Abstract
Baculovirus expression vector system (BEVS) is widely known as a mass-production tool to produce functional recombinant glycoproteins except that it may not be always suitable for medical practice due to the differences in the structure of N-linked glycans between insects and mammalian. Currently, various approaches have been reported to alter N-linked glycan structures of glycoproteins derived from insects into terminally sialylated complex-type N-glycans. In the light of those studies, we also proposed in vitro maturation of N-glycan with mass-produced and purified glycosyltransferases by silkworm–BEVS. β-1,4-Galactosyltransferase 1 (β4GalT1) is known as one of type II transmembrane enzymes that transfer galactose in a β-1, 4 linkage to accepter sugars, and a key enzyme for further sialylation of N-glycans. In this study, we developed a large-scale production of recombinant human β4GalT1 (rhβ4GalT1) with N- or C-terminal tags in silkworm–BEVS. We demonstrated that rhβ4GalT1 is N-glycosylated and without mucin-type glycosylation. Interestingly, we found that purified rhβ4GalT1 from silkworm serum presented higher galactosyltransferase activity than that expressed from cultured mammalian cells. We also validated the UDP-galactose transferase activity of produced rhβ4GalT1 proteins by using protein subtracts from silkworm silk gland. Taken together, rhβ4GalT1 from silkworms can become a valuable tool for producing high-quality recombinant glycoproteins with mammalian-like N-glycans.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The migration of cells to tissues/organs is controlled by a specific cell surface sugar decoration, mainly sialyl Lewisx/a (sLex/a). This key epitope is created through the activity of specific glycosyltransferases (GTs). When these GTs are accumulated and/or activated in particular cells or are introduced ex vivo, they migrate to specific locations where the counter-receptors (selectins) are expressed. Selectin-dependent tethering and rolling decreases cellular velocity below that of the prevailing hemodynamic stream. P- and E-selectin are membrane-bound lectins that bind to glycoprotein or glycolipid ligands (e.g., PSGL-1 and CD43) on the cell in flow via their lectin domains. The specialized glycan determinant for selectin ligand is comprised of sialofucosylations containing an α (2,3)-linked sialic acid substitution on galactose, and an α(1,3)-linked fucose modification on N-acetylglucosamine, prototypically displayed as the terminal tetrasaccharide sLex (or its isomer sLea).
In order for selectin ligands to be formed, GTs responsible for creating the sLea/x structures must be endogenously expressed and active. The native display of these glycan structures on selectin ligands requires the various active GTs, including the action of α1,3- or α1,4-fucosyltransferases, α2,3-sialyltransferases, β1,4-galactosyltransferases, and β1,6-N-acetyl-glucosaminyltransferases. The knowledge of which GTs are required to create particular structures allows researchers to manipulate cells in order to create ligands on cells’ surface ex vivo with the intention of helping to direct the migration of therapeutic cells to specific tissues that express the corresponding selectins [1,2,3]. Selectin ligands must express sLex/a structures on extended lactosamines of N- or O-glycans. Adult stem cells are often inadequately α1,3-fucosylated, this being the case with mesenchymal stem cells (MSC) [1], hematopoietic stem cells (HSC) [4,5,6,7] and neural stem cells [2] and so exhibit migration defects. Indeed, ex vivo treatment of stem cells with FTs, particularly FT-VI and FT-VII, increases cell surface sLex determinants, boosts binding to E-selectin and P-selectin, and enhances homing and engraftment into bone marrow of immunocompromised mice [4,5,6,7]. By generating a comprehensive set of tools (i.e., β1,4-galactosyltransferases, β1,6-N-acetyl-glucosaminyltransferases, α1,3- or α1,4-fucosyltransferases and α2,3-sialyltransferases) for ex vivo design of sLex structures on the surface of a therapeutic cell, strategies to enhance the migration of these cells for the treatment of diseases can be further anticipated.
The β1,4-galactosyltransferase (β4GalT1) gene family consists of seven members that transfer galactose in a β-1, 4 linkage to accepter sugars. β4GalT1 (NCBI accession number NP_001488) is unique among the seven enzymes because it can be expressed either as membrane associated form or secreted form [8,9,10]. It is also one of the key enzymes responsible for extending the lactosamine structures on N- and O-glycans as well as adding the terminal galactose that will be capped with α(2,3)-linked sialic acid to generate the sLex selectin ligand. Thus, expression and purification of this enzyme would be beneficial for the ex vivo creation of selectin ligands on therapeutic cells.
