–195
Sohl G, Maxeiner S, Willecke K (2005) Expression and functions of neuronal gap junctions. Nat Rev Neurosci 6(3):191–200
Corriden R, Insel PA (2010) Basal release of ATP: an autocrine-paracrine mechanism for cell regulation. Sci Signal 3(104):re1
Le-Corronc H, Rigo JM, Branchereau P, Legendre P (2011) GABA (A) receptor and glycine receptor activation by paracrine/autocrine release of endogenous agonists: more than a simple communication pathway. Mol Neurobiol 44(1):28–52
Ogorevc E, Kralj-Iglic V, Veranic P (2013) The role of extracellular vesicles in phenotypic cancer transformation. Radiol Oncol 47(3):197–205
Vader P, Breakefield XO, Wood MJ (2014) Extracellular vesicles: emerging targets for cancer therapy. Trends Mol Med 20(7):385–393
Gupta A, Pulliam L (2014) Exosomes as mediators of neuroinflammation. J Neuroinflammation 11(1):68
Simons M, Raposo G (2009) Exosomes—vesicular carriers for intercellular communication. Curr Opin Cell Biol 21(4):575–581
Gyorgy B, Szabo TG, Pasztoi M, Pal Z, Misjak P, Aradi B, Laszlo V, Pallinger E, Pap E, Kittel A et al (2011) Membrane vesicles, current state-of-the-art: emerging role of extracellular vesicles. Cell Mol Life Sci CMLS 68(16):2667–2688
Sustar V, Bedina-Zavec A, Stukelj R, Frank M, Ogorevc E, Jansa R, Mam K, Veranic P, Kralj-Iglic V (2011) Post-prandial rise of microvesicles in peripheral blood of healthy human donors. Lipids Health Dis 10:47
Xin H, Li Y, Cui Y, Yang JJ, Zhang ZG, Chopp M (2013) Systemic administration of exosomes released from mesenchymal stromal cells promote functional recovery and neurovascular plasticity after stroke in rats. J Cereb Blood Flow Metab Off J Int Soc Cereb Blood Flow and Metab 33(11):1711–1715
Record M, Carayon K, Poirot M, Silvente-Poirot S (2014) Exosomes as new vesicular lipid transporters involved in cell-cell communication and various pathophysiologies. Biochim Biophys Acta 1841(1):108–120
Faure C, Nallet F, Roux D, Milner ST, Gauffre F, Olea D, Lambert O (2006) Modeling leakage kinetics from multilamellar vesicles for membrane permeability determination: application to glucose. Biophys J 91(12):4340–4349
Lachenal G, Pernet-Gallay K, Chivet M, Hemming FJ, Belly A, Bodon G, Blot B, Haase G, Goldberg Y, Sadoul R (2011) Release of exosomes from differentiated neurons and its regulation by synaptic glutamatergic activity. Mol Cell Neurosci 46(2):409–418
Yang X, Weng Z, Mendrick DL, Shi Q (2014) Circulating extracellular vesicles as a potential source of new biomarkers of drug-induced liver injury. Toxicol Lett 225(3):401–406
Cheng L, Sun X, Scicluna BJ, Coleman BM, Hill AF (2013) Characterization and deep sequencing analysis of exosomal and non-exosomal miRNA in human urine. Kidney Int 86(2):433–444
Graves LE, Ariztia EV, Navari JR, Matzel HJ, Stack MS, Fishman DA (2004) Proinvasive properties of ovarian cancer ascites-derived membrane vesicles. Cancer Res 64(19):7045–7049
Admyre C, Grunewald J, Thyberg J, Gripenback S, Tornling G, Eklund A, Scheynius A, Gabrielsson S (2003) Exosomes with major histocompatibility complex class II and co-stimulatory molecules are present in human BAL fluid. Eur Respir J 22(4):578–583
Vojtech L, Woo S, Hughes S, Levy C, Ballweber L, Sauteraud RP, Strobl J, Westerberg K, Gottardo R, Tewari M et al (2014) Exosomes in human semen carry a distinctive repertoire of small non-coding RNAs with potential regulatory functions. Nucleic Acids Res 42(11):7290–7304
Melnik BC, John SM, Schmitz G (2014) Milk: an exosomal microRNA transmitter promoting thymic regulatory T cell maturation preventing the development of atopy? J Transl Med 12(1):43
Ogawa Y, Kanai-Azuma M, Akimoto Y, Kawakami H, Yanoshita R (2008) Exosome-like vesicles with dipeptidyl peptidase IV in human saliva. Biol Pharm Bull 31(6):1059–1062
Fruhbeis C, Frohlich D, Kramer-Albers EM (2012) Emerging roles of exosomes in neuron-glia communication. Front Physiol 3:119
Von Bartheld CS, Altick AL (2011) Multivesicular bodies in neurons: distribution, protein content, and trafficking functions. Prog Neurobiol 93(3):313–340
