Abstract
Metastasis is the most complex and deadly event. Tumor-stromal interface is a place where invasion of tumor cells in the form of single-cell or collective migration occurs, with the latter being less common but more efficient. Initiation of metastasis relies on the tumor cell cross-talking with stromal cells and taking an epithelial-mesenchymal transition (EMT) in single cells, and a hybrid EMT in collective migratory cells. Stromal cross-talking along with an abnormal leaky vasculature facilitate intravasation of tumor cells, here the cells are called circulating tumor cells (CTCs). Tumor cells isolated from the primary tumor exploit several mechanisms to maintain their survival including rewiring metabolic demands to use sources available within the new environments, avoiding anoikis cell death when cells are detached from extracellular matrix (ECM), adopting flow mechanic by acquiring platelet shielding and immunosuppression by negating the activity of suppressor immune cells, such as natural killer (NK) cells. CTCs will adhere to the interstituim of the secondary organ/s, within which the newly arrived disseminative tumor cells (DTCs) undergo either dormancy or proliferation. Metastatic outgrowth is under the influence of several factors, such as the activity of macrophages, impaired autophagy and secondary site inflammatory events. Metastasis can be targeted by multiple ways, such as repressing the promoters of pre-metastatic niche (PMN) formation, suppressing environmental contributors, such as hypoxia, oxidative and metabolic stressors, and targeting signaling and cell types that take major contribution to the whole process. These strategies can be used in adjuvant with other therapeutics, such as immunotherapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Metastasis is the deadliest event in tumorigenesis, and distant metastasis is regarded as the end result in tumorigenesis [1]. In prostate cancer, for instance, virtually all deaths occur as a result of metastasis [2]. Previously, metastasis was viewed as the stage of an advanced tumor, namely happening mostly at the time of tumor progression [3]; however, recent investigations approved the occurrence of metastasis often early in tumorigenesis. This infers that metastasis can occur both at early and late tumorigenesis, but the two represent distinct pathogenesis in which cells that elicit early metastasis just carry truncal mutations, whereas late-arising metastatic cells show subclonal mutations [1].
Metastasis is an organ-selective and multi-stepping process that is started by escape of tumor cells from the primary tumor and ended with colonizing secondary tumors in the distant sites [4]. The multi-stepping process of metastasis accounts for the complexity of the whole event, which imposes a huge burden on effective tumor therapy. This is also a reason for failure of developing drugs (so called migrastatics) to combat tumor metastasis with high efficiency. A number of promoters of metastasis has been identified so far (see Table 1). Developing drugs against the whole process of metastasis is not applicable. This is a reason for an ongoing research in the area. Every year more insights into the complexity of this phenomenon will come to the understanding. Knowing more about the whole process and the major drivers of this key phenomenon is critical for identification of the main targets in tumor metastasis. This may let us to think of factors and cells that take major responsibility in tumor metastasis. Novel approaches for suppressing tumor metastasis or reducing the extent of this process are the current concern in the area. In this review, we are focusing particularly over the key steps in metastasis of solid tumors by interpreting papers published recently in the relevant subject. The steps in metastasis are as followings: escape of cancer cells from primary tumor, intravasation, survival maintenance, extravasation (secondary site seeding) and outgrowth (colonization) [5,6,7,8,9]. The whole process is illustrated in Fig. 1.
Sequential steps in metastasis. a Cellular escaping. Acquiring an epithelial-mesenchymal transition (EMT) enhances invasive behavior of cancer cells and ECM degradation required for cell escape. Transforming growth factor (TGF)-β released from cancer-associated fibroblasts (CAFs) fosters EMT mediated invasion. Physical forces exerted from CAFs over tumor cells change physical properties of basement membrane (BM) in the interface of tumor and stroma to make it permissive for invasion of tumor cells. Macrophage type 2 (M2) cells and CAFs release ECM degrading factors. M2 cells release pro-angiogenic factors to stimulate angiogenesis and lymphangiogenesis, which are for providing a path for metastasis. M2 cells also support metastatic niche formation. b Vascular entrance. Exosomes possibly derived from CAFs increase vascular permeability. Circulating tumor cells (CTCs) with EMT phenotype form invadopodia to pass through ECM (via releasing ECM degrading proteases), dissociate from the Edge front, and to intrude tumor vasculature. Neutrophils facilitate bondage between cancer cells and endothelium, and hypoxia inducible C-X-C chemokine ligand 12 (CXCL12)/C-X-C chemokine receptor type 4 (CXCR4) is a key promoter of cancer cell intravasation. c Survival maintenance. CTCs have a potential to avoid anoikis cell death within circulation. EMT is a possible contributor. Flow mechanics also influences cancer cell survival. Rewiring metabolic pathways and reprograming gene expression profile are contributed to cancer cell survival within circulation. Signals of survival are directed by exosomes. Cell-to-cell interactions between cancer cells with CAFs, neutrophils and platelets favors immune escaping. d Extravasation (secondary site seeding. Blood platelets through release of TGF-β enable extravasation by disrupting cell-to-cell endothelial junctions. Extravasation is also potentiated by factors released from TME in distant organs into the circulation, such as vascular endothelial growth factor (VEGF). Disseminative tumor cells (DTCs) with EMT phenotype undergo intravascular arrest and evolve cellular protrusions to help trans-endothelial migration (TEM) of cancer cells and their entrance into the ECM of the metastatic site. Exosomes increase vascular permeability (leakiness) and cellular seeding. Physical forces within the circulation would determine the success of distant metastatic seeding. Here, cancer cells may remain dormant for a period prior to colonization, which gives cells the ability to escape immunosurveillance, and to colonize successfully in the distant organ/s. e Tumor outgrowth (colonization). DTCs acquire a MET phenotype for proliferation into forming secondary tumors. Exosomes derived from cancer cells restructure the metastatic sites through supporting dynamic interplay with the TME in order for promoting tumor colonization (outgrowth). One of the outcomes of these interactions is reprogramming of glucose metabolism in cancer cells
Invasion
Invasion takes an early step and a pre-requisite to metastatic dissemination [10] so that cancer cell escape is a known characteristic of most advanced tumors [11]. Cancer cells are required to take a plastic phenotype to attain an invasive state [12]. Harnessing this inherent plasticity through evoking a systemic inflammatory response, which is occurring in certain primary tumors like breast, can impede metastatic establishment [13]. Higher frequency of driver mutations (alterations in the somatic copy numbers and mutational burden) [2, 14], and the presence of abnormal chromosomes (called aneuploidy) [14] can also lead cancer cells to drive a metastatic potential.
Single-cell migration vs. collective invasion
Invasion occurs at the tumor-stromal interface (also called Edge or invasive front) of tumor by either single-cell migration (monoclonal metastasis) or collective invasion (polyclonal metastasis). For the former, tumoral cells to attain an invasive phenotype will modify their shape and attachment profiles. For the latter, invasion occurs as a cohesive multi-cellular strands still maintaining cell-to-cell adhesions. Colon, breast, thyroid, prostate, lung and glioblastoma cancer cells take features of collective invasion [10, 15]. Collective invasion is less common but more efficient for taking an efficient metastatic route, as compared to the single-cell migration [16]. E-cadherin is a marker of epithelialization (cell-to-cell adhesion) that is generally downregulated in single-cell invasion. Padmanaban and colleagues in an invasive ductal breast cancer has come to the finding that E-cadherin expression was negatively related to invasion, while its relation with metastasis was positive. The negative relation can be interpreted by collective invasion in which E-cadherin mediated cell-to-cell adhesion is reported to be critical for reinforcing cancer cell survival during the early step of metastasis [17]. Thus, in the collective invasion two or more tumor cells retain some properties of epithelial cells, such as cell-to-cell adhesion; this enables them to take a collective migration and enter blood circulation as multi-cellular circulating tumor cell (CTC) clusters. Detection of these cellular clusters within the circulation indicates worse prognosis, compared to the detection of single CTCs by solo [15]. Generally, ˂ 5 tumor cells are dissociated from the invasive (Edge) area of tumor as clusters. Tumor cells dissociated as clusters higher than this amount (5 or higher) without aggregating as glandular structures are called poorly differentiated clusters (PDCs). In colorectal cancer (CRC), PDCs are linked positively with high local and distant metastasis, tumor grade and patient mortality [18]. Components of Wnt/PCP signaling are dysregulated abundantly in solid tumors, and mediate collective cell migration, acting possibly for generation of CTC clusters and their maintenance inside the circulatory system. Targeting Wnt/PCP signaling is thus being a promising for metastatic intervention [19].
