Abstract
The aim of this study is to find Iranian tall fescue accessions that tolerate drought stress and investigation on phylogenetical, morphological, and physiological characterization of them. For this propose, inter-simple sequence repeats (ISSR) markers were used to examine the genetic variability of accessions from different provinces of Iran. Of 21 primers, 20 primers generated highly reproducible fragments. Using these primers, 390 discernible DNA fragments were produced with 367 (93.95 %) being polymorphic. The polymorphic information content (PIC) values ranged from 0.948 to 0.976, with a mean PIC value of 0.969. Probability identity (PI) and discriminating power (D = 1 − PI) among the primers ranged from 0.001 to 0.004 and 0.998 to 0.995, respectively. A binary qualitative data matrix was constructed. Data analyses were performed using the NTSYS software and the similarity values were used to generate a dendrogram via UPGMA. To study the drought stress, plants were irrigated at 25 % FC condition for three times. Fresh leaves were collected to measure physiological characters including: superoxide dismutase, catalase, and peroxidase activities and proline and total chlorophyll content at two times, before and after stress application. Relative water content, fresh and dry weight ratio, survival percentage, and visual quality were evaluated after stress. Morphological and physiological characters were assessed in order to classify accessions as either tolerant or sensitive using Ward’s method of Hierarchical cluster analysis in SPSS software. The results of present study demonstrated that the ISSR markers are useful for studying tall fescue genetic diversity. Convergence of morphological and physiological characterizations during drought stress and phylogenetic relationship results showed that accessions can be grouped into four clusters; drought-tolerant accessions that collected from west of Iran, drought-tolerant accessions collected from northwest of Iran, drought semi-tolerant accessions collected from center of Iran, and drought-sensitive accessions collected from north of Iran. Data presented could be used to classify the tall fescue accessions based on suitability of cultivation in the regions studied or the regions with the similar environmental condition.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tall fescue (Festuca arundinacea Schreb.) is a common cool-season perennial grass that is under cultivation in urban landscape throughout the transitional climates [1]. This species has a relatively deep root system that acclimates itself to drought stress better than other cool-season grasses [2]. Tall fescue is cross-pollinated, allohexaploid (2n = 6x = 42) with the genomic constitution of PPG1G1G2G2 [3]. This species grows in natural pastures of central, western, and northeast regions [4]. One well-known method for proving crop breeding, especially in studies on genetic diversity and gene mapping, is using DNA markers [5]. Inter-simple sequence repeats (ISSR)-PCR has recently emerged as a powerful technique in the characterization of genomes, and is specifically useful in the differentiation of closely related individuals or species [6–9] that based on random nucleic acid analysis. This type of PCR screening uses the inter-simple sequence repeats present in all eukaryotic species to anchor random oligonucleotide primers. ISSR-PCR produced markers tend to exhibit co-dominant Mendelian inheritance. However, the same as RAPD markers, tend to occur in non-coding regions of the genome, produce bands on electrophoresis gel that each represents a locus of the genome, and therefore, are a valuable and effective tool for screening [10, 11]. ISSR-PCR studies have been performed to determine the diversity of various plant species, such as wheat (Triticum aestivum L.) [12], radish (Raphanus sativus L.) [13], perennial ryegrass (Lolium perenne L.) [14], and bermudagrass (Cynodon dactylon (L.) Pers. and Cynodon × transvaalensis) [15]. However, there are few reports on genetic diversity of tall fescue using ISSR markers [16]. Few of other molecular markers such as RFLP were used to study the phylogenetic relationship of tall fescues with related grass species [17]. Drought is the most serious abiotic stress affecting plant growth, which is caused by lack of water in arid or semi-arid areas or due to irregular distribution of precipitation [18]. During drought stress, Reactive Oxygen Species (ROSs) were increased that these ROSs adversely affected growth and development [19]. ROSs highly deactivate cellular metabolism by oxidative damage to membranes, proteins, and nucleic acids; they also cause lipid peroxidation, protein denaturation, and DNA mutation [20]. Activity and content of antioxidant enzymes such as superoxide dismutase (SOD), catalase (CAT), and peroxidase (APX), and proline increases in plant cells as a response to drought stress and play a key role in the defense against oxidative stress [19]. However, tolerance to drought stress is a complex quantitative character controlled by several small effect genes [21, 22]. The results of investigation on important morphological and physiological traits of tall fescue accessions can be used in breeding programs to find tolerant accessions. Thus, in this report we investigated the physiological and morphological characters (before and after drought stress) and ISSR markers in 45 Iranian accessions along with one foreign commercial cultivar (as control) of tall fescue.