Kramer-Albers EM, Bretz N, Tenzer S, Winterstein C, Mobius W, Berger H, Nave KA, Schild H, Trotter J (2007) Oligodendrocytes secrete exosomes containing major myelin and stress-protective proteins: Trophic support for axons? Proteomics Clin Appl 1(11):1446–1461
Cai J, Han Y, Ren H, Chen C, He D, Zhou L, Eisner GM, Asico LD, Jose PA, Zeng C (2013) Extracellular vesicle-mediated transfer of donor genomic DNA to recipient cells is a novel mechanism for genetic influence between cells. J Mol Cell Biol 5(4):227–238
Valencia K, Luis-Ravelo D, Bovy N, Anton I, Martinez-Canarias S, Zandueta C, Ormazabal C, Struman I, Tabruyn S, Rebmann V et al (2014) miRNA cargo within exosome-like vesicle transfer influences metastatic bone colonization. Mol Oncol 8(3):689–703
Lehmann SM, Kruger C, Park B, Derkow K, Rosenberger K, Baumgart J, Trimbuch T, Eom G, Hinz M, Kaul D et al (2012) An unconventional role for miRNA: let-7 activates Toll-like receptor 7 and causes neurodegeneration. Nat Neurosci 15(6):827–835
Lespagnol A, Duflaut D, Beekman C, Blanc L, Fiucci G, Marine JC, Vidal M, Amson R, Telerman A (2008) Exosome secretion, including the DNA damage-induced p53-dependent secretory pathway, is severely compromised in TSAP6/Steap3-null mice. Cell Death Differ 15(11):1723–1733
Eirin A, Riester SM, Zhu XY, Tang H, Evans JM, O’Brien D, van Wijnen AJ, Lerman LO (2014) MicroRNA and mRNA cargo of extracellular vesicles from porcine adipose tissue-derived mesenchymal stem cells. Gene 551(1):55–64
Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO (2007) Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol 9(6):654–659
Pigati L, Yaddanapudi SC, Iyengar R, Kim DJ, Hearn SA, Danforth D, Hastings ML, Duelli DM (2010) Selective release of microRNA species from normal and malignant mammary epithelial cells. PLoS One 5(10):e13515
Osman A (2012) MicroRNAs in health and disease—basic science and clinical applications. Clin Lab 58(5–6):393–402
Zhang Y, Liao Y, Wang D, He Y, Cao D, Zhang F, Dou K (2011) Altered expression levels of miRNAs in serum as sensitive biomarkers for early diagnosis of traumatic injury. J Cell Biochem 112(9):2435–2442
Muro EM, Mah N, Andrade-Navarro MA (2011) Functional evidence of post-transcriptional regulation by pseudogenes. Biochimie 93(11):1916–1921
Hayes CN, Akamatsu S, Tsuge M, Miki D, Akiyama R, Abe H, Ochi H, Hiraga N, Imamura M, Takahashi S et al (2012) Hepatitis B virus-specific miRNAs and Argonaute2 play a role in the viral life cycle. PLoS One 7(10):e47490
Decembrini S, Bressan D, Vignali R, Pitto L, Mariotti S, Rainaldi G, Wang X, Evangelista M, Barsacchi G, Cremisi F (2009) MicroRNAs couple cell fate and developmental timing in retina. Proc Natl Acad Sci U S A 106(50):21179–21184
Song J, Hu B, Qu H, Bi C, Huang X, Zhang M (2012) Mechanical stretch modulates microRNA 21 expression, participating in proliferation and apoptosis in cultured human aortic smooth muscle cells. PLoS One 7(10):e47657
Meunier J, Lemoine F, Soumillon M, Liechti A, Weier M, Guschanski K, Hu H, Khaitovich P, Kaessmann H (2013) Birth and expression evolution of mammalian microRNA genes. Genome Res 23(1):34–45
Da Costa Martins PA, De Windt LJ (2012) Targeting microRNA targets. Circ Res 111(5):506–508
Bartel DP (2009) MicroRNAs: target recognition and regulatory functions. Cell 136(2):215–233
Voinnet O (2009) Origin, biogenesis, and activity of plant microRNAs. Cell 136(4):669–687
Krol J, Loedige I, Filipowicz W (2010) The widespread regulation of microRNA biogenesis, function and decay. Nat Rev Genet 11(9):597–610
Carthew RW, Sontheimer EJ (2009) Origins and mechanisms of miRNAs and siRNAs. Cell 136(4):642–655
Zinovyev A, Morozova N, Gorban AN, Harel-Belan A (2013) Mathematical modeling of microRNA-mediated mechanisms of translation repression. Adv Exp Med Biol 774:189–224
Katoh T, Sakaguchi Y, Miyauchi K, Suzuki T, Kashiwabara S, Baba T, Suzuki T (2009) Selective stabilization of mammalian microRNAs by 3′ adenylation mediated by the cytoplasmic poly (A) polymerase GLD-2. Genes Dev 23(4):433–438
Kai ZS, Pasquinelli AE (2010) MicroRNA assassins: factors that regulate the disappearance of miRNAs. Nat Struct Mol Biol 17(1):5–10