Epithelial-mesenchymal transition in tumor cell invasion
Epithelial-mesenchymal transition (EMT) is known as the key mechanism in promotion of cancer metastasis. Generally, a cell type with mesenchymal phenotype is more prone to acquire drug resistance and invasion [20]. The point is that both single cells and collective migratory cells show EMT and stem cell-like characteristics with some differences. Single tumor cells will lose cell-to-cell adhesion and undergo EMT [10]. EMT seemingly drives metastasis initiation [21], and induction of this phenotypic state in tumors cells requires cross-talking with stromal cells, especially with cancer-associated fibroblasts (CAFs) [22], known as the most abundant cell types within the microenvironment of tumors that their presence within the stroma indicates poor prognosis [23].
Tumor cell clusters in collective migration take a partial (hybrid) EMT [24]. In this type, some cells are served as leaders (in the front-line of migration) and others are followers [10]. The EMT phenotype is more pronounced in leader cells compared to those following them [25]. In an animal model of lung cancer, it has shown that the activity of transforming growth factor (TGF)-β-Smad signaling is important for metastasis. TGF-β acts through diminishing E-cadherin expression [26]. The impedance of EMT by A-Kinase Anchor Protein (AKAP8) is reported to suppress metastasis of breast cancer [20].
There is evidence that tumor cells expressing a mixture of mesenchymal and epithelial phenotypes have more potency to complete metastatic steps [27]. The EMT phenotype in some collective migratory cells infers the heterogeneity of the cellular clusters, and that the pack of cells with such phenotype may suggest the presence of cancer stem cells (CSCs) and/or cancer cells acquiring the EMT phenotype. Compared to the non-EMT cancer cells, cells with EMT phenotype have more developed anti-apoptotic systems and show more resistance to therapy [28, 29].
Mechanical forces in collective migration
Physical forces exerted by CAFs are required for collective migration of tumor cells [30]. Contractile forces exerted by CAFs (independent on matrix metalloproteinases [MMPs]) can pull and stretch the basement membrane segregating cancer cells from the nearby stroma, which finally alter physical properties of this barrier to become permissive for cellular invasion [31].
Intravasation
Cancer cells intruded toward the blood vessels are called CTCs, while their extrusion from the blood and rooted toward the secondary cite/s of metastasis is called disseminative tumor cells (DTCs). Intravasation is a complex process involving numerous intrinsic and extrinsic factors. Tumoral cell intrinsic factors include EMT and production of proteases. Extrinsic factors include the activity of pro-tumor neutrophils (N2 type), fibroblasts (CAFs) and macrophages (M2 type) [32]. Intravasation of cancer cells is facilitated possibly by the abnormal leaky vasculature [33]. Endothelial cells (ECs) secrete C-X-C chemokine ligand 12 (CXCL12), which attracts C-X-C chemokine receptor type 4 (CXCR4)-expressing cancer cells toward the CXCL12 gradient, thus facilitating their further intravasation. The point is that both ligand and receptor in the CXCL12/CXCR4 axis are hypoxia inducible, which indicates a key role taken by hypoxia in intravasation [34] of tumor cell clusters [35]. Perivascular tumor-associated macrophages (TAMs) promote cancer cell intravasation through upregulating EGF [36] and MMP-9 [37] expressions. The stiffened extracellular matrix (ECM) induces invadopodia formation in cancer cells [38]; cancer cells then required to path through ECM in order for intruding the blood vessels. Invadopodia are actin-rich special protrusions from cancer cell membrane effective for taking a pass through ECM by releasing proteases, such as MMPs at the tip of these unique structures. Here, TAMs and CAFs are acting together to cleave the ECM through releasing MMPs (2 and 9) [39,40,41,42]. The activity of CAFs in reorganizing the ECM and drilling the holes is important in paving the paths smoother for invading tumor cells [43, 44]. ECM degradation is required not only for creating tumoral cell tracks (paths) [9, 45] but also for liberation of growth factors implicated in promotion of angiogenesis [46] and lymphangiogenesis [47], as well as promoting extravasation of cancer cells [45]. These are indicative that how both ECM stiffness and degradation are functional for promoting tumor metastasis.
Survival maintenance in tumor cells isolated from the primary tumor
CTCs have a half-life of about 2.4 h in human subjects. CTCs although are under exposure to the nutrient rich bloodstream, the cells still exhibit the same invasive phenotype undertaken upon sequestration from the nutrient-low, invasive (Edge) area of tumor. This is possibly due to the short time lived within the circulation, which is not sufficient to make a change in the phenotype of CTCs in spite of accessing to the high amounts of nutrients within the blood [48]. This infers the importance of timing in determining cellular plasticity. The cells rooted toward circulation or when firstly reside within the secondary site/s of metastasis are under continuous exposure to the destructing signals. Within the circulation, CTCs confront several environmental stressors including oxidative stress, shear forces and an assault imposed by immune system [15], so many of the CTCs are condemned to die within circulation. Only a few number of cells remain alive and take the next step, namely secondary site seeding, among these secondary site seeding cells there are also a considerable rate of cell death, so the fraction of cells achieving the final metastatic fate is too low. However, these low fractions of DTCs are highly competent to take an effective metastatic fate. Effective metastasis relies heavily on developing mechanisms of survival, highly efficient to keep the cells ‘hale and hearty’ from the harm conditions encountered by. There are four potential mechanisms to maintain survival of cancer cells within circulation (i.e. CTCs) and in the metastatic site/s (i.e. DTCs) as followings:
Rewiring metabolic demands
Metabolic rewiring leads cancer cell survival within circulation and their adaptation to the foreign metastatic milieu [49]. The differences in the metabolic predilection between cancer cells from primary tumor with micrometastatic cells in the secondary organ are important therapeutically. Reduced capacity for glucose uptake and further blockade of glycolysis in CTCs can potentially cause anoikis cell death [16, 50, 51]. CTCs either exploit mechanisms to retrieve their glucose uptake systems [50] or to use other sources of energy available within circulation, such as fatty acids [52].
Higher OXPHOS/glycolysis in micrometastatic breast cancer cells deposited into the lung, and the reversed ratio for primary breast cancer cells reported by Davis et al. highlight the potential of targeting OXPHOS to preclude metastasis of breast cancer [53]. How about primary tumors? Are they more responsive to glycolysis targeting? The response to this question is somewhat challenging, which may be covered by another question. Is there a strategy to enhance glucose content in the TME (for potentiating immune activation), while simultaneously reducing glycolysis (for avoiding cancer cell growth and resistance)? Although TME factors involved in the promotion of cancer cell glycolysis are known to an extent, such as hyaluronan [54], we leave this question open for forthcoming research and see how it can be translated into clinic.
Avoiding anoikis cell death
Anoikis resistance is known as a cornerstone step for a cell attaining a metastatic feature [10]. The capacity to avoid anoikis cell death when cells are detached from ECM is a distinctive characteristic of most epithelial tumors, isolating them from normal epithelial cells [55], and enabling cancer cells to maintain their survival within circulation [10]. The activity of TGF-β signaling is contributed to the anoikis resistance [56], possibly by maintaining an EMT state in CTCs [50]. TGF-β signaling increases the activity of extracellular-signal-regulated kinase (ERK), the activity of which promotes Slug upregulation and resulting inhibition of E-cadherin [57].
CTCs highly express hypoxia inducible factor (HIF)-1α activated in a mechanism independent on hypoxia (CTCs have access to the O2 within the blood). HIF-1α protects CTCs from anoikis cell death possibly through promotion of metabolic reprogramming (increasing cellular uptake of glucose) [51]. Due to the key roles taken by HIF-1 [58] and TGF-β [29] in maintaining EMT, it is fair to postulate that HIF-1α are activated in CTCs possibly under the influence of TGF-β. It has found that patients with HIF-1α+ tumors have lower overall survival (OS) and 5-year survival rates [59]. Topotecan is an inhibitor of HIF-1 that its application in tumors like cervical [60] and endometrial [61] cancers, and the results were promising to an extent.