To this end, the mammalian expression system is often applied for the production of glycoproteins with high activity and stability, although it offers suffers from several disadvantages, such relatively poor productivity and higher cost. To date, various approaches have been reported to alter the N-linked glycan structure of insect-derived proteins into terminally sialylated complex-type N-glycans by transgenic expression of mammalian glycosyltransferases in insect cells [11,12,13]. However, the efficiency in the formation of matured complex-type N-glycans from the insect cells is still low because the activity of introduced mammalian β-1, 4-galactosyltransferase 1 (β4GalT1) is unstable in insect cell [14]. Additionally, the negative effect of exogenously expressedβ4GalT1 in insect cells must be taken into account especially in transgenic insects. Recently, the baculovirus expression vector system (BEVS) using lepidopteran insects and cells has been reported to be suitable for mass-production of glycoproteins with mammalian-like post-translational modifications and safety profiles [15,16,17,18,19]. Therefore, we propose another approach in which the recombinant glycoproteins produced by silkworm–BEVS are further processed by in vitro N-glycosylation using mammalian glycosyltransferases purified from larvae. Because galactosylated N-glycans provide substrates for sialylation by sialyltransferases, it is desirable to mass-produce the recombinant β4GalT1 by silkworm–BEVS. In this study, we constructed the recombinant BmNPV to express the secreted form human β4GalT1 in cultured silkworm cells and larvae. We also report the expression, purification and characterization of this recombinant human β4GalT1 secreted from silkworm larvae.
Materials and Methods
Cells and Silkworm Larvae
The NIAS-Bm-oyanagi2 (BmO2, kindly provided from Dr. Imanishi) and Bme21 cells [20] were cultured in IPL-41 medium (Sigma, St. Louis, MO) supplemented with 10% fetal bovine serum (Gibco, Grand Island, NY); the silkworm larvae were provided from the Institute of Genetic Resources, Kyushu University Graduate School. The larvae were reared on fresh mulberry leaves at 25–27 °C.
Construction of Recombinant Human β4GalT1
Matured form of human β4GalT1 (a.a.77–398) gene was amplified using the specific primers β4GalT1-matured-5 (5′-CGGACCGGAGGGGCCCGGCC-3′) and β4GalT1-3-Xho1 (5′-GTCCCTCGAGCTCGGTGTCCCGATGTCCAC-3′). The amplified PCR product without 1–77 amino acid residues of β4GalT1 including native cytosolic (C) and transmembrane (T) domains was digested by Xho1, and further inserted into the EcoRV and XhoI double-digested site of the modified pENTR11 vectors (pENTR11L2130KTEVH8STREP and pENTR11L2130KH8STREPTEV) containing a lobster L21 sequence at the N-terminal, and the tobacco etch virus (TEV) protease cleavage site and the tobacco etch virus (TEV) protease cleavage site for removing the affinity tag from the recombinant protein in case terminal tags affect the protein stability and/or activity, the 8 × histidine tag and the Strep-tag at the N- or C-terminal. The resulting constructs were named pENTR11/30K_rhβ4GalT1 (N-tag) and pENTR11/30K_rhβ4GalT1 (C-tag), respectively.
Generation of Recombinant BmNPV
The transfer plasmids for generating the recombinant baculoviruses were subsequently constructed by LR Gateway reaction between the pENTR11/30K_rhβ4GalT1 (N-tag) and pENTR11/30K_rhβ4GalT1 (C-tag) and pDEST8 vector (Invitrogen, Carlsbad, California) according to the manufacture’s protocol. The recombinant baculoviruses were generated using BmNPV/T3 bacmid system as described previously [21]. A 200 ng of the bacmid DNA was transfected into 1 × 105 BmO2 cells in 24-well plates using FuGENE HD transfection reagent (Promega, Madison, WI) to yield the recombinant virus particles, BmNPV/polh-30K_rhβ4GalT1 (N-tag) and BmNPV/polh-30K_rhβ4GalT1 (C-tag), respectively. Before transfection, the culture medium was replaced by FBS-free COSMEDIUM 009X medium (Cosmo-Bio, Tokyo, Japan). At 4-day post-infection (dpi), cell culture mediums containing P1 virus particles were collected and stored at 4 °C in the dark. High-titer virus (P3) stocks were prepared by serial infection following the protocols recommended in the manufacturer’s manual (Invitrogen).