de Sousa E, Walter LT, Higa GS, Casado OA, Kihara AH (2013) Developmental and functional expression of miRNA-stability related genes in the nervous system. PLoS One 8(5):e56908
Kinjo ER, Higa GS, de Sousa E, Casado OA, Damico MV, Britto LR, Kihara AH (2013) A possible new mechanism for the control of miRNA expression in neurons. Exp Neurol 248:546–558
Valiunas V, Polosina YY, Miller H, Potapova IA, Valiuniene L, Doronin S, Mathias RT, Robinson RB, Rosen MR, Cohen IS et al (2005) Connexin-specific cell-to-cell transfer of short interfering RNA by gap junctions. J Physiol 568(Pt 2):459–468
Brink PR, Valiunas V, Gordon C, Rosen MR, Cohen IS (2012) Can gap junctions deliver? Biochim Biophys Acta 1818(8):2076–2081
Higa GS, de Sousa E, Walter LT, Kinjo ER, Resende RR, Kihara AH (2014) MicroRNAs in neuronal communication. Mol Neurobiol 49(3):1309–1326
Stoorvogel W (2012) Functional transfer of microRNA by exosomes. Blood 119(3):646–648
Zernecke A, Bidzhekov K, Noels H, Shagdarsuren E, Gan L, Denecke B, Hristov M, Koppel T, Jahantigh MN, Lutgens E et al (2009) Delivery of microRNA-126 by apoptotic bodies induces CXCL12-dependent vascular protection. Sci Signal 2(100):ra81
Arroyo JD, Chevillet JR, Kroh EM, Ruf IK, Pritchard CC, Gibson DF, Mitchell PS, Bennett CF, Pogosova-Agadjanyan EL, Stirewalt DL et al (2011) Argonaute2 complexes carry a population of circulating microRNAs independent of vesicles in human plasma. Proc Natl Acad Sci U S A 108(12):5003–5008
Kosaka N, Iguchi H, Yoshioka Y, Takeshita F, Matsuki Y, Ochiya T (2010) Secretory mechanisms and intercellular transfer of microRNAs in living cells. J Biol Chem 285(23):17442–17452
Ksiazek-Winiarek DJ, Kacperska MJ, Glabinski A (2013) MicroRNAs as novel regulators of neuroinflammation. Mediators Inflamm 2013:172351
Gantier MP (2010) New perspectives in MicroRNA regulation of innate immunity. J Interf Cytokine Res off J Int Soc Interferon Cytokine Res 30(5):283–289
Quinn SR, O’Neill LA (2011) A trio of microRNAs that control Toll-like receptor signalling. Int Immunol 23(7):421–425
Taganov KD, Boldin MP, Chang KJ, Baltimore D (2006) NF-kappaB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc Natl Acad Sci U S A 103(33):12481–12486
Cui JG, Li YY, Zhao Y, Bhattacharjee S, Lukiw WJ (2010) Differential regulation of interleukin-1 receptor-associated kinase-1 (IRAK-1) and IRAK-2 by microRNA-146a and NF-kappaB in stressed human astroglial cells and in Alzheimer disease. J Biol Chem 285(50):38951–38960
Medzhitov R (2001) Toll-like receptors and innate immunity. Nat Rev Immunol 1(2):135–145
Takeda K, Akira S (2004) TLR signaling pathways. Semin Immunol 16(1):3–9
Miyake K (2007) Innate immune sensing of pathogens and danger signals by cell surface Toll-like receptors. Semin Immunol 19(1):3–10
Hashimoto C, Hudson KL, Anderson KV (1988) The Toll gene of Drosophila, required for dorsal-ventral embryonic polarity, appears to encode a transmembrane protein. Cell 52(2):269–279
Blasius AL, Beutler B (2010) Intracellular toll-like receptors. Immunity 32(3):305–315
Kawai T, Akira S (2010) The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol 11(5):373–384
Benias PC, Gopal K, Bodenheimer H Jr, Theise ND (2012) Hepatic expression of toll-like receptors 3, 4, and 9 in primary biliary cirrhosis and chronic hepatitis C. Clinics Res Hepatol Gastroenterol 36(5):448–454
Gerondakis S, Grumont RJ, Banerjee A (2007) Regulating B-cell activation and survival in response to TLR signals. Immunol Cell Biol 85(6):471–475
Iwamura C, Nakayama T (2008) Toll-like receptors in the respiratory system: their roles in inflammation. Curr Allergy Asthma Rep 8(1):7–13
Eriksson M, Meadows SK, Basu S, Mselle TF, Wira CR, Sentman CL (2006) TLRs mediate IFN-gamma production by human uterine NK cells in endometrium. J Immunol 176(10):6219–6224
Duan X, Kang E, Liu CY, Ming GL, Song H (2008) Development of neural stem cell in the adult brain. Curr Opin Neurobiol 18(1):108–115
Sabroe I, Whyte MK (2007) Toll-like receptor (TLR)-based networks regulate neutrophilic inflammation in respiratory disease. Biochem Soc Trans 35(Pt 6):1492–1495
Yoshimoto T, Nakanishi K (2006) Roles of IL-18 in basophils and mast cells. Allergol Int Off J Jpn Soc Allergol 55(2):105–113