It is presumable that anoikis resistance might has negative relation with Hippo pathway. Wu and colleagues have found an increase in the rate of metastasis in breast cancer tissues upregulating Zinc finger protein 367 (ZNF367). ZNF367 is a transcriptional factor that its upregulation can promote metastasis via suppression of Hippo pathway. Anoikis resistance can be an outcome of the inhibition in the Hippo pathway [62]. The question here is that whether Hippo reactivation can be used as an approach for reducing the number of CTCs and the subsequent reduction of the chance of tumor metastasis? The answer to this question is yes. From what discussed above, and adhering the results of clinical trials in the relevant context activation of the Hippo pathway can be a good prognostic value and an effective approach for retarding tumor invasion. Generally, malignant mesothelial cells inactivate the Hippo pathway, the result of which is the YAP activation [63]. Maille and colleagues carried out a study in patients with malignant pleural mesothelioma, and they found a link between MST1/hippo kinase inactivation with the poor prognosis. Loss of expression for the MST1/hippo pathway resulted in the nuclear accumulation of YAP, silencing of which reduced invasion in MST1-depleted cells [64]. Hippo reactivation by statin has found to be effective for reducing the proliferative activity of hepatocellular carcinoma cells, thus improving the prognosis in the treated patients [65].
Adapting flow mechanics
Flow mechanic and co-option between lymphatic and blood circulatory systems influences the effective transit of cancer cells from a primary tumor, their survival within circulation, as well as extravasation and seeding in the metastatic site/s. Vascular size, flow rates and shear stress can potentially influence survival of cancer cells within circulation and their organotropic seeding. High flow velocity and shear forces (as in arterial vessels) can cause mechanical stress and cancer cell death, while moderate velocity and shear stress (as in venous vessels) favor intravascular arrest of CTCs and their extravasation [66]. CTCs must endure the pressures imposed by blood vessels to maintain their survival [27]. Platelets within the circulation form aggregates around the CTCs in response to stimulatory signals received from cancer cells; these aggregates provide a shield to protect the CTCs from shear stress and immune responses [16, 67, 68].
Immune suppression
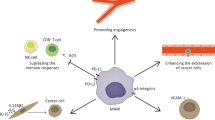
To avoid destruction by immune system upon taking a circulatory route, CTCs promote interactions with CAFs, neutrophils and platelets. It seems that the physical impact of CTC clusters can augment the recruitment of these immunosuppressive cells, protecting them from being attacked by anti-tumor immune cells, such as NK cells [15]. A recent study by Owen and colleagues has shown that prostatic cancer cells seeded within the bone will lose their intrinsic interferon 1 (IFN1) signaling; this allows them to repress tumor immunogenicity and to dampen tumor responses to immunotherapy, which indicates a reason for failure of therapy in metastatic solid tumors [69]. The activity of natural killer (NK) is important for suppression of tumor metastasis. Higher expression of epithelial genes and cell-to-cell adhesion markers in CTC clusters is related negatively with the expression of ligands responsible for activation of NK cells. This along with an increase in the rate of monocolonal versus polyclonal metastasis upon depletion of NK cells infers the lower sensitivity of CTC clusters than single-cell tumor cells to NK cell-mediated metastasis blockade [15].
Prognostic value of CTCs
Liquid biopsies can be obtained from cancer patients to track CTCs. Counting the total CTCs and the fraction of mesenchymal-type (M+) CTCs can be exploited for monitoring therapeutic resistance and for predicting the prognosis in patients with advanced cancers, as for breast cancer [70]. In renal cell carcinoma (RCC) it was found that the initial CTC count (one day prior to the operation) was not correlated with the cancer relapse or metastasis, attested by no considerable difference between metastasis-free patients with metastasis cases. However, counting the number of epithelial (E+) M+ (mixed) CTCs at 12 months after surgery showed a significant increase, as compared to that performed at 6 months’ post-operation or one day pre-operation [71]. Cai and colleagues in a study analyzed the peripheral blood from 91 CRC cancer patients, and they noticed a noticeable relevance between the number of M+ CTCs with the distance metastasis. The authors collected M+ CTCs from 73 patients for assessing the expression of cyclooxygenase (COX)-2. 38 patients showed COX-2 expression in the CTCs, and its expression in M+ CTCs showed higher rates in the metastatic patients, compared to that for non-metastatic cases [72]. Overexpression of COX-2 is linked positively with the invasive behavior of CRC cells [73]. These data indicate that evaluation of CTCs can be used as diagnostic marker and a marker for predicting the efficacy of therapy.
Extravasation
Release of cytokines into the systemic circulation facilitates extravasation of cancer cells within the distant organs. The activity of platelets is important for extravasation of tumor cells. Platelet shielding of cancer cells can cause an imbalance in the homeostatic control over coagulation, thus promoting blood clotting varied from micro-thrombi to pulmonary emboli [8]. The cells promote coagulation and weaken endothelial barrier [68] possibly through releasing TGF-β [67]. Coagulation abnormalities is experienced in more than 50% of all cancer cases and in 90% of patients with metastatic tumors [74]. In non-small cell lung cancer (NSCLC) patients with cancer emboli and the subsequent cerebral infarction the risk of brain metastasis will be increased considerably, as documented in a recent study by Kim and coworkers [75].
TME in distant organs also send signals (such as vascular endothelial growth factor (VEGF) to promote cancer cell extravasation [33]. N2 cells [76] and M2 cells [77] are active in such process. When cells leaving the primary tumor and metastasize to an organ including lung, it is important to be survived for an extended period of time. This means that the metastatic cells to survive for longer times are needed to activate pro-survival signals in the organ of target. Interactions between fibronectin fibrils (promoted by metastatic breast cancer cells) with integrin (in pneumocyte type 1 cells) is an example in this context [78].
Extravasation relies on interactions between circulating tumor cells with endothelium and the secondary site
A key event in metastasis is the interaction between CTCs with endothelium (adhesion between CTCs and ECs is a required step for the subsequent extravasation), and then with the secondary site (the requirement of which is the shedding of endothelial glycocalyx) [79, 80]. Cell adhesion molecules (CAMs) are expressed on both CTCs and ECs [79], and these molecules mediate endothelial interactions, either directly or indirectly, within the specific tissues [81], inferring their role in organ tropism. Barbazán and colleagues in mouse colon cancer model liver metastasis reported the presence of fibronectin deposits in the luminal liver vasculature as a site for attachment of talin1+ CTCs, and the depletion of this focal adhesive component on CTCs has found to impair endothelial adhesion and cellular migration through the endothelium, and the further reduction of liver metastasis [82]. CTCs exploit low-energy adhesion (integrin β3 and CD44 involvement) and stronger adhesions (integrin β1 involvement) for the respective initiation of transient vascular arrest, and the stable bonds to the endothelium and extravasation [83, 84]. The point is that a combination of biological (EC interactions) and physical (flow dynamics) factors are involved in the vascular colonization of CTCs, namely CTC intravascular arrest of CTCs [83, 85]. Blood flow may cause shear stress in arrest CTCs arrested within the vessels; to oppose the stable bond between CTCs and endothelium is evolved [84].
Besides the adhesion between CTCs with endothelium, CTC-ECM interaction is also important for the final cellular seeding within the secondary site/s. Adhesion between ECM with tumor cells is mediated through integrin family [86]. Tumor cells form protrusions called invadopodia at endothelial junctions to extend between ECs [87]. Williams and colleagues in breast cancer brain metastasis mouse model have found the chemosensing activity of these cellular protrusion. In this model, they notified the presence of chemokine receptors EGFR and GABA receptor in the invadopodia protrusions, which are responding to their respective ligand available within the brain environment. This infers a positive relation between invadopodia protrusions with organotropism (here is brain) of metastatic tumor cells. PAK1 mediates this chemotaxis potential by controlling responses from invadopodia to the ligands in the brain environment, as its suppression renders tumor cells irresponsive to the stromal chemotactic stimuli. Therefore, the ‘soil fertility’ (brain in this study) is important for extravasation of tumor cells toward the microenvironment of distant metastatic site [88]. Structurally, invadopodia are in fact protrusions of actin filaments, which also include assembly of the intermediate filaments and the cytoskeletal linking proteins. Yoneyamaa and colleagues in a study have found that the assembly of the intermediate filament vimentin and cytoskeletal linker plectin was necessary for generation and stabilization of invadopodia of highly metastatic bladder cancer cells, and that the disruption of the link between vimentin, plectin and actin filaments has found to reduce the capacity of the cells for migrating toward the endothelium and their further intrusion into the site of metastasis [89]. From these results, it could be understood that targeting invadopodia by either addressing their interactions with the stroma of the secondary site or disruption of these protrusions structurally can be effective approaches to repress extravasation and secondary site seeding of tumoral cells.