Materials and Methods
Plant Material and Examination of Morphological and Physiological Characteristics
One foreign cultivar, “Starlet” (as control) and 45 Iranian accessions of F. arundinacea were collected from different geographical regions (Fig. 1) and moved to greenhouse of Department of Horticultural Science at Shiraz University. They were maintained under ambient temperature at 25 ± 2 °C, 70–80 % relative humidity. Photosynthetic photon flux (PPF) density was set at 60 ± 5 μmol m−2 s−1 provided by white fluorescent lamps with 16-h photoperiod. The experiment was arranged as a completely randomized design (CRD) with three replications. After 2 months of growing, fresh leaves were collected to measure their physiological characters including; SOD [23], CAT [24], APX [25] activities, and proline and total chlorophyll content. After that, plants were irrigated to 25 % FC condition for three times and then after, fresh leaves were collected again for measuring SOD, CAT, and APX activities, proline and total chlorophyll content, and morphological traits. Morphological traits including relative water content (RWC), fresh and dry weight ratio, survival percentage, and visual quality were evaluated. Morphological and physiological characters were assessed in order to classify accessions as either drought tolerant or sensitive using Ward’s method of Hierarchical cluster analysis in SPSS software.
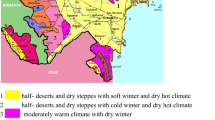
The sampling locations for tall fescue accessions collected from different regions of Iran 1 Borojen, 2 Tiran, 3 Hashtrod, 4 Saghez, 5 Hamedan, 6 Aligodarz, 7 Divandareh I, 8 Alisadr, 9 Boloran, 10 Bostanabad, 11 Golpayegan, 12 Islamabad, 13 Arak, 14 Kamyaran, 15 Khoramabad, 16 Salmas, 17 Darehsari, 18 Sanandaj, 19 Divandareh II, 20 Pakdasht, 21 Garmsar, 22 Shahrod, 23 Tonekabon, 24 Manjil, 25 Azadshahr, 26 Rasht, 27 Mahmodabad, 28 Bandargaz, 29 Tehran, 30 Chalos, 31 Khoshyeylagh, 32 Aliabad, 33 Karaj, 34 Babol, 35 Chaboksar, 36 Sari, 37 Neka, 38 Qaemshahr, 39 Qazvin, 40 Lahijan, 41 Kohein, 42 Semnan, 43 Gorgan, 44 Damghan, 45 Behshahr
ISSR Analysis
Samples (4 g of fresh mature leaves) were rinsed in tap water followed by sterilized distilled water. They were then air-dried and stored at −80 °C until used. The leaves were ground in liquid nitrogen in a sterile prechilled mortar with pestle. Plant genomic DNA was extracted by a CTAB (cetyltrimethylammonium bromide) protocol. Leaf tissues (100 mg) were ground in 1000 μL of CTAB extraction buffer [100 mM Tris (pH 8.0), 1.4 M NaC1, 20 mM EDTA (pH 8.0), 0.2 % (p/v) β-mercaptoethanol, 2 % (p/v) CTAB], and heated at 60 °C for 30 min. DNA was extracted with one volume of a chloroform:isoamyl alcohol mix (24:1) and precipitated in presence of isopropanol [40 % (v/v) final concentration]. The DNA pellet was washed with 5 mM ammonium acetate and 70 % ethanol, dried, and dissolved in 100 μL of TE [10 mM Tris-HCL (pH 8.0), 1 mM EDTA (pH 8.0)]. After addition of 1 μL of RNase (10 mg/mL), DNA concentrations were determined with a spectrophotometer (Biochrom, France).