Gibson FC 3rd, Ukai T, Genco CA (2008) Engagement of specific innate immune signaling pathways during Porphyromonas gingivalis induced chronic inflammation and atherosclerosis. Front Biosci J Virtual libr 13:2041–2059
Prehaud C, Megret F, Lafage M, Lafon M (2005) Virus infection switches TLR-3-positive human neurons to become strong producers of beta interferon. J Virol 79(20):12893–12904
Qi J, Buzas K, Fan H, Cohen JI, Wang K, Mont E, Klinman D, Oppenheim JJ, Howard OM (2011) Painful pathways induced by TLR stimulation of dorsal root ganglion neurons. J Immunol 186(11):6417–6426
Mishra BB, Gundra UM, Teale JM (2008) Expression and distribution of Toll-like receptors 11–13 in the brain during murine neurocysticercosis. J Neuroinflammation 5:53
Tang SC, Arumugam TV, Xu X, Cheng A, Mughal MR, Jo DG, Lathia JD, Siler DA, Chigurupati S, Ouyang X et al (2007) Pivotal role for neuronal Toll-like receptors in ischemic brain injury and functional deficits. Proc Natl Acad Sci U S A 104(34):13798–13803
Barajon I, Serrao G, Arnaboldi F, Opizzi E, Ripamonti G, Balsari A, Rumio C (2009) Toll-like receptors 3, 4, and 7 are expressed in the enteric nervous system and dorsal root ganglia. J Histochem Cytochem Off J Histochem Soc 57(11):1013–1023
Sloane JA, Batt C, Ma Y, Harris ZM, Trapp B, Vartanian T (2010) Hyaluronan blocks oligodendrocyte progenitor maturation and remyelination through TLR2. Proc Natl Acad Sci U S A 107(25):11555–11560
Lehnardt S, Henneke P, Lien E, Kasper DL, Volpe JJ, Bechmann I, Nitsch R, Weber JR, Golenbock DT, Vartanian T (2006) A mechanism for neurodegeneration induced by group B streptococci through activation of the TLR2/MyD88 pathway in microglia. J Immunol 177(1):583–592
Taylor DL, Pirianov G, Holland S, McGinnity CJ, Norman AL, Reali C, Diemel LT, Gveric D, Yeung D, Mehmet H (2010) Attenuation of proliferation in oligodendrocyte precursor cells by activated microglia. J Neurosci Res 88(8):1632–1644
Bsibsi M, Nomden A, van Noort JM, Baron W (2012) Toll-like receptors 2 and 3 agonists differentially affect oligodendrocyte survival, differentiation, and myelin membrane formation. J Neurosci Res 90(2):388–398
Jack CS, Arbour N, Manusow J, Montgrain V, Blain M, McCrea E, Shapiro A, Antel JP (2005) TLR signaling tailors innate immune responses in human microglia and astrocytes. J Immunol 175(7):4320–4330
Bsibsi M, Ravid R, Gveric D, van Noort JM (2002) Broad expression of Toll-like receptors in the human central nervous system. J Neuropathol Exp Neurol 61(11):1013–1021
Carpentier PA, Begolka WS, Olson JK, Elhofy A, Karpus WJ, Miller SD (2005) Differential activation of astrocytes by innate and adaptive immune stimuli. Glia 49(3):360–374
El-Hage N, Podhaizer EM, Sturgill J, Hauser KF (2011) Toll-like receptor expression and activation in astroglia: differential regulation by HIV-1 Tat, gp120, and morphine. Immunol Invest 40(5):498–522
Cassiani-Ingoni R, Cabral ES, Lunemann JD, Garza Z, Magnus T, Gelderblom H, Munson PJ, Marques A, Martin R (2006) Borrelia burgdorferi induces TLR1 and TLR2 in human microglia and peripheral blood monocytes but differentially regulates HLA-class II expression. J Neuropathol Exp Neurol 65(6):540–548
Lehnardt S, Lehmann S, Kaul D, Tschimmel K, Hoffmann O, Cho S, Krueger C, Nitsch R, Meisel A, Weber JR (2007) Toll-like receptor 2 mediates CNS injury in focal cerebral ischemia. J Neuroimmunol 190(1–2):28–33
Yoon HJ, Jeon SB, Kim IH, Park EJ (2008) Regulation of TLR2 expression by prostaglandins in brain glia. J Immunol 180(12):8400–8409
Suzuki M, Sugimoto Y, Ohsaki Y, Ueno M, Kato S, Kitamura Y, Hosokawa H, Davies JP, Ioannou YA, Vanier MT et al (2007) Endosomal accumulation of Toll-like receptor 4 causes constitutive secretion of cytokines and activation of signal transducers and activators of transcription in Niemann-Pick disease type C (NPC) fibroblasts: a potential basis for glial cell activation in the NPC brain. J Neurosci the Off J Soc Neurosci 27(8):1879–1891
Du M, Butchi NB, Woods T, Peterson KE (2011) Poly-thymidine oligonucleotides mediate activation of murine glial cells primarily through TLR7, not TLR8. PLoS One 6(7):e22454