Outgrowth (colonization)
From the whole cancer cells routed toward circulation a vast majority are condemned to die, and among them only a subset (0.01%) stays alive and form secondary tumors [22, 90]; the survived cells take either one of the two routes: dormancy or proliferation. Generally, DTCs upon entering the secondary site/s undergo dormancy for a while. The proliferative cancer cells are able to grow macrometastatic tumors [14, 91, 92]. Acquisition of either route is determined by multiple intracellular and extracellular signals, as well as by factors rendered from the bone marrow niche [27]. Actin assembly has found to play a critical role for promotion of a dormancy-to-proliferation switch in DTCs. Gau and colleagues in a study attested the positive role for the actin cytoskeleton regulator myocardin-related transcription factor (MRTF) in survival and outgrowth of DTCs from breast cancer. MRTF loss of function has found to reduce the activity of Profilin-1 (Pfn1). Pfn1 activity is essential for regulation of the actin dynamics [93]. 6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 3 (Pfkfb3) is an essential mediator of glycolysis that its elevated expression has found to be positively related to the metastatic outgrowth of breast cancer. This is mediated through the positive impact of Pfkfb3 on emergence of CSCs from the dormant metastatic state toward reactivating programs related to the cellular proliferation and outgrowth [94].
Evaluation of p38/ERK ratio can determine whether the cells take the either phenotypes, with the higher p38-to-ERK ratio causing cellular reawaking, while the higher ERK-to-p38 ratio causing cellular dormancy [27]. It has found that activation of p38 can suppress metastasis of breast cancer. Guereño and colleagues exploited the efficacy of Glypican-3 (GPC3) on this process, and they found a switch from mesenchymal-to-epithelial in breast cancer cells mediated by activation of p38, which further impairs metastasis and induce dormancy in tumor cells [92]. This infers how switching from one phenotype to another will define the metastatic potency of a cell type.
The point here is that DTCs chose specific target organs where they can promote metastatic colonization. The term ‘organ tropism’ is used in this context. Rodrigues and colleagues in a study found that tumor-derived exosomes containing cell migration-inducing and hyaluronan-binding protein (CEMIP) are more prone to take a metastatic predilection toward the brain than the other organs including bone and lung. They noticed that tumoral cell depletion of CEMIP impaired metastasis into the brain [95]. This infers how the microenvironment in the secondary organ is preferentially influenced by signals from primary tumor organ to direct organ tropism.
Promoters and suppressors of metastatic outgrowth
The activity of macrophages
A number of factors are related to the colonization or outgrowth of DTCs (see Table 2). Macrophages play important roles for metastatic colonization within the bone [96]. Macrophages have two phenotypes: anti-tumor M1 (so called classically activated cells) and pro-tumor M2 cells (so called alternatively activated cells) [97]. M1 macrophages are implicated for improving the recruitment of T cells, supporting normalization of tumor vessels, and suppressing the activity of M2 cells, whereas M2 cells are responsible for promotion of cancer cell proliferation, angiogenesis, immune escape, invasion and metastasis [98]. A study by Ma and colleagues documented that macrophages expressing CC chemokine receptor 2 (CCR2) and IL4R are more prone to promote metastatic colonization of breast cancer cells within the bone. They noticed high amount of macrophages in the bone of breast cancer in both human and mice, and that ablation of CCR2 or IL4R suppressed metastatic outgrowth within the bone [96]. CCR2 is a chemokine that its interaction with CCL2 (the CCL2/CCR2 axis) acts for recruitment of immunosuppressive M2 cells into the TME, and its blockade can be an approach for restoring anti-tumor immunity [99]. The two clinical trials are performed in this context: in the first study, the immunosuppressive (M2) cells were targeted by CSF-1 inhibition (by emactuzumab) in advanced solid tumors. Roca and colleagues in this study found the attenuation of the immunosuppressive TAMs, but its administration either alone or in combination with paclitaxel did not show anti-tumor activity from the clinical standpoint [100]. In another study, the CCR2 inhibitor PF-04136309 was used in combination with FOLFIRINOX for targeting pancreatic ductal adenocarcinoma (PDAC), and the combination therapy considerably improved the local tumor control (in 32/33 patients) [99].
A suggested strategy is to polarize macrophages from a suppressive into immune activating M1 phenotype. M1 polarization can normalize tumor vasculature, through which more infiltration of CD8+ T cells into the tumor area is possible; the higher presence of CD8+ tumor infiltrating lymphocytes (TILs) will be effective for hampering immune escape capacity of tumor cells, thus reducing the chance of tumor metastasis. This is applicable by targeting factors responsible for polarization of macrophages into M2 cell phenotype, such as VEGF and ILs 4, 10 & 13 [98].
Autophagy
The ideas behind the roles for contribution of autophagy in metastatic systems are somewhat controversial, which can be interpreted differently from what reported in experimental studies with that published for clinical trials. Outcomes of the two experimental studies in the relevant concept are favoring the use of enforced autophagic systems as a potential approach for preventing metastatic outgrowth: Marsh and colleagues reported that the autophagy system acts differently in primary and advanced tumors in which in an autophagic competent tumor, induction of autophagy promotes the growth of primary tumor. A deficient autophagy system has found to cause accumulation of Neighbor to BRCA1 (NBR1), an autophagic cargo receptor, accumulation of which mediates the impact of autophagy suppression on metastasis, as authors noticed that the degradation of NBR1 will restrict the outgrowth of breast tumor cells, and suggest targeting this receptor as a potential strategy to combat outgrowth of tumor cells in the metastatic sites. The authors noticed that the enforced autophagy took a preventive role on colonization of DTCs. This presumably infers the role for autophagy for promoting a dormant state in the DTCs. The authors also evaluated the outcomes on the OS in human breast cancers, and they noticed the positive relation between decreased autophagy with the reduced survival in these patients [101]. In line, Flynn and colleagues in an experimental model of dormant breast cancer have found a diverse relation between autophagy with emergence of breast CSCs from dormant metastatic state. The authors declared that the autophagic machinery can be activated in order for extending the OS by maintaining disseminative CSCs in perpetual dormancy, inhibition of which drives the escape of tumoral cells from metastatic dormancy thus rendering them competent to promote metastatic colonization [94].
Hydroxychloroquine (HCQ) is an inhibitor of autophagy used in clinic. Jyoti and colleagues exploited the role for HCQ when is used in combination with chemotherapy (paclitaxel, carboplatin with or without bevacizumab) in metastatic NSCLC. The authors noticed that the combination therapy improved objective response rate (ORR) and progression-free survival (PFS), KRAS+ tumors in particular, and they declared that HCQ addition to the standard chemotherapy regimen may be an effective approach for overcoming tumor resistance to chemotherapy [102]. Improvement in ORR by addition of HCQ to the chemotherapy regimen (gemcitabine plus nab-paclitaxel) has also been approved in advanced pancreatic cancer by Karasic and coworkers. However, the authors found no improvement in the OS upon addition of this autophagy inhibitor to the chemotherapy [103]. The results of the two clinical trials are mostly objective, so it is impossible to compare the results with that found in the experimental studies, discussed above. This indicates the requirement for more studies both on human cancers and animal models to extract more knowledge about the impact of autophagy machinery on tumors at both lower and higher (or metastatic) stages.