Twenty-one ISSR primers were purchased in lyophilized form from Cinnagen Co (Iran) and optimum annealing temperature was determined for each primer. Amplifications were carried out by using a DNA thermal cycler (Eppendorf, mastercycler gradient, USA), program for each primer is shown in Table 1.
The PCR products were analyzed by electrophoresis using a 1.5 % agarose gel. DNA was stained by soaking the gels in a 0.5 μg/mL ethidium bromide solution and observed under UV light and photographed using gel documentation unit. Amplified ISSR markers were scored as present (1) or absent (0) and ambiguous bands, which could not be easily distinguished, were not scored. A binary qualitative data matrix was constructed. Data analyses were performed using the NTSYS PC version 2.02 software [26]. The similarity values were used to generate a dendrogram via the un-weighted pair group method with arithmetic average (UPGMA) [27]. Polymorphism information content (PIC) value, probability identity (PI), and discriminating power (D = 1-PI) of primers was estimated using HET software [28].
PIC described by Oliviera et al. [29], and PI described by Pollefeys and Bousquet [30] were calculated as follows:
where P i and P j are the frequencies of the ith and jth alleles within each locus, respectively.
Results and Discussion
Out of 21 ISSR primers, 20 of them produced DNA fragments which were scorable and all the 20 scorable primers produced polymorphic bands. However, ISSR-13 produced no band. ISSR-19 produced maximum bands (27) while, ISSR-2, ISSR-4, ISSR-8, ISSR-11, ISSR-16, ISSR-17, and ISSR-21 primers produced 100 % polymorphic bands (Table 1). Annealing temperature ranged from 49 °C (ISSR-10 and ISSR-12) to 58 °C (ISSR-7 and ISSR-8). The PIC of primers was high and varied from 0.948 for ISSR-16 to 0.976 for ISSR-7 (K15) with an average PIC of 0.969. Furthermore, minimum D and maximum P i was belonged to ISSR-7 and maximum D and minimum P i was belonged to ISSR-16 (Table 1).
The UPGMA clustering algorithm from ISSR analysis grouped the accession into five clusters at a similarity index value of 0.22 (Fig. 2). Tall fescues collected from Rasht, Alisadr, Chalos, and Khoramabad were included in the separate groups. Other accessions made a major group that was separated to three subgroups. The first subgroup had 18 accession including: Borojen, Tiran, Hashtrod, Hamedan, Control, Divandare I, Boloran, Bostanabad, Golpaygan, Kamyaran, Salmas, Darehsari, Divandare II, Sanandaj, Eslamabad, Pakdasht, Garmsar and Semnan. The Second subgroup had 23 accession including: Saghez, Aligodarz, Shahrod, Tonekabon, Kohin, Qazvin, Mahmodabad, Aliabad, Behshahr, Khoshyeylagh, Damghan, Gorgan, Karaj, Tehran, Azadshahr, Chaboksar, Bandargaz, Babol, Sari, Neka, Qaemshahr, Lahijan, and Manjil. The Third subgroup included only the accession collected from Arak.
Dendrogram of the genetic relationships between 45 accession of tall fescue and control cultivar using the data generated from the Jaccard similarity matrix based on ISSR profiles. For the accession numbers correspondence see the numbers listed in Fig. 1
Relations between tall fescue accessions were also revealed by PCoA (Fig. 3). In the two-dimensional PCoA plot, in general, similar groupings with the UPGMA dendrogram and geographical information were also revealed (e.g., the accessions were placed in one group by their phylogenetic relationships and geographical distance). The first two principal axes accounted for 9.5 and 5.22 % of the total variation, respectively, representing the complex multidimensional nature of ISSR variation. The two-dimensional projection of genotypes along the first principal axes revealed the climate and location relationships among accessions (Fig. 3). The accessions that isolated from Khazari, East Zagros, and Central climates produced tight clusters and exhibited considerable separation. The factor loadings along the second axis (5.22 %) cannot contribute accessions.