Glezer I, Simard AR, Rivest S (2007) Neuroprotective role of the innate immune system by microglia. Neuroscience 147(4):867–883
McCusker RH, Kelley KW (2013) Immune-neural connections: how the immune system’s response to infectious agents influences behavior. J Exp Biol 216(Pt 1):84–98
Olivieri F, Rippo MR, Prattichizzo F, Babini L, Graciotti L, Recchioni R, Procopio AD (2013) Toll like receptor signaling in “inflammaging”: microRNA as new players. Immun Ageing I A 10(1):11
Tsai SY, Segovia JA, Chang TH, Morris IR, Berton MT, Tessier PA, Tardif MR, Cesaro A, Bose S (2014) DAMP molecule S100A9 acts as a molecular pattern to enhance inflammation during influenza A virus infection: role of DDX21-TRIF-TLR4-MyD88 pathway. PLoS Pathog 10(1):e1003848
Fang H, Ang B, Xu X, Huang X, Wu Y, Sun Y, Wang W, Li N, Cao X, Wan T (2014) TLR4 is essential for dendritic cell activation and anti-tumor T-cell response enhancement by DAMPs released from chemically stressed cancer cells. Cell Mol Immunol 11(2):150–159
Peng Y, Zhang L (2014) Activation of the TLR1/2 pathway induces the shaping of the immune response status of peripheral blood leukocytes. Exp Ther Med 7(6):1708–1712
Marsh BJ, Williams-Karnesky RL, Stenzel-Poore MP (2009) Toll-like receptor signaling in endogenous neuroprotection and stroke. Neuroscience 158(3):1007–1020
Kaczorowski DJ, Mollen KP, Edmonds R, Billiar TR (2008) Early events in the recognition of danger signals after tissue injury. J Leukoc Biol 83(3):546–552
Marsh BJ, Stevens SL, Hunter B, Stenzel-Poore MP (2009) Inflammation and the emerging role of the toll-like receptor system in acute brain ischemia. Stroke J Cereb Circ 40(3 Suppl):S34–S37
Chotirmall SH, Low TB, Hassan T, Branagan P, Kernekamp C, Flynn MG, Gunaratnam C, McElvaney NG (2011) Cystic fibrosis, common variable immunodeficiency and Aspergers syndrome: an immunological and behavioural challenge. Ir J Med Sci 180(2):607–609
O’Neill LA, Bowie AG (2007) The family of five: TIR-domain-containing adaptors in Toll-like receptor signalling. Nat Rev Immunol 7(5):353–364
Nada M, Ohnishi H, Tochio H, Kato Z, Kimura T, Kubota K, Yamamoto T, Kamatari YO, Tsutsumi N, Shirakawa M et al (2012) Molecular analysis of the binding mode of Toll/interleukin-1 receptor (TIR) domain proteins during TLR2 signaling. Mol Immunol 52(3–4):108–116
Ko MK, Saraswathy S, Parikh JG, Rao NA (2011) The role of TLR4 activation in photoreceptor mitochondrial oxidative stress. Invest Ophthalmol Vis Sci 52(8):5824–5835
He X, Jing Z, Cheng G (2014) MicroRNAs: new regulators of Toll-like receptor signalling pathways. Biomed Res Int 2014:945169
Fehniger TA (2013) Extracellular microRNAs turn on NK cells via TLR1. Blood 121(23):4612–4613
He S, Chu J, Wu LC, Mao H, Peng Y, Alvarez-Breckenridge CA, Hughes T, Wei M, Zhang J, Yuan S et al (2013) MicroRNAs activate natural killer cells through Toll-like receptor signaling. Blood 121(23):4663–4671
Nahid MA, Satoh M, Chan EK (2011) Mechanistic role of microRNA-146a in endotoxin-induced differential cross-regulation of TLR signaling. J Immunol 186(3):1723–1734
Ceppi M, Pereira PM, Dunand-Sauthier I, Barras E, Reith W, Santos MA, Pierre P (2009) MicroRNA-155 modulates the interleukin-1 signaling pathway in activated human monocyte-derived dendritic cells. Proc Natl Acad Sci U S A 106(8):2735–2740
Fabbri M, Paone A, Calore F, Galli R, Gaudio E, Santhanam R, Lovat F, Fadda P, Mao C, Nuovo GJ et al (2012) MicroRNAs bind to Toll-like receptors to induce prometastatic inflammatory response. Proc Natl Acad Sci U S A 109(31):E2110–E2116
Li Y, Shi X (2013) MicroRNAs in the regulation of TLR and RIG-I pathways. Cell Mol Immunol 10(1):65–71
Fabbri M, Paone A, Calore F, Galli R, Croce CM (2013) A new role for microRNAs, as ligands of Toll-like receptors. RNA Biol 10(2):169–174
Tan JY, Marques AC (2014) The miRNA-mediated cross-talk between transcripts provides a novel layer of posttranscriptional regulation. Adv Genet 85:149–199
Grasso M, Piscopo P, Confaloni A, Denti MA (2014) Circulating miRNAs as biomarkers for neurodegenerative disorders. Molecules 19(5):6891–6910