Inflammation
Inflammation plays a key role in tumorigenesis, representing a tight link with the incidence of over half of human cancers [104]. Inflammation plays a key role in all aspects of tumorigenesis (development and progression of tumor) [105] including its inducible effect on metastatic colonization. The study by Rodrigues and colleagues showed that CEMIP inducible effect on microglial cells and the resulting inflammation is associated with the brain colonization of metastatic breast cancer cells [95]. The link between inflammation with metastatic colonization is also depicted in the study by Du and colleagues. They noticed the positive link between activation of nuclear factor kappa B (NF-κB) in local fibroblasts with the intra-pulmonary colonization of lung cancer cells [106]. NF-κB is a master regulator of inflammation that shows constitutive activity in several advanced-stage cancers [107, 108]. Activation of NF-κB leads to the generation of pro-inflammatory cytokines [109]. Granulocyte colony-stimulating factor (G-CSF) is a pro-inflammatory cytokine that its upregulation in brain tissue has found to be contributed to the recruitment of immunosuppressive neutrophils, which act for driving metastatic colonization [110]. The study performed by So and colleagues showed that colonization of breast cancer cells at distant sites of metastasis requires epigenetic reprogramming. They noticed a positive relation between the activity of inflammatory mediators IL-6 and prostaglandin E2 (PGE2) with altering pathways required for proliferation, survival and colonization of tumor cells at distant sites, mediated through induction of DNA methyltransferase 3B (DNMT3B) [111]. Pein and colleagues documented that breast cancer lung colonization requires orchestration of an inflammatory phenotype in lung CAFs in a mechanism mediated by NF-κB [112].
Surgical resection of primary tumor may sometimes deteriorate the condition of patients by predisposing them to overt metastasis. Evidence of which is in the study by Miarka and coworkers who found a positive relation between surgical resection of primary pancreatic ductal adenocarcinoma (PDAC) with the outgrowth of micrometastatic lesions seeded within the liver. They notified that abdominal surgery can induce inflammation within the liver, which is a trigger for hepatic stellate cell (HSC) activation into myofibroblasts, enabling the metastatic cells to escape growth arrest [113].
How to combat cancer metastasis?
The complexity of tumor metastasis is a major concern. Despite huge efforts, there is no drug or agent approved specifically to suppress tumor metastasis until now. Some inducers of metastasis in solid tumors is presented in Table 1, and the clinical trials carried out to target mediators of metastasis is presented in Table 3. Over viewing of the whole process is required to seek for more appropriate modality to target this devastating condition. The point here is that tumor cells taking an invasive phase reside more within the Edge of tumor, and the cells are more resistant to therapy than the ones not taking the invasive step. The current strategies to combat cancer metastasis are focusing on combating tumor relevant signaling pathways, as well as addressing tumor promoting cell types, in brief targeting signaling and cell types that take major contribution to the whole process. CAFs and platelets are examples of cells important for metastasis, and among the pathways TGF-β, MMPs and signaling related to the EMT are the key contributors, so they can be targeted using appropriate regimen. For example, SMAD4 can be targeted in the TGF-β/SMAD4 pathway using SIRT7, and the results are promising for retarding breast cancer lung metastasis [114]. Due to the important contribution taken by tumor-mediated immunosuppression in metastasis, it is reasonable to think of exploiting adjuvant immunotherapy with the target signaling of metastasis, for example TGF-β inhibition plus PD-L1 blockade, as it has been under the current focus. PRC1 is known to promote metastasis of prostatic cancer cells by inducing stemness and recruiting the immunosuppressive M2 and Treg cells, thus being an appropriate choice to be used in combination with immune checkpoint inhibitor (ICI), such as PD-L1 blockade therapy for suppression of tumor metastasis [115]. As discussed, the architecture of tumor vessels is abnormal. The leaky vasculature in the pre-metastatic niche facilitates tumor outgrowth in the metastatic site. A study by He and colleagues showed that a repair in the leaky vasculature through a cytokine-based therapy will preclude the possibility of metastatic outgrowth and sensitizes established metastatic tumors to ICI [116].
Environmental events such as hypoxic, oxidative and metabolic stresses are the key contributors to the tumor progression and metastasis, so they can be a target [107, 117,118,119]. Relationship between mitochondrial oxidative stress and immunosuppression within the TME is positive [117], and application of oxidative modulators, such as melatonin [120,121,122,123], resveratrol [124], metformin [125] and curcumin [126] has shown promising results.
Tumor metabolism can be targeted to avoid further metastasis, and in tumors forming PMNs, strategies can be expanded to target the main promoters of PMN formation. CAFs take the key role in PMN formation. In fact, primary tumor cells send signals to either recruit or activate fibroblasts in the secondary sites to form PMNs for their subsequent metastasis [127]. For tumors initiating the process without any evidence of detectable tumoral cells in the target secondary organs, strategies can be switched to target signaling implicated in early metastatic cascade, such as EMT. Both single-cell invasion and collective migration use EMT at the early metastatic event, so EMT inhibitors can be used in combination with PD-1/PD-L1 blockade therapy with the aim of retarding the whole events. However, failure of response in the long-term to such therapies is expectable, so knowledge in the area must be expanded to seek for strategies that are more effective, more durable and less invasive.
Conclusion
Metastasis is a complicated event which may occur early at tumorigenesis or upon tumor taking a progressive step. Metastasis is viewed as a systemic disease, being different from a local primary tumor which is treatable by surgery or chemo/radiation therapy [81]. Metastasis is started by selection of some cancer cells taking an invasive phase at the Edge area of tumor, and is finished by colonization and outgrowth in the secondary area. Despite the real progresses in the field, which resulted in the identification of key drivers of the metastatic steps, research in the area is still continuing, which are to shed more lights in regard with the cross-communications occurring between contributing factors in each step with another.
References
Graham TA, Shibata D. Navigating the path to distant metastasis. Nat Genet. 2020;52(7):642–3.
Aytes A, et al. NSD2 is a conserved driver of metastatic prostate cancer progression. Nat Commun. 2018;9(1):1–14.
Hu Z, Curtis C. Looking backward in time to define the chronology of metastasis. Nat Commun. 2020;11(1):1–4.
Mortezaee K. CXCL12/CXCR4 axis in the microenvironment of solid tumors: a critical mediator of metastasis. Life Sci. 2020:117534.
Müller A, et al. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410(6824):50.
Ding Y, Du Y. Clinicopathological significance and prognostic role of chemokine receptor CXCR4 expression in pancreatic ductal adenocarcinoma, a meta-analysis and literature review. Int J Surg. 2019;
López-Soto A, et al. Control of metastasis by NK cells. Cancer Cell. 2017;32(2):135–54.
Lambert AW, Pattabiraman DR, Weinberg RA. Emerging biological principles of metastasis. Cell. 2017;168(4):670–91.
Paul CD, Mistriotis P, Konstantopoulos K. Cancer cell motility: lessons from migration in confined spaces. Nat Rev Cancer. 2017;17(2):131.
Kim YH, et al. Senescent tumor cells lead the collective invasion in thyroid cancer. Nat Commun. 2017;8:15208.
Sennino B, McDonald DM. Controlling escape from angiogenesis inhibitors. Nat Rev Cancer. 2012;12(10):699.
Laudato, S., A. Aparicio, and F.G. Giancotti, Clonal evolution and epithelial plasticity in the emergence of AR-independent prostate carcinoma. Trends in cancer, 2019.
Castaño Z, et al. IL-1β inflammatory response driven by primary breast cancer prevents metastasis-initiating cell colonization. Nat Cell Biol. 2018;20(9):1084–97.
Birkbak NJ, McGranahan N. Cancer genome evolutionary trajectories in metastasis. Cancer Cell. 2020;37(1):8–19.
Lo HC, et al. Resistance to natural killer cell immunosurveillance confers a selective advantage to polyclonal metastasis. Nat Cancer. 2020:1–14.
Strilic B, Offermanns S. Intravascular survival and extravasation of tumor cells. Cancer Cell. 2017;32(3):282–93.
Padmanaban V, et al. E-cadherin is required for metastasis in multiple models of breast cancer. Nature. 2019;573(7774):439–44.
Fujiyoshi K, et al. Tumour budding, poorly differentiated clusters, and T-cell response in colorectal cancer. EBioMedicine. 2020;57:102860.
VanderVorst K, et al. Wnt/PCP signaling contribution to carcinoma collective cell migration and metastasis. Cancer Res. 2019;79(8):1719–29.
Hu X, et al. The RNA-binding protein AKAP8 suppresses tumor metastasis by antagonizing EMT-associated alternative splicing. Nat Commun. 2020;11(1):1–15.
Dhamija S, Diederichs S. From junk to master regulators of invasion: lncRNA functions in migration, EMT and metastasis. Int J Cancer. 2016;139(2):269–80.
Steinbichler, T.B., et al. The role of exosomes in cancer metastasis. in Seminars in cancer biology. 2017. Elsevier.
