Abstract
It is estimated that more than half of cancer patients undergo radiotherapy during the course of their treatment. Despite its beneficial therapeutic effects on tumor cells, exposure to high doses of ionizing radiation (IR) is associated with several side effects. Although improvements in radiotherapy techniques and instruments could reduce these side effects, there are still important concerns for cancer patients. For several years, scientists have been trying to modulate tumor and normal tissue responses to IR, leading to an increase in therapeutic ratio. So far, several types of radioprotectors and radiosensitizers have been investigated in experimental studies. However, high toxicity of chemical sensitizers or possible tumor protection by radioprotectors creates a doubt for their clinical applications. On the other hand, the protective effects of these radioprotectors or sensitizer effects of radiosensitizers may limit some type of cancers. Hence, the development of some radioprotectors without any protective effect on tumor cells or low toxic radiosensitizers can help improve therapeutic ratio with less side effects. Melatonin as a natural body hormone is a potent antioxidant and anti-inflammatory agent that shows some anti-cancer properties. It is able to neutralize different types of free radicals produced by IR or pro-oxidant enzymes which are activated following exposure to IR and plays a key role in the protection of normal tissues. In addition, melatonin has shown the ability to inhibit long-term changes in inflammatory responses at different levels, thereby ameliorating late side effects of radiotherapy. Fortunately, in contrast to classic antioxidants, some in vitro studies have revealed that melatonin has a potent anti-tumor activity when used alongside irradiation. However, the mechanisms of its radiosensitive effect remain to be elucidated. Studies suggested that the activation of pro-apoptosis gene, such as p53, changes in the metabolism of tumor cells, suppression of DNA repair responses as well as changes in biosynthesis of estrogen in breast cancer cells are involved in this process. In this review, we describe the molecular mechanisms for radioprotection and radiosensitizer effects of melatonin. Furthermore, some other proposed mechanisms that may be involved are presented.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
For several years, scientists have been looking for strategies to reduce early and late effects of radiotherapy, as well as increasing tumor response to radiation treatment. Either of these aims can help improve therapeutic ratio, leading to the reduction of side effects and increasing survival rate of cancer patients [1]. The use of radioprotectors and radiosensitizers is two interesting strategies for alleviating side effects to normal tissues and reducing tumor resistance [2]. So far, several types of radioprotectors and radiosensitizers have been tested in experimental studies [3, 4]. An appropriate radioprotector should protect normal tissues selectively without adverse effects on tumor response. In addition, a good radiosensitizer should not increase the toxicity of normal tissues [2, 5].
Amifostine, a Food and Drug Administration (FDA) approved radioprotector, has been widely examined and used in clinical radiotherapy [6, 7]. It has shown the ability to protect various normal tissues such as oral mucosa and salivary glands in head and neck cancer, bone marrow, lungs, intestines and kidneys. [8, 9]. Several studies have confirmed that amifostine protects these organs selectively without any adverse effect on tumor response. However, amifostine has some side effects such as nausea and vomiting, which may lead to its discontinuation during the course of radiotherapy [10,11,12]. It is important to note that amifostine cannot protect all human organs against toxic effects of ionizing radiation (IR) [13] and, hence, the need for further research to discover alternative agents with both radioprotective and radiosensitization properties in radiation therapy applications.
Melatonin is a natural product of the human body that has shown impressive properties for protection against toxic effects of anti-cancer modalities such as chemotherapy and radiotherapy [14,15,16]. It is mostly secreted by the pineal gland in the brain, while several studies have identified other sources such as lymphocytes, retina and gastrointestinal system [17,18,19]. Although the major effect of melatonin in the body is control of sleep and wake cycle, several studies have shown that it has other interesting properties such as antioxidant, anti-inflammatory and anti-aging effects [20,21,22]. In the last two decades, several studies have been conducted to investigate the radioprotective effects of melatonin in different cells and organs. Due to the ease with which melatonin penetrates all cell types in the body, it can protect different organs against various side effects of radiation [23]. In recent years, some studies have shown that melatonin sensitizes some tumor cells to radiation. Both radioprotective and radiosensitization effects of melatonin make it an ideal candidate for use as an adjuvant in radiotherapy. On the other hand, the natural metabolisms of melatonin in human body cells lead to low toxicity compared to other chemical products such as amifostine [24]. In this review, we clarify the mechanisms through which melatonin can act as both radioprotector and radiosensitizer as well as possible future applications in cancer radiotherapy.
Radiation-induced DNA damage and cell death
DNA is the most crucial target for toxic effects of radiation. After transmission of an ionizing radiation from vital cells, it may interact with DNA directly, leading to chromosomal aberrations or cells death. Although, more than two-third of toxic effects of ionizing radiation results from the production of free radical due to interaction of ionizing radiations with water molecules [25]. Free radicals including reactive oxygen species (ROS) and reactive nitrogen species are very active molecules that are able to attack DNA and other vital organelles within cells. If DNA damages overwhelm the responses, it may lead to cell death or development of neoplasia [26].
It has been confirmed that radiation can trigger cell death through different mechanisms such as apoptosis, mitotic catastrophe, necrosis, necroptosis, autophagy, and senescence [27]. The type of cell death depends on the cell type [28]. Furthermore, as radiation dose increases, necrosis is also increased [29]. After DNA damage and cell death following irradiation, some danger alarms are released from damaged cells. Danger alarms can be recognized by macrophages and lymphocytes, leading to several signaling pathways involved in inflammation, DNA repair and reduction/oxidation (redox) metabolism [30,31,32]. These processes are associated with a massive production of free radicals including ROS and NO, as well as an increase in the level of several cytokines and chemokines such as TNF-α, TGF-β, IL-1, IL-4, IL-6, IL-8, IL-13, and others [33]. Moreover, these changes can cause suppression of antioxidant defense in cells, leading to more oxidative stress, DNA breaks and cell death [34,35,36].