Drouin-Ouellet J, Cicchetti F (2012) Inflammation and neurodegeneration: the story ‘retolled’. Trends Pharmacol Sci 33(10):542–551
Ulrich H, do Nascimento IC, Bocsi J, Tarnok A (2014) Immunomodulation in stem cell differentiation into neurons and brain repair. Stem Cell Rev (in press)
Shastri A, Bonifati DM, Kishore U (2013) Innate immunity and neuroinflammation. Mediators Inflamm 2013:342931
Hayward CP, Moffat KA, Graf L (2014) Technological advances in diagnostic testing for von Willebrand disease: new approaches and challenges. Int J Lab Hematol 36(3):334–340
Noelker C, Morel L, Lescot T, Osterloh A, Alvarez-Fischer D, Breloer M, Henze C, Depboylu C, Skrzydelski D, Michel PP et al (2013) Toll like receptor 4 mediates cell death in a mouse MPTP model of Parkinson disease. Sci Rep 3:1393
Letiembre M, Liu Y, Walter S, Hao W, Pfander T, Wrede A, Schulz-Schaeffer W, Fassbender K (2009) Screening of innate immune receptors in neurodegenerative diseases: a similar pattern. Neurobiol Aging 30(5):759–768
Stridh L, Mottahedin A, Johansson ME, Valdez RC, Northington F, Wang X, Mallard C (2013) Toll-like receptor-3 activation increases the vulnerability of the neonatal brain to hypoxia-ischemia. J Neurosci Off J Soc Neurosci 33(29):12041–12051
Guo L, Duggan J, Cordeiro MF (2010) Alzheimer’s disease and retinal neurodegeneration. Curr Alzheimer Res 7(1):3–14
Querfurth HW, LaFerla FM (2010) Alzheimer’s disease. N Engl J Med 362(4):329–344
Rajendran L, Honsho M, Zahn TR, Keller P, Geiger KD, Verkade P, Simons K (2006) Alzheimer’s disease beta-amyloid peptides are released in association with exosomes. Proc Natl Acad Sci U S A 103(30):11172–11177
Vingtdeux V, Hamdane M, Loyens A, Gele P, Drobeck H, Begard S, Galas MC, Delacourte A, Beauvillain JC, Buee L et al (2007) Alkalizing drugs induce accumulation of amyloid precursor protein by-products in luminal vesicles of multivesicular bodies. J Biol Chem 282(25):18197–18205
Emmanouilidou E, Melachroinou K, Roumeliotis T, Garbis SD, Ntzouni M, Margaritis LH, Stefanis L, Vekrellis K (2010) Cell-produced alpha-synuclein is secreted in a calcium-dependent manner by exosomes and impacts neuronal survival. J Neurosci 30(20):6838–6851
Frank S, Copanaki E, Burbach GJ, Muller UC, Deller T (2009) Differential regulation of toll-like receptor mRNAs in amyloid plaque-associated brain tissue of aged APP23 transgenic mice. Neurosci Lett 453(1):41–44
Richard KL, Filali M, Prefontaine P, Rivest S (2008) Toll-like receptor 2 acts as a natural innate immune receptor to clear amyloid beta 1–42 and delay the cognitive decline in a mouse model of Alzheimer’s disease. J Neurosci 28(22):5784–5793
Yu X, Wang Q, Lin Y, Zhao J, Zhao C, Zheng J (2012) Structure, orientation, and surface interaction of Alzheimer amyloid-beta peptides on the graphite. Langmuir ACS J Surf colloid 28(16):6595–6605
Ohshima K, Inoue K, Fujiwara A, Hatakeyama K, Kanto K, Watanabe Y, Muramatsu K, Fukuda Y, Ogura S, Yamaguchi K et al (2010) Let-7 microRNA family is selectively secreted into the extracellular environment via exosomes in a metastatic gastric cancer cell line. PLoS One 5(10):e13247
Cogswell JP, Ward J, Taylor IA, Waters M, Shi Y, Cannon B, Kelnar K, Kemppainen J, Brown D, Chen C et al (2008) Identification of miRNA changes in Alzheimer’s disease brain and CSF yields putative biomarkers and insights into disease pathways. J Alzheimer’s Dis JAD 14(1):27–41
Kumar P, Dezso Z, MacKenzie C, Oestreicher J, Agoulnik S, Byrne M, Bernier F, Yanagimachi M, Aoshima K, Oda Y (2013) Circulating miRNA biomarkers for Alzheimer’s disease. PLoS One 8(7):e69807
Nagpal N, Kulshreshtha R (2014) miR-191: an emerging player in disease biology. Front Genet 5:99
Hebert SS, Papadopoulou AS, Smith P, Galas MC, Planel E, Silahtaroglu AN, Sergeant N, Buee L, De Strooper B (2010) Genetic ablation of Dicer in adult forebrain neurons results in abnormal tau hyperphosphorylation and neurodegeneration. Hum Mol Genet 19(20):3959–3969
Lang AE, Lozano AM (1998) Parkinson’s disease. Second of two parts. N Engl J Med 339(16):1130–1143
Lees AJ (2009) The Parkinson chimera. Neurology 72(7 Suppl):S2–S11