Expression of rhβ4GalT1 in Cultured Silkworm Cell
2 × 106 Bme21 cells in a 12-well plate were infected with the recombinant BmNPV at a multiplicity of infection (MOI) of 10. At 4 dpi, after centrifugation at 1000g for 10 min at 4 °C, the supernatant was subjected to Western blotting analysis. The cell pellet was suspended in ice-cold lysis buffer (20 mM Tris–HCl pH 7.4, 0.5 M NaCl, 1% Triton X-100, 10% glycerol, 10 mM 2-mercaptoethanol, 1 mM PMSF) and homogenized. After centrifugation at 15,000g for 30 min at 4 °C, the supernatant containing soluble proteins was also subjected to Western blotting analysis as cell lysate.
Purification of rhβ4GalT1 from Silkworm Larvae
The recombinant virus was injected into the 5th instar of n17 silkworm larvae. At 4 dpi, 10 ml of the serum from roughly 25 silkworm larvae was collected into a 15-ml tube containing 20 mM 1-phenyl-2-thiourea, followed by centrifugation at 10,000g for 30 min at 4 °C. Then, the supernatant was transferred into a new tube. The resulting serum was diluted by buffer A (20 mM Tris–HCl pH 7.4, 0.5 M NaCl, 1 mM PMSF), and centrifuged at 20,000g for 30 min at 4 °C. After the filtration through a 0.45-μm filter (Millipore, USA), the supernatant containing rhβ4GalT1 was loaded to a nickel affinity chromatography with 5 ml HisTrap excel column (GE Healthcare Bioscience, USA), and eluted by 500 mM imidazole solution buffers. After concentrated by ultrafiltration using Amicon 10 K filters (Millipore, USA) in buffer B (100 mM Tris–HCl pH 8.4, 150 mM NaCl, 1 mM EDTA), the rhβ4GalT1 was applied to StrepTrap HP column (GEHealthcare Bioscience, USA), and then eluted by the buffer containing 2.5 mM desthiobiotin. The purified proteins were determined of quantity by using DC™ Protein Assay (BioRad).
Glycosylation Analysis with Glycosidase Digestions
The purified rhβ4GalT1s and the commercially available rhβ4GalT1 (a.a.44–398; R&D systems, Inc., Mineapolis, USA) expressed in mouse myeloma cell lines were incubated in Glycoprotein Denaturing Buffer (NEB, USA) at 100 °C for 10 min. Subsequently, the denatured protein samples were treated by adding PNGase F (NEB, USA), or O-glycosidase (NEB, USA) with α2-3,6,8 Neuraminidase (NEB, USA) for 1 h at 37 °C according to the manufacturer’s protocol.
SDS-PAGE and Western Blotting
The purified or deglycosylated rhβ4GalT1 was subjected to SDS-PAGE (with 10% polyacrylamide gel) and visualized by Coomassie Brilliant Blue R-250 staining or transferred to a polyvinylidene difluoride (PVDF) membrane for Western blotting. As for Western blotting, the membrane was blocked for 1 h in TBST buffer (20 mM Tris–HCl pH 7.6, 500 mM NaCl, 0.1% w/v Tween-20) with 5% w/v skim milk (Wako, Japan) followed by incubation with HisProbe-HRP (1:5000 v/v; Thermo Scientific, USA) for 1 h. After washing, the HRP signals were detected by the Super Signal West Pico Chemiluminescent Substrate (Thermo Scientific, USA).
Galactosyltransferase Activity Against N-acetylglucosamin
The transfer activity of rhβ4GalT1 was determined in vitro using the Glycosyltransferase Activity Kit (R&D Systems, Inc., Minneapolis, USA), following the assay procedure of rhβ4GalT1 from R&D systems. Briefly, the dissociative UDP after the galactosyltransferase reaction is further hydrolyzed by the calcium-dependent nucleotidase ENTPD3/CD39L3 giving rise to free inorganic phosphate, which can be detected by the malachite green reagent. The reaction was initiated by incubating 5 nmol of UDP-galactose (UDP-Gal, SIGMA) as a donor substrate, 500 nmol N-Acetyl-alpha-D-glucosamine (GlcNAc, SIGMA) as an acceptor substrate, 200 ng of ENTPD3/CD39L3, 150 ng of the recombinant proteins in the assay buffer (25 mM Tris–HCl pH 7.5, with 10 mM CaCl2, 10 mM MnCl2) using 96-well microplate for 15 min at 37 °C, and terminated by adding the malachite green development solution. After 20 min incubation with Malachite Green Reagent B, the microplate was read absorbance at 620 nm in endpoint mode of Synergy HTX Plate Reader (BioTek, Japan). As a negative control, recombinant human alpha-1-acid glycoprotein (α1AGP) purified in our previous study [22] was used. All reactions were performed in triplicate independently.