Two-dimensional projection of inter simple sequence repeat variation calculated by principal coordinate analysis for 45 accessions of tall fescue and control cultivar. For the accession numbers correspondence see the numbers listed in Fig. 1
The UPGMA clustering algorithm from physiological characters before drought stress grouped accessions into two major clusters. The first group that had more antioxidant enzymes activity, and proline and protein content were 9 accessions including: Borojen, Khoramabad, Tiran, Pakdasht, Karaj, Kamyaran, Garmsar, Shahrod, and Tehran. Other accessions were in second group and had low antioxidant enzymes activity, and proline and protein content (Fig. 4).
Dendrogram of the physiological relationships between 45 accession of tall fescue and control cultivar under water-sufficient condition. For the accession numbers correspondence see the numbers listed in Fig. 1
UPGMA clustering algorithm from physiological and morphological characters after drought stress grouped accessions into three major clusters. The first group is foreign cultivar, the second group included 25 accessions, and third group included 20 accessions. First group that is drought-tolerant, had most antioxidant enzymes activity; proline and protein content; highest visual quality, RWC, dry and fresh weight ratio, and survival percentage. The second group that are drought semi-tolerant had lower pointed characters than first group and higher than third group, i.e., drought-sensitive group (Fig. 5).
Dendrogram of the physiological and morphological relationships between 45 accession of tall fescue and control cultivar under water-deficient condition. For the accession numbers correspondence see the numbers listed in Fig. 1
Principal component analysis (PCA) of morphological and physiological characteristics in tall fescue accessions grown under water-sufficient and deficient condition were revealed in Figs. 6 and 7 by the two-dimensional PCA plots. It was found that the first and second component accounted for 96.5, 2.5 % of variance under water-sufficient condition and 96.4, 2.8 % of variance under water-deficient condition. The accessions that isolated from Khazari and shore Khazari exhibited considerable separations under two water availability conditions.
Principal component analysis (PCA) of physiological characteristics in 45 tall fescue accessions and control cultivar grown under water-sufficient condition. For the accession numbers correspondence see the numbers listed in Fig. 1
Principal component analysis (PCA) of morphological and physiological characteristics in 45 tall fescue accessions and control cultivar grown under water-deficient condition. For the accession numbers correspondence see the numbers listed in Fig. 1
Results showed that ISSR markers are efficient tool for identification of tall fescue accessions and presenting high polymorphism, PIC, D, and low PI. Tessier et al. [31] reported the discriminating power, which is an extension of PIC available from the frequencies of the different banding patterns. Sivaprakash et al. [32] have reported that the ability to resolve genetic variation may be more directly related to the degree of polymorphism detected by the marker system. Our PIC values were high, which could be due to the fact that the accessions used in our study have a widespread genetic base. Twenty ISSR markers produced 380 bands totally and the average of polymorphism was high (93.95 %). In an investigation on genetic diversity of Iranian tall fescue accessions by AFLP, Majidi et al. [33] reported that of the 493 bands scored, 421 (85.4 %) were polymorphic. Motawei and Ghumaiz [16] reported that 15 ISSR primers among the six turfgrass cultivars [two tall fescue cultivars, two perennial ryegrass cultivars, and two orchardgrass (Dactylis glomerata L.) cultivars] produced 77 bands, 66 (85.7 %) of them were polymorphic. However, in that study amplified bands were lower than the present study. Totally, most of the primers used in present experiment revealed workable patterns that can be tested in further investigations for analysis of phylogenetic relationships between tall fescue populations.