Reynolds AD, Kadiu I, Garg SK, Glanzer JG, Nordgren T, Ciborowski P, Banerjee R, Gendelman HE (2008) Nitrated alpha-synuclein and microglial neuroregulatory activities. J Neuroimmune Pharmacol Off J Soc NeuroImmune Pharmacol 3(2):59–74
Reynolds AD, Glanzer JG, Kadiu I, Ricardo-Dukelow M, Chaudhuri A, Ciborowski P, Cerny R, Gelman B, Thomas MP, Mosley RL et al (2008) Nitrated alpha-synuclein-activated microglial profiling for Parkinson’s disease. J Neurochem 104(6):1504–1525
Chang C, Lang H, Geng N, Wang J, Li N, Wang X (2013) Exosomes of BV-2 cells induced by alpha-synuclein: important mediator of neurodegeneration in PD. Neurosci Lett 548:190–195
Maciotta S, Meregalli M, Torrente Y (2013) The involvement of microRNAs in neurodegenerative diseases. Front Cell Neurosci 7:265
Junn E, Lee KW, Jeong BS, Chan TW, Im JY, Mouradian MM (2009) Repression of alpha-synuclein expression and toxicity by microRNA-7. Proc Natl Acad Sci U S A 106(31):13052–13057
Beraud D, Twomey M, Bloom B, Mittereder A, Ton V, Neitzke K, Chasovskikh S, Mhyre TR, Maguire-zeiss KA (2011) Alpha-synuclein alters toll-like receptor expression. Front Neurosci 5:80
Beraud E, Chandy KG (2011) Therapeutic potential of peptide toxins that target ion channels. Inflamm Allergy Drug Targets 10(5):322–342
Lomen-Hoerth C (2008) Amyotrophic lateral sclerosis from bench to bedside. Semin Neurol 28(2):205–211
Beers DR, Henkel JS, Xiao Q, Zhao W, Wang J, Yen AA, Siklos L, McKercher SR, Appel SH (2006) Wild-type microglia extend survival in PU.1 knockout mice with familial amyotrophic lateral sclerosis. Proc Natl Acad Sci U S A 103(43):16021–16026
Gomes C, Keller S, Altevogt P, Costa J (2007) Evidence for secretion of Cu, Zn superoxide dismutase via exosomes from a cell model of amyotrophic lateral sclerosis. Neurosci Lett 428(1):43–46
Koval ED, Shaner C, Zhang P, du Maine X, Fischer K, Tay J, Chau BN, Wu GF, Miller TM (2013) Method for widespread microRNA-155 inhibition prolongs survival in ALS-model mice. Hum Mol Genet 22(20):4127–4135
Parisi C, Arisi I, D’Ambrosi N, Storti AE, Brandi R, D’Onofrio M, Volonte C (2013) Dysregulated microRNAs in amyotrophic lateral sclerosis microglia modulate genes linked to neuroinflammation. Cell Death Dis 4:e959
Zhou F, Guan Y, Chen Y, Zhang C, Yu L, Gao H, Du H, Liu B, Wang X (2013) miRNA-9 expression is upregulated in the spinal cord of G93A-SOD1 transgenic mice. Int J Clin Exp Pathol 6(9):1826–1838
Sta M, Sylva-Steenland RM, Casula M, de Jong JM, Troost D, Aronica E, Baas F (2011) Innate and adaptive immunity in amyotrophic lateral sclerosis: evidence of complement activation. Neurobiol Dis 42(3):211–220
Casula M, Iyer AM, Spliet WG, Anink JJ, Steentjes K, Sta M, Troost D, Aronica E (2011) Toll-like receptor signaling in amyotrophic lateral sclerosis spinal cord tissue. Neuroscience 179:233–243
Zhao W, Beers DR, Henkel JS, Zhang W, Urushitani M, Julien JP, Appel SH (2010) Extracellular mutant SOD1 induces microglial-mediated motoneuron injury. Glia 58(2):231–243
Teasell R, Hussein N, McClure A, Meyer M (2014) Stroke: more than a ‘brain attack’. Int J Stroke Off J Int Stroke Soc 9(2):188–190
Ouyang Q, Zhou W, Zhang CM (2007) The key of increasing the therapeutic effect of scalp acupuncture on hemiplegia due to stroke. Zhongguo zhen jiu Chin Acupunct Moxibustion 27(10):773–776
Jickling GC, Ander BP, Zhan X, Noblett D, Stamova B, Liu D (2014) microRNA expression in peripheral blood cells following acute ischemic stroke and their predicted gene targets. PLoS One 9(6):e99283
Dharap A, Bowen K, Place R, Li LC, Vemuganti R (2009) Transient focal ischemia induces extensive temporal changes in rat cerebral microRNAome. J Cereb blood flow Metab Off J Int Soc Cereb Blood Flow Metab 29(4):675–687
Lee ST, Chu K, Jung KH, Yoon HJ, Jeon D, Kang KM, Park KH, Bae EK, Kim M, Lee SK et al (2010) MicroRNAs induced during ischemic preconditioning. Stroke J Cereb Circulation 41(8):1646–1651
Jeyaseelan K, Lim KY, Armugam A (2008) MicroRNA expression in the blood and brain of rats subjected to transient focal ischemia by middle cerebral artery occlusion. Stroke J Cereb Circulation 39(3):959–966