Galactosyltransferase Activity Against Endogenous Glycoproteins Extracted from Silk Glands of Silkworm Larvae
The endogenous glycoproteins in posterior silk glands (PSG) from 5 silkworm larvae (5th instar) were lysed in 5 ml ice-cold extraction buffer (20 mM Tris–HCl pH 7.4, 0.5 M NaCl, 1% Triton X-100, 10% glycerol, 10 mM 2-mercaptoethanol, 1 mM PMSF) for 6 h. Subsequently, 300 µL crude extraction of PSG was precipitated in acetone (1:3, v/v) at −20 °C for 2 h. After centrifugation at 12,000g in 4 °C for 5 min, the protein pellet was dried and re-resolved in 100 µL the assay buffer (25 mM Tris–HCl pH 7.5, with 10 mM CaCl2, 10 mM MnCl2). The resulting solution including endogenous glycoprotein in silk gland was named PSG. The in vitro glycosylation assay was initiated by incubating 1 µL SG with 100 ng of rhβ4GalT1 (C-tag or N-tag) and 10 mM UDP-galactose (UDP-Gal, SIGMA) as a donor in a total volume of 10 µL assay buffer for 1 h at 37 °C. After incubation, the resulting solution was subjected to SDS-PAGE and transferred to a PVDF membrane for lectin blotting. Briefly, the membrane was blocked for 30 min in a carbo-free blocking solution (Vector Laboratories, USA), followed by incubation with Ricinus Communis Agglutinin I (RCA120, specifically for terminal β-D-galactosyl residues; HRP-conjugated, 2 ppm, J-OILMILLS, Japan) diluted in PBST buffer (PBS pH 7.5, 0.05% Tween-20) for 1 h. After washing twice with PBST buffer, the HRP signals were visualized by the Super Signal West Pico Chemiluminescent Substrate (Thermo Scientific, USA).
Results
Construction of Expression Vectors
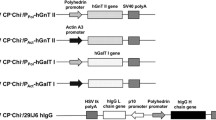
As illustrated in Fig. 1a, human β4GalT1 reported to be composed of 398 amino acid residues, contains a cytoplasmic domain (a.a.1–24), a type-2 transmembrane domain (a.a.25–44) and a luminal domain (a.a.45–398). Generally, human β4GalT1 is localized primarily in the plasma membrane and exists in the trans cisternae of the Golgi complex [10]. Previous studies suggest that the mature form of the human β4GalT1 is secreted into body fluids by cleaving its catalytic domain between L77 and R78 [8, 9] (Fig. 1a). Accordingly, it is reasonable to consider that a significant level of the recombinant β4GalT1 flows out from Golgi apparatus, and the intracellular GT activity becomes relatively lower [14], even when a large amount of protein is generated using the BEVS. Here, we created the expression construct for the catalytic domain of human β4GalT1 (a.a.78–398) (rhβ4GalT1) with a silkworm 30 K signal peptide to induce effective secretion [23]. In addition to aid in the efficient purification of the enzyme, 8 × His-tag and Strep-tag were added to the N- or C-terminus of the recombinant human β4GalT1. Therefore, we generated two types of expression vectors for the expression of recombinant human β4GalT1 (Fig. 1b).
Functional domains of full-length human β4GalT1 are shown, including the cytoplasmic domain (C), transmembrane (T) and lumenal domain containing β4GalT1 catalytic domain. By cleaving between L77 and R78, matured form of human β4GalT1 was generated (a). Construction of the vectors for expression the rhβ4GalT1 (matured form). The plasmid pDEST8/polh 30 K_rhβ4GalT1 (N-tag) and pDEST8/polh 30K_rhβ4GalT1 (C-tag) were generated from pENTR and pDEST8 vector by Gateway reaction. These vectors were under the control of polyhedron (polh) promoter and followed by SV40 polyadenylation signal (polyA). L21: leader sequence for enhancing translation efficiency; 30K: signal peptide of silkworm 30 kDa protein; TEV: tobacco etch virus protease cleavage site; His: 8 x histidine tag; ST: strep-tag (b). Expression of the rhβ4GalT1 in Bme21 cells. At 4 dpi, the rhβ4GalT1 s were detected from culture medium (M) and cell lysate (C) by Western blotting using His-Probe (c)
Expression Verification of rhβ4GalT1 in BmNPV-Infected Cultured Silkworm Cells
The expressions of two kinds recombinant baculoviruses of rhβ4GalT1 were firstly examined in BmNPV-hypersensitive Bme21 cells [20]. The predicted molecular masses of the rhβ4GalT1 s with N-terminal or C-terminal tags were 39.7 and 39.9 kDa, respectively. As demonstrated in Fig. 1c, both rhβ4GalT1 s with N-tag or C-tag were expressed and secreted into the culture medium (M). Interestingly, the molecular weight of the N-tagged rhβ4GalT1 estimated from the mobility on SDS-PAGE was higher than the predicted size. Besides, the C-tagged rhβ4GalT1 secreted into culture medium generated three bands containing 8 x His-tag, indicating the possibilities that C-tagged rhβ4GalT1 was hydrolyzed from its N-terminus or simply because alternative ATG start codons were used for protein translation. Furthermore, the expression level of the C-tagged rhβ4GalT1 was significant higher than of the N-tagged, suggesting that the C-terminus is ideal for improving the yields of rhβ4GalT1.