Najafi M, Mortezaee K, Majidpoor J. Stromal reprogramming: a target for tumor therapy. Life Sci. 2019;239:117049.
Pastushenko I, Blanpain C. EMT transition states during tumor progression and metastasis. Trends Cell Biol. 2019;29(3):212–26.
Derynck, R., S.J. Turley, and R.J. Akhurst, TGFβ biology in cancer progression and immunotherapy. Nat Rev Clin Oncol, 2020: p. 1–26.
Tong L, et al. Proteasome-dependent degradation of Smad7 is critical for lung cancer metastasis. Cell Death Differ. 2020;27(6):1795–806.
Fares J, et al. Molecular principles of metastasis: a hallmark of cancer revisited. Signal Transduct Target Ther. 2020;5(1):1–17.
Najafi M, Mortezaee K, Majidpoor J. Cancer stem cell (CSC) resistance drivers. Life Sci. 2019;234:116781.
Najafi M, Mortezaee K, Ahadi R. Cancer stem cell (a) symmetry & plasticity: tumorigenesis and therapy relevance. Life Sci. 2019;
Labernadie A, et al. A mechanically active heterotypic E-cadherin/N-cadherin adhesion enables fibroblasts to drive cancer cell invasion. Nat Cell Biol. 2017;19(3):224–37.
Glentis A, et al. Cancer-associated fibroblasts induce metalloprotease-independent cancer cell invasion of the basement membrane. Nat Commun. 2017;8(1):1–13.
Anderson RL, et al. A framework for the development of effective anti-metastatic agents. Nat Rev Clin Oncol. 2019;16(3):185–204.
Fukumura D, et al. Enhancing cancer immunotherapy using antiangiogenics: opportunities and challenges. Nat Rev Clin Oncol. 2018;15(5):325.
Jin F, et al. New insight into the SDF-1/CXCR4 axis in a breast carcinoma model: hypoxia-induced endothelial SDF-1 and tumor cell CXCR4 are required for tumor cell intravasation. Mol Cancer Res. 2012;10(8):1021–31.
Donato C, et al. Hypoxia triggers the Intravasation of clustered circulating tumor cells. Cell Rep. 2020;32(10):108105.
Williams CB, Yeh ES, Soloff AC. Tumor-associated macrophages: unwitting accomplices in breast cancer malignancy. NPJ breast cancer. 2016;2(1):1–12.
Cassetta L, Pollard JW. Targeting macrophages: therapeutic approaches in cancer. Nat Rev Drug Discov. 2018;17(12):887–904.
Kai F, Laklai H, Weaver VM. Force matters: biomechanical regulation of cell invasion and migration in disease. Trends Cell Biol. 2016;26(7):486–97.
Yang L, Zhang Y. Tumor-associated macrophages: from basic research to clinical application. J Hematol Oncol. 2017;10(1):58.
Aras S, Zaidi MR. TAMeless traitors: macrophages in cancer progression and metastasis. Br J Cancer. 2017;117(11):1583–91.
Erdogan B, Webb DJ. Cancer-associated fibroblasts modulate growth factor signaling and extracellular matrix remodeling to regulate tumor metastasis. Biochem Soc Trans. 2017;45(1):229–36.
Chronopoulos A, et al. ATRA mechanically reprograms pancreatic stellate cells to suppress matrix remodelling and inhibit cancer cell invasion. Nat Commun. 2016;7(1):1–12.
Zeltz, C., et al. Cancer-associated fibroblasts in desmoplastic tumors: emerging role of integrins. in Seminars in cancer biology. 2020. Elsevier.
Farhood B, Najafi M, Mortezaee K. Cancer-associated fibroblasts: secretions, interactions, and therapy. J Cell Biochem. 2019;120(3):2791–800.
Mollinedo F. Neutrophil degranulation, plasticity, and cancer metastasis. Trends Immunol. 2019;
Chen X, Song E. Turning foes to friends: targeting cancer-associated fibroblasts. Nat Rev Drug Discov. 2019;18(2):99–115.
Su SC, et al. Cancer metastasis: mechanisms of inhibition by melatonin. J Pineal Res. 2017;62(1):e12370.
García-Jiménez C, Goding CR. Starvation and pseudo-starvation as drivers of cancer metastasis through translation reprogramming. Cell Metab. 2019;29(2):254–67.
Tang, Q., et al., Mutant p53 on the Path to Metastasis. Trends in Cancer, 2019.
Kim Y-N, et al. Anoikis resistance: an essential prerequisite for tumor metastasis. Int J cell Biol. 2012;2012
De Bock K, Mazzone M, Carmeliet P. Antiangiogenic therapy, hypoxia, and metastasis: risky liaisons, or not? Nat Rev Clin Oncol. 2011;8(7):393.
Beloribi-Djefaflia S, Vasseur S, Guillaumond F. Lipid metabolic reprogramming in cancer cells. Oncogenesis. 2016;5(1):e189.
Davis RT, et al. Transcriptional diversity and bioenergetic shift in human breast cancer metastasis revealed by single-cell RNA sequencing. Nat Cell Biol. 2020;22(3):310–20.
Zhu J, Thompson CB. Metabolic regulation of cell growth and proliferation. Nat Rev Mol Cell Biol. 2019;20(7):436–50.
Takahashi N, et al. Cancer cells co-opt the neuronal redox-sensing channel TRPA1 to promote oxidative-stress tolerance. Cancer Cell. 2018;33(6):985–1003. e7
Wheeler LJ, et al. Multi-omic approaches identify metabolic and autophagy regulators important in ovarian cancer dissemination. iScience. 2019;19:474–91.
Najafi M, Ahmadi A, Mortezaee K. Extracellular-signal-regulated kinase/mitogen-activated protein kinase signaling as a target for cancer therapy: an updated review. Cell Biol Int. 2019;43(11):1206–22.
Mortezaee K. Human hepatocellular carcinoma: protection by melatonin. J Cell Physiol. 2018;233(10):6486–508.
Ren W, et al. The expression of hypoxia-inducible factor-1α and its clinical significance in lung cancer: a systematic review and meta-analysis. Swiss Med Wkly. 2013;143(3536)
Coronel J, et al. Weekly topotecan as second-or third-line treatment in patients with recurrent or metastatic cervical cancer. Med Oncol. 2009;26(2):210–4.
Wadler S, et al. Topotecan is an active agent in the first-line treatment of metastatic or recurrent endometrial carcinoma: eastern cooperative oncology group study E3E93. J Clin Oncol. 2003;21(11):2110–4.
Wu X, et al. Zinc finger protein 367 promotes metastasis by inhibiting the hippo pathway in breast cancer. Oncogene. 2020;39(12):2568–82.
Tanaka K, et al. Statin suppresses hippo pathway-inactivated malignant mesothelioma cells and blocks the YAP/CD44 growth stimulatory axis. Cancer Lett. 2017;385:215–24.
Maille E, et al. MST1/hippo promoter gene methylation predicts poor survival in patients with malignant pleural mesothelioma in the IFCT-GFPC-0701 MAPS phase 3 trial. Br J Cancer. 2019;120(4):387–97.
Higashi T, et al. Statin attenuates cell proliferative ability via TAZ (WWTR1) in hepatocellular carcinoma. Med Oncol. 2016;33(11):123.
Follain G, et al. Fluids and their mechanics in tumour transit: shaping metastasis. Nat Rev Cancer. 2019:1–18.
Marcolino E, et al. Blood platelets stimulate cancer extravasation through TGFβ-mediated downregulation of PRH/HHEX. Oncogenesis. 2020;9(2):1–12.
Gaertner F, Massberg S. Patrolling the vascular borders: platelets in immunity to infection and cancer. Nat Rev Immunol. 2019:1–14.
Owen KL, et al. Prostate cancer cell-intrinsic interferon signaling regulates dormancy and metastatic outgrowth in bone. EMBO Rep. 2020:e50162.
Guan X, et al. The prognostic and therapeutic implications of circulating tumor cell phenotype detection based on epithelial–mesenchymal transition markers in the first-line chemotherapy of HER2-negative metastatic breast cancer. Cancer Commun. 2019;39(1):1–10.
Wang Z-L, et al. Dynamic changes of different phenotypic and genetic circulating tumor cells as a biomarker for evaluating the prognosis of RCC. Cancer Biol Ther. 2019;20(4):505–12.