From the UPGMA clustering results, tall fescue accessions were obviously separated. Because tall fescue is cross-pollinated and self-incompatible [34], the accessions lead to many different groups and genetic diversity between them is affected by the self-incompatibility [35–37]. However, in small populations self-incompatibility causes reduction in genetic diversity due to a reduced availability of compatible mates [38, 39]. UPGMA clustering and two-dimensional PCoA plot indicated that the accessions grouping is closely based on their origin. As mentioned before, tall fescues were isolated from different climates and the climate affected the flowering time. Therefore, accessions are fertilized by accessions that their anthesis takes place at the same time. The factors that affect the tall fescue flowering have not been extensively investigated. One most important factor that enhances floral development is cold temperature during the winter [34]. Furthermore, this matter was found that tall fescue will flower under a range of photoperiods; short days for flower induction (not an absolute requirement when vernalization had occurred during the winter) and long days resulted in earlier flowering with fewer panicles per plant [40]. Hicks and Mitchell [41] reported that tall fescue need short photoperiods or low temperature for floral induction and long photoperiods (16 h) for floral initiation. As can be observed in Fig. 1, accessions were collected from regions with different latitudes and altitudes. Latitude can affect photoperiod and temperature and altitude can affect temperature.
Principal component analysis (PCA) is one of the most general explorative methods used to reduce multivariate data complexity. This is a method of choice for identifying patterns, and expressing data in ways that highlight similarities and differences between samples [42]. The results of CAT, POD, and APX activities in accessions showed significant increase during drought stress (data not shown). Other studies have reported similar changes in maize [43], caper [44], and wheat [45]. Plants have a wide range of defense systems to survive against drought stress [46, 47]. One of the earliest responses of plants to stresses such as water-deficiency is the accumulation of ROSs such as superoxide, hydroxyl radicals, hydrogen peroxide, and singlet oxygen that can damage the plant cells [48]. ROS accumulation initiates pre-emptive defense responses such as increasing activity of antioxidant enzymes [49, 50]. CAT, SOD, and POD are common and important antioxidant enzymes that have high ability to destroy ROSs [49, 51] and many researchers reported that these antioxidant enzymes are higher in resistant plants than sensitive ones during drought stress [47, 52, 53]. In present study, CAT, SOD, and POD were increased by drought stress, especially in tolerant accessions (data not shown). Furthermore, water stress induced proline and protein content. It is well known that the free proline level increases in response to drought. Proline is one of the osmotic adjustment (OA) materials and has important function as osmoprotectant [54] for resistance and adaptation under drought stress conditions [55]. It may play a role in protection from desiccation and from the harmful effects resulted from solute accumulation. The level of free proline in plants depends to water stress. Drought stress increases ROSs, resulting in oxidative injury at cellular level. ROSs can disrupt electron transport system [56, 57], therefore limit photosynthesis and usually accompanied with the formation of ROSs such as O2−, H2O2, and OH− in chloroplasts [58, 59] causing decrease in chlorophyll content.
According to phylogenetical, morphological, and physiological investigations and origins of accessions we can cluster them into four groups: drought-tolerant accessions that collected from west of Iran, drought-tolerant accessions collected from northwest of Iran, drought semi-tolerant accessions collected from center of Iran, and drought-sensitive accessions collected from north of Iran. However, foreign cultivar was the most drought-tolerant population compared to Iranian accessions. Data presented could be used to classify the tall fescue accessions based on suitability of cultivation in the regions studied or the regions with the similar environmental condition.
References
Christians, N. (2004). Fundamentals of turfgrass management. Hoboken, NJ: Wiley. 359.
Sheffer, K. M., Dunn, J. H., & Minner, D. D. (1987). Summer drought responses and rooting depth of three cool-season turfgrass. HortScience, 22, 296–297.
Barnes, R. F. (1990). Importance and problems of tall fescue. In M. J. Kasperbauer (Ed.), Biotechnology in tall Fescue improvement (pp. 1–12). Boca Raton, FL: CRC.
Khayyam-Nekouei, M. (2001). Germplasm collection and molecular detection of endophytic fungi in Iranian tall fescue (Festuca arundinacea Schreb). Ph.D. Thesis, University of Putra, Malaysia.
Williams, J. G. K., Kubelik, A. R., Livak, K. J., Rafalski, J. A., & Tingey, S. V. (1990). DNA polymorphisms amplified by arbitrary primers are useful as genetic markers. Nucleic Acids Research, 18, 6231–6235.