Purification of rhβ4GalT1 from Silkworm Larvae
The rhβ4GalT1 s secreted into silkworm hemolymph were collected at 4 dpi of the indicated recombinant BmNPV, and then purified using two-step affinity chromatography. As a result, approximately 124 µg of the N-tagged (Fig. 2a) and 239 µg of the C-tagged (Fig. 2b) highly purified rhβ4GalT1 were recovered from 10 ml of hemolymph from silkworm larvae. As far as we are aware, this is the first study reporting the expression and purification of human β4GalT1 on this scale using this system. Unlike the expression in Bme21 cells illustrated in Fig. 1c, the purified N-tagged and C-tagged rhβ4GalT1 s generated one major band at approximately 42 or 40 kDa, and smear multiply bands ranging from 42–45 to 40–45 kDa, respectively (Fig. 2a, b). These results suggest that the resulting proteins were potentially with certain post-translational modifications (PTMs), e.g., glycosylation [24].
Purification of the N- or C-tagged rhβ4GalT1 from sera of infected silkworm larvae (a C-tagged; b N-tagged). The His and Strep double-tagged rhβ4GalT1 were purified through nickel affinity chromatography (lanes IP, 1, 2, 3) and Strep-Tactin affinity chromatography (lanes 4, 5, 6) as described in Methods. Each of the fractions was resolved on 10% SDS-PAGE and visualized by Coomassie Brilliant Blue (CBB) R-250. M molecular mass markers; Lane 1 flow-through fraction; Lane 2 wash fraction; Lane 3 eluent fraction (500 mM imidazole); Lane 4 flow-through fraction; Lane 5 wash fraction; Lane 6 eluent fraction (2.5 mM desthiobiotin)
Molecular Characterization of rhβ4GalT1 s from Silkworm Larvae
Human β4GalT1 is expected to contain 1 N-glycosylation site (N113) and the previously study showed its N-linked glycan has triantennary or biantennary complex-type structures [24]. In addition, 5 potential O-glycosylation sites are predicted in the amino acid sequence of human β4GalT1, but confirmation of the existence of these O-glycans in human β4GalT1 has not been reported until now. Therefore, we compared the N- or O-linked glycan structures of rhβ4GalT1 s from silkworm larvae with the commercially available rhβ4GalT1 (a.a.44–398; R&D systems, Inc., Mineapolis, USA) expressed in mouse myeloma cell lines as control. As shown in Fig. 3, the rhβ4GalT1 s and the commercial β4GalT1 were treated with PNGase F to cleave potential N-linked glycans, or O-glycosidase along with α2-3,6,8 Neuraminidase to cleave possible O-linked glycans. As shown in Fig. 3, all C-tagged, N-tagged and commercial rhβ4GalT1s were sensitive and cleavable by PNGase F, which proved that rhβ4GalT1s secreted from silkworm larvae were N-glycosylated similarly to that expressed by mouse cells. On the other hand, after treatment with O-glycosidase, the electrophoretic mobility of C-tagged or N-tagged rhβ4GalTs was not changed. Besides, even with treatment of the PNGase F, the rhβ4GalT1 s from silkworm larvae did not show a single product, which we considered that the products from smear bands were not generated by glycosylation, possibly an unknown PTMs.