Cai J, et al. Associations between the cyclooxygenase-2 expression in circulating tumor cells and the clinicopathological features of patients with colorectal cancer. J Cell Biochem. 2019;120(4):4935–41.
Chen EY, et al. A phase II study of celecoxib with irinotecan, 5-fluorouracil, and leucovorin in patients with previously untreated advanced or metastatic colorectal cancer. Am J Clin Oncol. 2018;41(12):1193.
Egeblad M, Nakasone ES, Werb Z. Tumors as organs: complex tissues that interface with the entire organism. Dev Cell. 2010;18(6):884–901.
Kim J, et al. Role of cancer emboli as a metastatic core on the growth of brain metastasis in patients with non-small cell lung cancer. J Neurointensive Care. 2020;
Silvestre-Roig C, et al. Neutrophil diversity in health and disease. Trends Immunol. 2019;
Biswas SK. Metabolic reprogramming of immune cells in cancer progression. Immunity. 2015;43(3):435–49.
Montagner M, et al. Crosstalk with lung epithelial cells regulates Sfrp2-mediated latency in breast cancer dissemination. Nat Cell Biol. 2020:1–8.
Roberts S, Agrawal N. Temporal analysis of CTC-endothelium interactions during early metastasis. in 2015 41st Annual Northeast Biomedical Engineering Conference (NEBEC). 2015. IEEE.
Bersini S, et al. A combined microfluidic-transcriptomic approach to characterize the extravasation potential of cancer cells. Oncotarget. 2018;9(90):36110.
Li J, King MR. Adhesion receptors as therapeutic targets for circulating tumor cells. Front Oncol. 2012;2:79.
Barbazán J, et al. Liver metastasis is facilitated by the adherence of circulating tumor cells to vascular fibronectin deposits. Cancer Res. 2017;77(13):3431–41.
Osmani N, et al. Metastatic tumor cells exploit their adhesion repertoire to counteract shear forces during intravascular arrest. Cell Rep. 2019;28(10):2491–500. e5
Osmani N, et al, Intravascular arrest of circulating tumor cells is a two-step process exploiting their adhesion repertoire. Available at SSRN 3272239, 2018.
Hynes W, et al. Examining metastatic behavior within 3D bioprinted vasculature for the validation of a 3D computational flow model. Sci Adv. 2020;6(35):eabb3308.
Gkretsi V, Stylianopoulos T. Cell adhesion and matrix stiffness: coordinating cancer cell invasion and metastasis. Front Oncol. 2018;8:145.
Leong HS, et al. Invadopodia are required for cancer cell extravasation and are a therapeutic target for metastasis. Cell Rep. 2014;8(5):1558–70.
Williams KC, et al. Invadopodia are chemosensing protrusions that guide cancer cell extravasation to promote brain tropism in metastasis. Oncogene. 2019;38(19):3598–615.
Yoneyama MS, et al. Vimentin intermediate filament and plectin provide a scaffold for invadopodia, facilitating cancer cell invasion and extravasation for metastasis. Eur J Cell Biol. 2014;93(4):157–69.
Diepenbruck M, Christofori G. Epithelial–mesenchymal transition (EMT) and metastasis: yes, no, maybe? Curr Opin Cell Biol. 2016;43:7–13.
Achrol AS, et al. Brain metastases. Nat Rev Dis Primers. 2019;5(1):1–26.
Guereño M, et al. Glypican-3 (GPC3) inhibits metastasis development promoting dormancy in breast cancer cells by p38 MAPK pathway activation. Eur J Cell Biol. 2020;99(6):151096.
Gau DM, et al. Abstract LB-043: MRTF| Profilin is an important signaling axis for metastatic outgrowth of triple negative breast cancer cells. 2019, AACR.
Flynn ALB, et al. Autophagy inhibition elicits emergence from metastatic dormancy by inducing and stabilizing Pfkfb3 expression. Nat Commun. 2019;10(1):1–15.
Rodrigues G, et al. Tumour exosomal CEMIP protein promotes cancer cell colonization in brain metastasis. Nat Cell Biol. 2019;21(11):1403–12.
Ma R-Y, et al. Monocyte-derived macrophages promote breast cancer bone metastasis outgrowth. J Exp Med. 2020;217(11)
Farhood B, et al. Disruption of the redox balance with either oxidative or anti-oxidative overloading as a promising target for cancer therapy. J Cell Biochem. 2019;120(1):71–6.
Mortezaee K. Immune escape: a critical hallmark in solid tumors. Life Sci. 2020:118110.
Nywening TM, et al. Targeting tumour-associated macrophages with CCR2 inhibition in combination with FOLFIRINOX in patients with borderline resectable and locally advanced pancreatic cancer: a single-Centre, open-label, dose-finding, non-randomised, phase 1b trial. Lancet Oncol. 2016;17(5):651–62.
Gomez-Roca C, et al. Phase I study of emactuzumab single agent or in combination with paclitaxel in patients with advanced/metastatic solid tumors reveals depletion of immunosuppressive M2-like macrophages. Ann Oncol. 2019;30(8):1381–92.
Marsh T, et al. Autophagic degradation of NBR1 restricts metastatic outgrowth during mammary tumor progression. Dev Cell. 2020;52(5):591–604. e6
Malhotra J, et al. Phase Ib/II study of hydroxychloroquine in combination with chemotherapy in patients with metastatic non-small cell lung cancer (NSCLC). Cancer Treat Res Commun. 2019;21:100158.
Karasic TB, et al. Effect of gemcitabine and nab-paclitaxel with or without hydroxychloroquine on patients with advanced pancreatic cancer: a phase 2 randomized clinical trial. JAMA Oncol. 2019;5(7):993–8.
Mortezaee K, et al. NADPH oxidase as a target for modulation of radiation response; implications to carcinogenesis and radiotherapy. Curr Mol Pharmacol. 2019;12(1):50–60.
Nandi P, et al. PGE2 promotes breast cancer-associated lymphangiogenesis by activation of EP4 receptor on lymphatic endothelial cells. BMC Cancer. 2017;17(1):1–17.
Du C, et al. Tumour-derived exosomal miR-3473b promotes lung tumour cell intrapulmonary colonization by activating the nuclear factor-κB of local fibroblasts. J Cell Mol Med. 2020;
Najafi M, et al. Hypoxia in solid tumors: a key promoter of cancer stem cell (CSC) resistance. J Cancer Res Clin Oncol. 2020;146(1):19–31.
Mortezaee K, et al. NF-κB targeting for overcoming tumor resistance and normal tissues toxicity. J Cell Physiol. 2019;234(10):17187–204.
Mortezaee K. Nicotinamide adenine dinucleotide phosphate (NADPH) oxidase (NOX) and liver fibrosis: a review. Cell Biochem Funct. 2018;36(6):292–302.
Zhang L, et al. Blocking immunosuppressive neutrophils deters pY696-EZH2–driven brain metastases. Sci Transl Med. 2020;12(545)
Yang L, et al. Induction of DNMT3B by PGE2 and IL6 at distant metastatic sites promotes epigenetic modification and breast cancer colonization. Cancer Res. 2020;
Pein M, et al. CXCR3-expressing metastasis-initiating cells induce and exploit a fibroblast niche in the lungs to fuel metastatic colonization. bioRxiv, 2019: p. 546952.
Miarka L, et al. The hepatic microenvironment and TRAIL-R2 impact outgrowth of liver metastases in pancreatic Cancer after surgical resection. Cancers. 2019;11(6):745.
Tang X, et al. SIRT7 antagonizes TGF-β signaling and inhibits breast cancer metastasis. Nat Commun. 2017;8(1):1–14.
Su W, et al. The polycomb repressor complex 1 drives double-negative prostate cancer metastasis by coordinating stemness and immune suppression. Cancer Cell. 2019;36(2):139–55. e10
He B, et al. Remodeling of metastatic vasculature reduces lung colonization and sensitizes overt metastases to immunotherapy. Cell Rep. 2020;30(3):714–24. e5
Kuo C-L, et al. Mitochondrial oxidative stress by Lon-PYCR1 maintains an immunosuppressive tumor microenvironment that promotes cancer progression and metastasis. Cancer Lett. 2020;474:138–50.