Zietkiewicz, E., Rafalski, A., & Labuda, D. (1994). Genome fingerprinting by simple sequence repeat (SSR)-anchored polymerase chain reaction amplification. Genomics, 20, 176–183.
Gupta, P. K., & Varshney, R. K. (2000). The development and use of microsatellite markers for genetic analysis and plant breeding with emphasis on bread wheat. Euphytica, 113, 163–185.
Wu, K., Jones, R., Dannaeberger, L., & Scolnik, P. A. (1994). Detection of microsatellite polymorphisms without cloning. Nucleic Acids Research, 22, 3257–3258.
Meyer, W., Michell, T. G., Freedman, E. Z., & Vilgalys, R. (1993). Hybridization probes for conventional DNA fingerprinting used as single primers in polymerase chain reaction to distinguish strain of Cryptococcus neoformans. Journal of Clinical Biology, 31, 2274–2280.
Kantelry, R. V., Zeng, X., Bennetzen, J. L., & Zehr, B. E. (1995). Assessment of genetic diversity in dent and popcorn (Zea mays L.) inbred lines using inter-simple sequence repeat (ISSR) amplification. Molecular Breeding, 1, 365–373.
Sankar, A. A., & Moore, G. A. (2001). Evaluation of inter-simple sequence repeat analysis for mapping in Citrus and extension of the genetic linkage map. Theoretical and Applied Genetics, 102, 206–214.
Motawei, M. I., Al-Doss, A. A., & Moustafa, K. A. (2007). Genetic diversity among selected wheat lines differing in heat tolerance using molecular markers. Journal of Food and Agricultural Environment, 5, 180–183.
Wang, L. L., Zhao, L., Gong, Y., Wang, M., Chen, L. M., Yang, J. L., et al. (2008). DNA fingerprinting and genetic diversity analysis of late-bolting radish cultivars with RAPD, ISSR and SRAP markers. Scientia Horticulturae, 116, 240–247.
Pivoriene, O., & Pasakinskiene, I. (2008). Inter-simple sequence repeat (ISSR) loci mapping in the genome of perennial ryegrass. Biologiae, 54, 17–21.
Al-Humaid, A., Motawei, M. I., Abdalla, M. Y., & Al-Mana, F. (2004). Detection of genetic variation and Fusarium resistance in turfgrass genotyoes using PCR-based markers (ISSR and SCAR). Journal of Food, Agriculture and Environment, 2, 225–229.
Motawei, M., & AL-Ghumaiz, N. S. (2012). Genetic diversity in some introduced pasture grass cultivars revealed by inter-simple sequence repeats (ISSR) markers. South African Journal of Biotechnology, 11, 3531–3536.
Xu, W. W., & Sleper, D. A. (1994). Phylogeny of tall fescue and related species using RFLPs. Theoretical and Applied Genetics, 88, 685–690.
Toker, C., Lluch, C., Tejera, N. A., Serraj, R., & Siddique, K. H. M. (2007). Abiotic stresses. Chickpea breeding and management, 474–496.
Rahimizadeh, M., Habibi, D., Madani, H., Mohammadi, G. N., Mehraban, A., & Sabet, A. M. (2007). The effect of micronutrients on antioxidant enzymes metabolism in sunflower (Helianthus annuus L.) under drought stress. HELIA, 30, 167–174.
Baby, J., & Jini, D. (2011). Development of salt stress- tolerant plants by gene manipulation of antioxidant enzymes. Asian Journal of Agricultural Research, 5, 17–27.
Barnabas, B., Jager, K., & Feher, A. (2008). The effect of drought and heat stress on reproductive processes in cereals. Plant, Cell and Environment, 31, 11–38.
Fleury, D., Jefferies, S., Kuchel, H., & Langridge, P. (2010). Genetic and genomic tools to improve drought tolerance in wheat. Journal of Experimental Botany, 61, 3211–3222.
Beauchamp, C., & Fridovich, I. (1971). Superoxide dismutases: Improved assays and an assay predictable to acrylamide gels. Annals of Biochemesitry, 44, 276–287.