Characterization of the C-tagged and N-tagged rhβ4GalT1 secreted in silkworm larval serum. The commercial rhβ4GalT1 (with N-terminal tag) from mouse cell lines, C-tagged and N-tagged rhβ4GalT1 produced by BEVS were incubated with (+) or without (−) PNGase F or O-glycosidase with α 2-3,6,8 Neuraminidase for 1 h at 37 °C. After reaction, each mixture was resolved on 10% SDS-PAGE and visualized by Western blotting using His-Probe
Galactosyltransferase Activity Assay to Test the Activity
To compare the galactosyltransferase activities of the rhβ4GalT1 s from silkworm larvae with that from mouse cells, we performed an in vitro activity assay using a Glycosyltransferase Activity Kit (R&D Systems, Inc., Minneapolis, USA). As a negative control, the secretion protein from silkworm larvae, recombinant human α1AGP [22] was utilized in this study. As shown in Fig. 4a, both of the C-tagged and N-tagged rhβ4GalT1 exhibited much higher activity of about 1.6–2.0-fold than the rhβ4GalT1 expressed in mammalian cell lines did. Furthermore, we also learned that the N-tagged rhβ4GalT1 showed higher activity than the C-tagged protein, though the C-tagged construct provided more production, indicating C-terminal tags may slightly affect the glycol-transferring and thus it is rescannable to consider that N-terminal design is better for functional production of rhβ4GalT1. Moreover, from the results in Fig. 1c, the C-tagged rhβ4GalT1 appears to have less enzyme stability than that of N-tagged protein although it was expressed at a higher level.
Galactosyltransferase activity assay of the rhβ4GalT1. The specific activities of the commercial rhβ4GalT1 produced in mammalian cell lines, the C-tagged and N-tagged rhβ4GalT1 from silkworm larvae were measured by Glycosyltransferase Activity Kit (a). As a negative control of recombinant glycoprotein secreted silkworm larvae, we used the recombinant human α1AGP. Bars and error bars indicate the mean and SD of values, respectively. Significant differences in each rhβ4GalT1 activity were evaluated using one-way ANOVA and Turkey’s test. ***p < 0.05. Galactosyltransferase activities against N-glycan of endogenous glycoproteins from posterior silk glands in silkworm larvae were detected by lectin blotting using Ricinus communis agglutinin I (RCA120) (b). The extraction from posterior silk glands was incubated with (+) or without (−) the rhβ4GalT1 (N-tag or C-tag) and UDP-Galactose (UDP-Gal) as a donor for 1 h at 37 °C. Mock: the assay buffer (25 mM Tris–HCl pH 7.5, with 10 mM CaCl2, 10 mM MnCl2) instead of the extraction from posterior silk glands
Commonly, N-glycans of glycoproteins secreted from insect cells have paucimannosidic structures that lack the terminal GlcNAc that could act as an acceptor for galactose, likely due to the extensive β-N-acetylglucosaminidase (FDL) activity causing the removal of the terminal GlcNAc from the hybrid-type structure [25]. However, the previous research showed glycoproteins produced in silk glands in silkworm larvae have complex-type N-glycans with terminal GlcNAc [26]. In order to confirm the in vitro galactosyltransferase activity of rhβ4GalT1, N-glycans of glycoproteins from silkworm PSG with terminal GlcNAc and UDP-Galactose were used as a substrate and donor, respectively. We then extracted endogenous glycoproteins from PSG and incubated with UDP-Galactose together with indicated rhβ4GalT1. As shown in Fig. 4b, lectin blotting with galactose-specific detecting RCA120, both of the C- and N-tagged rhβ4GalT1 s exhibited galactosyltransferase activities against N-glycans of endogenous glycoprotein in PSG, verifying that rhβ4GalT1 from silkworm–BEVS provides both high yields and promising activity.