Liu DZ, Tian Y, Ander BP, Xu H, Stamova BS, Zhan X, Turner RJ, Jickling G, Sharp FR (2010) Brain and blood microRNA expression profiling of ischemic stroke, intracerebral hemorrhage, and kainate seizures. J Cereb Blood Flow Metab 30(1):92–101
Weng Q, Zhang J, Cao J, Xia Q, Wang D, Hu Y, Sheng R, Wu H, Zhu D, Zhu H et al (2011) Q39, a quinoxaline 1,4-Di-N-oxide derivative, inhibits hypoxia-inducible factor-1alpha expression and the Akt/mTOR/4E-BP1 signaling pathway in human hepatoma cells. Invest New Drugs 29(6):1177–1187
Chan YC, Banerjee J, Choi SY, Sen CK (2012) miR-210: the master hypoxamir. Microcirculation 19(3):215–223
Zeng J, Sun Y, Jiang L (2011) On-line ‘automatic pilot’ training for hand and arm motor rehabilitation after stroke. Med Hypotheses 76(2):197–198
Zhang W, Winder T, Ning Y, Pohl A, Yang D, Kahn M, Lurje G, Labonte MJ, Wilson PM, Gordon MA et al (2011) A let-7 microRNA-binding site polymorphism in 3′-untranslated region of KRAS gene predicts response in wild-type KRAS patients with metastatic colorectal cancer treated with cetuximab monotherapy. Ann Oncol Off J Eur Soc Med Oncol ESMO 22(1):104–109
Diao S, Zhang JF, Wang H, He ML, Lin MC, Chen Y, Kung HF (2010) Proteomic identification of microRNA-122a target proteins in hepatocellular carcinoma. Proteomics 10(20):3723–3731
Pan W, Zhu S, Yuan M, Cui H, Wang L, Luo X, Li J, Zhou H, Tang Y, Shen N (2010) MicroRNA-21 and microRNA-148a contribute to DNA hypomethylation in lupus CD4+ T cells by directly and indirectly targeting DNA methyltransferase 1. J Immunol 184(12):6773–6781
Li Y, Liu Z, Xin H, Chopp M (2014) The role of astrocytes in mediating exogenous cell-based restorative therapy for stroke. Glia 62(1):1–16
Fadakar K, Dadkhahfar S, Esmaeili A, Rezaei N (2014) The role of Toll-like receptors (TLRs) in stroke. Rev Neurosci 25(5):699–712
Gesuete R, Kohama SG, Stenzel-Poore MP (2014) Toll-like receptors and ischemic brain injury. J Neuropathol Exp Neurol 73(5):378–386
Arumugam TV, Okun E, Tang SC, Thundyil J, Taylor SM, Woodruff TM (2009) Toll-like receptors in ischemia-reperfusion injury. Shock 32(1):4–16
Mkaddem SB, Bens M, Vandewalle A (2010) Differential activation of Toll-like receptor-mediated apoptosis induced by hypoxia. Oncotarget 1(8):741–750
Leung PY, Stevens SL, Packard AE, Lessov NS, Yang T, Conrad VK, van den Dungen NN, Simon RP, Stenzel-Poore MP (2012) Toll-like receptor 7 preconditioning induces robust neuroprotection against stroke by a novel type I interferon-mediated mechanism. Stroke J Cereb Circ 43(5):1383–1389
Smiley ST, King JA, Hancock WW (2001) Fibrinogen stimulates macrophage chemokine secretion through toll-like receptor 4. J Immunol 167(5):2887–2894
Wang YC, Lin S, Yang QW (2011) Toll-like receptors in cerebral ischemic inflammatory injury. J Neuroinflammation 8:134
Cao CX, Yang QW, Lv FL, Cui J, Fu HB, Wang JZ (2007) Reduced cerebral ischemia-reperfusion injury in Toll-like receptor 4 deficient mice. Biochem Biophys Res Commun 353(2):509–514
Ziegler G, Harhausen D, Schepers C, Hoffmann O, Rohr C, Prinz V, Konig J, Lehrach H, Nietfeld W, Trendelenburg G (2007) TLR2 has a detrimental role in mouse transient focal cerebral ischemia. Biochem Biophys Res Commun 359(3):574–579
Vartanian K, Stenzel-Poore M (2010) Toll-like receptor tolerance as a mechanism for neuroprotection. Transl Stroke Res 1(4):252–260
Cooper JM, Wiklander PB, Nordin JZ, Al-Shawi R, Wood MJ, Vithlani M, Schapira AH, Simons JP, El-Andaloussi S, Alvarez-Erviti L (2014) Systemic exosomal siRNA delivery reduced alpha-synuclein aggregates in brains of transgenic mice. Mov Disord 29(12):1476–1485
Kawikova I, Askenase PW (2014) Diagnostic and therapeutic potentials of exosomes in CNS diseases. Brain Res S0006-8993(14)01343–2
Funding
This work was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP, #2014/16711-6) and Universidade Federal do ABC.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Paschon, V., Takada, S.H., Ikebara, J.M. et al. Interplay Between Exosomes, microRNAs and Toll-Like Receptors in Brain Disorders. Mol Neurobiol 53, 2016–2028 (2016). https://doi.org/10.1007/s12035-015-9142-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-015-9142-1