Dhindsa, R. S., Plumb-Dhindsa, P., & Thorpe, T. A. (1981). Leaf senescence: Correlated with increased levels of membrane permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalase. Journal of Experimental Botany, 32, 93–101.
Chance, B., & Maehly, A. C. (1995). Assay of catalase and peroxidase. In S. P. Culowic & N. O. Kaplan (Eds.), Methods in enzymology (Vol. 2, pp. 764–765). New York: Academic.
Rohlf, F. J. (1998). NTSYSpc: Numerical taxonomy and multivariate analysis system. Version 2.02. New York: Exeter Publications.
Rohlf, F. J. (1998). NTSYSpc-Numerical taxonomy and multivariate analysis system (Version 2.0). User guide. New York: Applied Biostatistics Inc.
Ott, J. (1988–2001). Program Het version 1.8. Utility programs for analysis of genetic linkage. Rockefeller University, New York. ftp://linkage.rockefeller.edu/software/utilities/.
Oliviera, E. J., Padua, J. G., Zucchi, M. I., & Venkovsky, R. (2006). Origin, evolution and genome distribution of microsatellites. Biology, 29, 294–307.
Pollefeys, P., & Bousquet, J. (2003). Molecular genetic diversity of the French-American grapevine hybrids cultivated in North America. Genome, 46, 1037–1048.
Tessier, C., David, J., This, P., Boursiquot, J. M., & Charrier, A. (1999). Optimization of the choice of molecular markers for varietal identification in Vitis viniferia L. Theoretical and Applied Genetics, 98, 171–177.
Sivaprakash, K. R., Prasanth, S. R., Mohanty, B. P., & Parida, A. A. (2004). Genetic diversity of black gram landraces as evaluated by AFLP markers. Current Science, 86, 1411–1415.
Majidi, M. M., Mirlohi, A. F., & Sayed- Tabatabaei, B. E. (2006). AFLP analyses of genetic variation in Iranian tall fescue accessions. Pakistan Journal of Biological Science, 9, 1869–1876.
Casler, M. D., & Duncan, R. R. (2003). Turfgrass biology, genetics, and breeding (p. 367). Hoboken, NJ: Wiley.
Halasz, J., Pedryc, A., & Hegedus, A. (2007). Origin and dissemination of the pollen-part mutated SC-haplotype that confers self-compatibility in apricot (Prunus armeniaca). New Phytology, 176, 793–803.
Milatovic, D., & Nikolic, D. (2007). Analysis of self-(in) compatibility in apricot cultivars using fluorescence microscopy. Journal of Horticultural Science & Biotechnology, 82, 170–174.
Pickup, M., & Young, A. G. (2008). Population size, self-incompatibility and genetic rescue in diploid and tetraploid races of Rutidosis leptorrhynchoides (Asteraceae). Heredity, 100, 268–274.
Byers, D. L., & Meagher, T. R. (1992). Mate availability in small populations of plant species with homomorhpic sporophytic self-incompatibility. Heredity, 68, 353–359.
Young, A. G., Brown, A. H. D., Murray, B. G., Thrall, P. H., & Miller, C. H. (2000). Genetic erosion, restricted mating and reduced viability in fragmented populations of the endangered grassland herb Rutidosis leptorrhynchoides. In A. G. Young & G. M. Clarke (Eds.), Genetics, demography and viability of fragmented populations (pp. 335–359). Cambridge: Cambridge University Press.
Templeton, W. C, Jr, Mott, G. O., & Bula, R. J. (1961). Some effects of temperature and light on growth and flowering of tall fescue, Festua arundinaceae Scherb. II. Floral development. Crop Science, 1, 283–286.
Hicks, D. H., & Mitchell, K. J. (1968). Flowering in pasture grasses. 1. Interactions of day length and temperature on inflorescence emergence for Festuca arundinacea Schreb. Variety Manade. New Zealand Journal of Botany, 6, 86–93.
Goodacre, R., Shann, B., Gilbert, R. J., Timmins, E. M., & McGovern, A. C. (2000). Detection of the dipicolinic acid biomarker in Bacillus spores using Curie-point pyrolysis mass spectrometry and Fourier transform infrared spectroscopy. Annals of Chemistry, 72, 119–127.