Discussion
In the current study, the N-tagged and C-tagged rhβ4GalT1 was expressed, purified and recovered at approximately 124 and 239 µg, respectively, from 10 ml sera of roughly 25 infected silkworm larvae. The rhβ4GalT1s were N-glycosylated with galactosyltransferase activities higher than that of rhβ4GalT1 expressed and purified from mammalian cell lines. Although the final yield of the C-tagged rhβ4GalT1 is higher than that of the N-tagged, it is better to choose N-tag for production of rhβ4GalT1 in silkworm–BEVS if taking the purity and overall activity into considerations. Besides, it was shown that the glycoproteins extracted from silk glands of silkworm larvae could be further galactosylated by in vitro processing with the rhβ4GalT1s. The production of these recombinant human β4GalT1 s from silkworm–BEVS will be valuable tools for both therapeutics as well as biotechnology. For therapeutics, rhβ4GalT1 s can be used along with α1,3- or α1,4-fucosyltransferases [1, 2, 6, 7] and α2,3-sialyltransferases to help create the sLex for the ex vivo on therapeutic cells such as stem cells in order to treat disease. In biotechnology, rhβ4GalT1 s can be used in the production of high-quality recombinant glycoproteins with mammalian-like N-glycans. Since there are several differences in N-linked glycosylation pathways between mammals and insects, proteins secreted from insect cells are paucimannosidic and generally not terminally galactosylated or sialylated [27] likely due to the extensive FDL activity which removes the terminal GlcNAc of the hybrid-type structure [25]. From this point of view, the recombinant glycoproteins generated using BEVS are presumably somewhat less stable in mammalian blood than those from native animals. Accordingly, it has been, for a long time, a problem to use recombinant glycoproteins produced from BEVS in practical medicine and clinical research. To date, various approaches have been reported to alter the N-linked glycan structure of insect-derived proteins into terminally sialylated complex-type N-glycans by transgenic expression of mammalian glycosyltransferases in insect cells [11,12,13]. However, the efficiency of formation of matured complex-type N-glycans in the insect cells is still low because the activity of mammalian β4GalT1 is unstable in insect cells [14]. In addition, the negative effect of exogenously expressed β4GalT1 on insect cells must be taken into account especially in transgenic insects. Here, we propose another approach in which the recombinant glycoproteins produced by BEVS are processed by in vitro N-glycosylation using mammalian glycosyltransferases mass-produced and purified from insect cells or larvae. Because galactosylated N-glycans provide substrates for sialylation by sialyltransferases, it is desirable to mass-produce the recombinant rhβ4GalT1 s by BEVS.
References
Sackstein, R., Merzaban, J. S., Cain, D. W., Dagia, N. M., Spencer, J. A., Lin, C. P., et al. (2008). Ex vivo glycan engineering of CD44 programs human multipotent mesenchymal stromal cell trafficking to bone. Nature Medicine, 14(2), 181–187.
Merzaban, J. S., Imitola, J., Starossom, S. C., Zhu, B., Wang, Y., Lee, J., et al. (2015). Cell surface glycan engineering of neural stem cells augments neurotropism and improves recovery in a murine model of multiple sclerosis. Glycobiology, 25(12), 1392–1409.
Sackstein, R. (2016). Fulfilling Koch’s postulates in glycoscience: HCELL, GPS and translational glycobiology. Glycobiology, 26(6), 560–570.
Hidalgo, A., Weiss, L. A., & Frenette, P. S. (2002). Functional selectin ligands mediating human CD34(+) cell interactions with bone marrow endothelium are enhanced postnatally. The Journal of Clinical Investigation, 110(4), 559–569.
Xia, L., McDaniel, J. M., Yago, T., Doeden, A., & McEver, R. P. (2004). Surface fucosylation of human cord blood cells augments binding to P-selectin and E-selectin and enhances engraftment in bone marrow. Blood, 104(10), 3091–3096.
Robinson, S. N., Simmons, P. J., Thomas, M. W., Brouard, N., Javni, J. A., Trilok, S., et al. (2012). Ex vivo fucosylation improves human cord blood engraftment in NOD-SCID IL-2Rgamma(null) mice. Experimental Hematology, 40(6), 445–456.
Wan, X., Sato, H., Miyaji, H., McDaniel, J. M., Wang, Y., Kaneko, E., et al. (2013). Fucosyltransferase VII improves the function of selectin ligands on cord blood hematopoietic stem cells. Glycobiology, 23(10), 1184–1191.
Masri, K. A., Appert, H. E., & Fukuda, M. N. (1988). Identification of the full-length coding sequence for human galactosyltransferase (β-N-acetylglucosaminide: β1,4-galactosyltransferase). Biochemical and Biophysical Research Communications, 157(2), 657–663.
Aoki, D., Appert, H. E., Johnson, D., Wong, S. S., & Fukuda, M. N. (1990). Analysis of the substrate binding sites of human galactosyltransferase by protein engineering. The EMBO Journal, 9(10), 3171–3178.
Lopez, L. C., Youakim, A., Evans, S. C., & Shur, B. D. (1991). Evidence for a molecular distinction between Golgi and cell surface forms of β1,4-galactosyltransferase. Journal of Biological Chemistry, 266(24), 15984–15991.
Jarvis, D. L. (2003). Developing baculovirus-insect cell expression systems for humanized recombinant glycoprotein production. Virology, 310(1), 1–7.