Mortezaee K. Hypoxia induces core-to-edge transition of progressive tumoral cells: a critical review on differential yet corroborative roles for HIF-1α and HIF-2α. Life Sci. 2020;242:117145.
Mortezaee K, et al. Redox interactions and genotoxicity of metal-based nanoparticles: a comprehensive review. Chem Biol Interact. 2019:108814.
Najafi M, et al. Adjuvant chemotherapy with melatonin for targeting human cancers: a review. J Cell Physiol. 2019;234(3):2356–72.
Farhood B, et al. Melatonin as an adjuvant in radiotherapy for radioprotection and radiosensitization. Clin Transl Oncol. 2019;21(3):268–79.
Mortezaee K, et al. Modulation of apoptosis by melatonin for improving cancer treatment efficiency: an updated review. Life Sci. 2019;
Mortezaee K, et al. Boosting immune system against cancer by melatonin: a mechanistic viewpoint. Life Sci. 2019:116960.
Mortezaee K, et al. Resveratrol as an adjuvant for normal tissues protection and tumor sensitization. Curr Cancer Drug Targets. 2019;
Mortezaee K, et al. Metformin as a radiation modifier; implications to normal tissue protection and tumor sensitization. Curr Clin Pharmacol. 2019;14(1):41–53.
Farhood B, et al. Curcumin as an anti-inflammatory agent: implications to radiotherapy and chemotherapy. J Cell Physiol. 2019;234(5):5728–40.
Ji Q, et al. Primary tumors release ITGBL1-rich extracellular vesicles to promote distal metastatic tumor growth through fibroblast-niche formation. Nat Commun. 2020;11(1):1–18.
Lin B, et al. CRMP2 is a therapeutic target that suppresses the aggressiveness of breast cancer cells by stabilizing RECK. Oncogene. 2020:1–17.
Bajaj R, et al. IMPAD1 and KDELR2 drive invasion and metastasis by enhancing Golgi-mediated secretion. Oncogene. 2020:1–16.
Ko C-J, et al. Inhibition of TMPRSS2 by HAI-2 reduces prostate cancer cell invasion and metastasis. Oncogene. 2020:1–14.
Zheng Y, et al. Insulin-like growth factor 1-induced enolase 2 deacetylation by HDAC3 promotes metastasis of pancreatic cancer. Signal Transduct Target Ther. 2020;5(1):1–14.
Zhang Z, et al. CDK4/6 inhibition blocks cancer metastasis through a USP51-ZEB1-dependent deubiquitination mechanism. Signal Transduct Target Ther. 2020;5(1):1–13.
Wu X, et al. Targeting the interaction between RNA-binding protein HuR and FOXQ1 suppresses breast cancer invasion and metastasis. Commun Biol. 2020;3(1):1–16.
Zhou Z, et al. VCAM-1 secreted from cancer-associated fibroblasts enhances the growth and invasion of lung cancer cells through AKT and MAPK signaling. Cancer Lett. 2020;473:62–73.
Sun Z, et al. Tenascin-C increases lung metastasis by impacting blood vessel invasions. Matrix Biol. 2019;83:26–47.
Seachrist DD, et al. The transcriptional repressor BCL11A promotes breast cancer metastasis. J Biol Chem. 2020;295(33):11707–19.
Liu S, et al. Deubiquitinase activity profiling identifies UCHL1 as a candidate oncoprotein that promotes TGFβ-induced breast cancer metastasis. Clin Cancer Res. 2020;26(6):1460–73.
Sjöberg E, et al. A novel ACKR2-dependent role of fibroblast-derived CXCL14 in epithelial-to-mesenchymal transition and metastasis of breast cancer. Clin Cancer Res. 2019;25(12):3702–17.
Zhang J, et al. The natural compound neobractatin inhibits tumor metastasis by upregulating the RNA-binding-protein MBNL2. Cell Death Dis. 2019;10(8):1–13.
Hendrikx S, et al. Endothelial calcineurin signaling restrains metastatic outgrowth by regulating Bmp2. Cell Rep. 2019;26(5):1227–41. e6
Howe EN, et al. Rab11b-mediated integrin recycling promotes brain metastatic adaptation and outgrowth. Nat Commun. 2020;11(1):1–15.
Sinha S, et al. Abstract A50: IMPACT inhibits metastatic outgrowth in pancreatic cancer by restraining GCN1-ATF4 signaling. 2019, AACR.
Hoj JP, Mayro B, Pendergast AM. A TAZ-AXL-ABL2 feed-forward signaling axis promotes lung adenocarcinoma brain metastasis. Cell Rep. 2019;29(11):3421–34. e8
Urosevic J, et al. ERK1/2 signaling induces upregulation of ANGPT2 and CXCR4 to mediate liver metastasis in colon cancer. Cancer Res. 2020;
Tulotta C, et al. Endogenous production of IL1B by breast cancer cells drives metastasis and colonization of the bone microenvironment. Clin Cancer Res. 2019;25(9):2769–82.
Yamaguchi N, et al. PCK1 and DHODH drive colorectal cancer liver metastatic colonization and nucleotide biosynthesis under hypoxia. bioRxiv, 2019: p. 833186.
McKernan CM, Pendergast AM. Abstract P3–03-04: Role of ABL kinase signaling in metastatic breast cancer colonization of the brain. 2020, AACR.
Li L, et al. EGR1 regulates angiogenic and osteoclastogenic factors in prostate cancer and promotes metastasis. Oncogene. 2019;38(35):6241–55.
Polireddy K, et al. High dose parenteral ascorbate inhibited pancreatic cancer growth and metastasis: mechanisms and a phase I/IIa study. Sci Rep. 2017;7(1):1–15.
Rodón J, et al. Pharmacokinetic, pharmacodynamic and biomarker evaluation of transforming growth factor-β receptor I kinase inhibitor, galunisertib, in phase 1 study in patients with advanced cancer. Investig New Drugs. 2015;33(2):357–70.
Kashiwagi S, et al. Mesenchymal–epithelial transition and tumor vascular remodeling in eribulin chemotherapy for breast cancer. Anticancer Res. 2018;38(1):401–10.
Li W, et al. Gold nanoparticles attenuate metastasis by tumor vasculature normalization and epithelial–mesenchymal transition inhibition. Int J Nanomedicine. 2017;12:3509.
Sorensen AG, et al. Increased survival of glioblastoma patients who respond to antiangiogenic therapy with elevated blood perfusion. Cancer Res. 2012;72(2):402–7.
Thomas RP, et al. Macrophage exclusion after radiation therapy (MERT): a first in human phase I/II trial using a CXCR4 inhibitor in glioblastoma. Clin Cancer Res. 2019;25(23):6948–57.
Shah MA, et al. Andecaliximab/GS-5745 alone and combined with mFOLFOX6 in advanced gastric and gastroesophageal junction adenocarcinoma: results from a phase I study. Clin Cancer Res. 2018;24(16):3829–37.
Giannelli G, et al. Biomarkers and overall survival in patients with advanced hepatocellular carcinoma treated with TGF-βRI inhibitor galunisertib. PLoS One. 2020;15(3):e0222259.
Kelley R, et al. A phase 2 study of galunisertib (TGF-β1 receptor type I inhibitor) and sorafenib in patients with advanced hepatocellular carcinoma. Clin Transl Gastroenterol. 2019;10(7)
Melisi D, et al. TGFβ receptor inhibitor galunisertib is linked to inflammation-and remodeling-related proteins in patients with pancreatic cancer. Cancer Chemother Pharmacol. 2019;83(5):975–91.
Sullivan RJ, et al. First-in-class ERK1/2 inhibitor ulixertinib (BVD-523) in patients with MAPK mutant advanced solid tumors: results of a phase I dose-escalation and expansion study. Cancer Discov. 2018;8(2):184–95.
Sun Y, et al. Phase I dose-escalation study of chiauranib, a novel angiogenic, mitotic, and chronic inflammation inhibitor, in patients with advanced solid tumors. J Hematol Oncol. 2019;12(1):9.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors of the paper have no potential conflict to interests, and authors have read and approve the final version.
Ethical approval
The paper received the ethical code: IR.MUK.REC.1399.106.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Majidpoor, J., Mortezaee, K. Steps in metastasis: an updated review. Med Oncol 38, 3 (2021). https://doi.org/10.1007/s12032-020-01447-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-020-01447-w