Bai, L. P., Sui, F. G., Ge, T. D., Sun, Z. H., Lu, Y. Y., & Zhou, G. S. (2006). Effect of soil drought stress on leaf water statu.s, membrane permeability and enzymatic antioxidant system of maize. Pedosphere, 16, 326–332.
Ozkur, O., Ozdemir, F., Bor, M., & Turkan, I. (2009). Physiochemical and antioxidant responses of the perennial xerophyte Capparis ovata Desf. to drought. Environmental and Experimental Botany, 66, 487–495.
Nayyar, H., & Gupta, D. (2006). Differential sensitivity of C3 and C4 plants to water deficit stress: Association with oxidative stress and antioxidants. Environmental and Experimental Botany, 58, 106–113.
Wang, W. X., Vinocur, B., & Altman, A. (2003). Plant responses to drought, salinity and extreme temperatures: Towards genetic engineering for stress tolerance. Planta, 218, 1–14.
Shao, H. B., Liang, Z. S., & Shao, M. A. (2005). Changes of some anti-oxidative enzymes under soil water deficits among 10 wheat genotypes at maturation stage. Colloids and Surface Biointerfaces, 45, 7–13.
Jiang, M. Y., & Zhang, J. H. (2004). Abscisic acid and antioxidant defense in plant cells. Acta Botanica Sinica, 46, 1–9.
Saba, J., Moghaddam, M., & Ghassemi, K. (2001). Genetic properties of drought resistance indices. Journal of Agricultural Science and Technology, 3, 43–49.
Munns, R. (2002). Comparative physiology of salt and water stress. Plant, Cell and Environment, 25, 239–252.
Capell, T., Bassie, L., & Christou, P. (2004). Modulation of the polyamine biosynthetic pathway in transgenic rice confers tolerance to drought stress. Proceedings of the National Academy of Sciences of the United States of America, 101, 9909–9914.
Gogorcena, Y., Iturbe-Ormaetxe, I., Escuredo, P. R., & Becana, M. (1995). Antioxidant defenses against activated oxygen in pea nodules subjected to water stress. Journal of Plant Physiology, 108, 753–758.
Bergmann, H., Lippmann, B., Leinhos, V., Tiroke, S., & Machelett, B. (1999). Activation of stress resistance in plants and consequences for product quality. Journal of Applied Botany, 73, 153–161.
Handa, S., Bressan, R. A., Handa, A. K., Carpita, N. C., & Hasegawa, P. M. (1983). Solutes contribution to osmotic adjustment in cultured plant cells adapt to water stress. Plant Physiology, 73, 834–843.
Hsiao, T. C. (1973). Plant responses to water stress. Annual Review of Plant Physiology, 24, 519–570.
Asada, K. (1999). The water–water cycle in chloroplasts: Scavenging of active oxygen and dissipation of excess photons. Annual Review of Plant Physiology and Plant Molecular Biology, 50, 601–639.
Van Breusegem, F., Vranovà, E., Dat, J. F., & Inzé, D. (2001). The role of active oxygen species in plant signal transduction. Plant Science, 161, 405–414.
Foyer, C. H., Descourvieres, P., & Kunert, K. J. (1994). Protection against oxygen radicals: An important defense mechanism studied in transgenic plants. Plant, Cell and Environment, 17, 507–523.
Asada, K. (1997). The role of ascorbate peroxidase and monodehydroascorbate reductase in H2O2 scavenging in plants. In J. G. Scandalios (Ed.), Oxidative stress and the molecular biology of antioxidant defenses (pp. 715–735). Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Salehi, M., Salehi, H., Niazi, A. et al. Convergence of Goals: Phylogenetical, Morphological, and Physiological Characterization of Tolerance to Drought Stress in Tall Fescue (Festuca arundinacea Schreb.). Mol Biotechnol 56, 248–257 (2014). https://doi.org/10.1007/s12033-013-9703-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12033-013-9703-3