Harrison, R. L., & Jarvis, D. L. (2006). Protein N-glycosylation in the baculovirus-insect cell expression system and engineering of insect cells to produce “mammalianized” recombinant glycoproteins. Advances in Virus Research, 68, 159–191.
Toth, A. M., Kuo, C. W., Khoo, K. H., & Jarvis, D. L. (2014). A new insect cell glycoengineering approach provides baculovirus-inducible glycogene expression and increases human-type glycosylation efficiency. Journal of Biotechnology, 182–183(1), 19–29.
Geisler, C., Mabashi-Asazuma, H., Kuo, C. W., Khoo, K. H., & Jarvis, D. L. (2015). Engineering β1,4-galactosyltransferase I to reduce secretion and enhance N-glycan elongation in insect cells. Journal of Biotechnology, 193, 52–65.
Seo, N., Hollister, J. R., & Jarvis, D. L. (2001). Mammalian glycosyltransferase expression allows sialoglycoprotein production by baculovirus-infected insect cells. Protein Expression and Purification, 241, 234–241.
Summers, M. D. (2006). Milestones leading to the genetic engineering of baculoviruses as expression vector systems and viral pesticides. Advances in Virus Research, 68(06), 3–73.
Kato, T., Kajikawa, M., Maenaka, K., & Park, E. Y. (2010). Silkworm expression system as a platform technology in life science. Applied Microbiology and Biotechnology, 85(3), 459–470.
Tauber, P., Krammer, F., Palmberger, D., Rendi, D., Wilson, I. B. H., & Grabherr, R. (2011). Insect cells for antibody production: Evaluation of an efficient alternative. Journal of Biotechnology, 153, 160–166.
Aumiller, J. J., Mabashi-asazuma, H., Hillar, A., Shi, X., & Jarvis, D. L. (2012). A new glycoengineered insect cell line with an inducibly mammalianized protein N-glycosylation pathway. Glycobiology, 22(3), 417–428.
Lee, J. M., Kawakami, N., Mon, H., Mitsunobu, H., Iiyama, K., Ninaki, S., et al. (2012). Establishment of a Bombyx mori nucleopolyhedrovirus (BmNPV) hyper-sensitive cell line from the silkworm e21 strain. Biotechnology Letters, 34, 1–7.
Ono, C., Nakatsukasa, T., Nishijima, Y., Asano, S., Sahara, K., & Bando, H. (2007). Construction of the BmNPV T3 bacmid system and its application to the functional analysis of BmNPV he65. Journal of Insect Biotechnology and Sericology, 167, 161–167.
Morokuma, D., Xu, J., Mon, H., Hirata, K., Hino, M., Kuboe, S., et al. (2015). Human alpha 1-acid glycoprotein as a model protein for glycoanalysis in baculovirus expression vector system. Journal of Asia-Pacific Entomology, 18(2), 303–309.
Soejima, Y., Lee, J. M., Nagata, Y., Mon, H., Iiyama, K., Kitano, H., et al. (2013). Comparison of signal peptides for efficient protein secretion in the baculovirus-silkworm system. Central European Journal of Biology, 8(1), 1–7.
Funita-Yamaguchi, Y., & Yoshida, A. (1981). Purification and characterization of human serum galactosyltransferase (lactose synthetase A protein). Journal of Biological Chemistry, 256(6), 2701–2706.
Kim, Y. K., Kim, K. R., Kang, D. G., Jang, S. Y., Kim, Y. H., & Cha, H. J. (2009). Suppression of β-N-acetylglucosaminidase in the N-glycosylation pathway for complex glycoprotein formation in Drosophila S2 cells. Glycobiology, 19(3), 301–308.
Iizuka, M., Ogawa, S., Takeuchi, A., Nakakita, S., Kubo, Y., Miyawaki, Y., et al. (2009). Production of a recombinant mouse monoclonal antibody in transgenic silkworm cocoons. FEBS Journal, 276(20), 5806–5820.
Marchal, I., Jarvis, D. L., Cacan, R., & Verbert, A. (2001). Glycoproteins from insect cells: Sialylated or not? Biological Chemistry, 382(2), 151–159.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Morokuma, D., Xu, J., Hino, M. et al. Expression and Characterization of Human β-1, 4-Galactosyltransferase 1 (β4GalT1) Using Silkworm–Baculovirus Expression System. Mol Biotechnol 59, 151–158 (2017). https://doi.org/10.1007/s12033-017-0003-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12033-017-0003-1