Melatonin as a radioprotector
For more than 2 decades, the radioprotective effect of melatonin has been confirmed, firstly by in vitro studies [37, 38]. Several in vivo and in vitro studies have been conducted to investigate its possible protection on different cells/organs. Melatonin has shown ability to alleviate various cytotoxic effects of IR such as DNA damage, apoptosis, inflammation, fibrosis, cataract, infertility and others [39]. Various studies have shown that melatonin can cause protection without any cytotoxic effect on the normal functions of other organs. It has shown radioprotection via some mechanisms such as neutralization of free radicals, boost response to DNA damage and amelioration of inflammatory responses through modulation of several signaling pathways that are involved in this process.
Melatonin and radiation-induced oxidative stress
Several studies have revealed the potent antioxidant effect of melatonin against various toxic agents such as IR, chemotherapy agents, metals and others [40]. Studies have proposed that the antioxidant effect of melatonin is as a result of two different processes known as the direct and indirect effects. The direct effect is due to scavenging free radicals such as reactive oxygen and nitrogen species while its indirect antioxidant effect results from several changes in gene transcription as well as the activities of antioxidants and ROS/NO producing enzymes [41,42,43]. Several in vitro and in vivo studies have shown that treatment with melatonin before exposure to IR can alleviate oxidative injury via upregulation of superoxide dismutase (SOD), glutathione (GSH), glutathione peroxidase (Gpx), and catalase (CAT) in different cells/organs [44,45,46,47,48,49,50]. It has been proposed that some upstream genes such as nrf2 [nuclear factor erythroid 2 (NF-E2)-related factor 2] or TGF-β are involved in upregulating and downregulating antioxidant enzymes. After interaction of free radicals with nrf2, it is upregulated, leading to the stimulation of antioxidant enzymes [51]. On the other hand, increased level of TGF-β which is predictable after irradiation may suppress SOD activity through upregulation of mir21 [52, 53]. Melatonin, via stimulation of NRF2-dependent pathways, can regulate antioxidant defense and neutralize free radicals [54, 55].
Another important antioxidant effect of melatonin is related to its inhibitory effects on ROS/NO producing enzymes. Melatonin has shown ability to attenuate COX-2 and iNOS enzyme expression in rat’s lung tissue, leading to the reduction of oxidative DNA [56]. Some recent studies have shown that redox activation by mitochondria is a main source of ROS production following exposure to IR [57,58,59]. This is associated with genomic instability and death of stem cells in critical organs such as hematopoietic and gastrointestinal system, leading to acute radiation syndrome and death [60, 61]. Melatonin has been proposed as mitochondria targeting agents, with the ability to neutralize mitochondria ROS via improvement in oxidative phosphorylation efficiency and electron leakage reduction [62]. These properties may make melatonin a potent radiation mitigator via modulation of ROS/NO metabolism after exposure to IR [63].
Melatonin modulates radiation-induced DNA damage/cell death and subsequent inflammation
For over two decades, it has been shown that melatonin protects against IR-induced DNA damage [37, 64]. In 1998, Vijayalaxmi et al. proposed that melatonin via scavenging of IR-induced ROS production and activation of DNA repair enzymes protects cells against IR [38]. In recent years, it has been shown that melatonin via modulation of DNA damage response can alleviate toxic effect of IR, leading to reduced cell death [65]. A study by Rezapoor et al. has shown that administering melatonin before whole body irradiation enhances BER response in circulating lymphocytes. After injecting melatonin, they irradiated rats with 2 or 8 Gy X-ray and then evaluated the expression of genes involved in BER pathway, including Xrcc1, 8-oxoguanine glycosylase1 (Ogg1), and Apex1. Their results showed that melatonin treatment augments the expression of all mentioned genes. In addition, pre-treatment with melatonin increases the expression of all three genes compared to irradiated non-treated rats. These changes were more obvious for 2 Gy, while stimulation of all genes was lower when melatonin was administered before exposure to 8 Gy [66]. Other studies proposed that pre-treatment with melatonin is able to enhance the expression of genes involved in other DNA repair pathways, including non-homologous end joining (NHEJ) and homologous recombination (HR) [67,68,69].
In addition to DNA repair modulation, melatonin has shown ability to change genes involved in apoptosis. The most common regulatory genes involved in apoptosis following exposure to radiation are Bcl-2 and Bax [70]. After exposure to IR, downregulation of Bcl-2 and upregulation of Bax stimulate apoptosis via stimulation of caspase-3 and release of cytochrome C from the mitochondria. Mohseni et al. evaluated the anti-apoptosis role of melatonin on rat’s peripheral blood lymphocytes. Their results showed that melatonin reduces apoptosis via reduction of bax/bcl-2 ratio. This was more obvious for higher doses of melatonin [71]. Similar results were obtained in an in vitro study by Jang et al. [72]. Khan et al. showed that pre-treatment with melatonin before irradiation with a lethal dose of gamma rays led to 100% survival and preservation of hematopoietic and gastrointestinal systems in mice. They showed that melatonin reversed the upregulation of Bax and p53 as pro-apoptosis genes and elevates Bcl-2 in these organs [73].
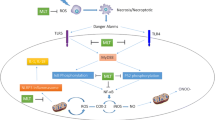
In addition to apoptosis in highly radiosensitive organs such as the bone marrows, necrosis and apoptosis in other organs can initiate some signaling pathways, leading to inflammation and fibrosis. The necrosis to apoptosis ratio has a direct relation with the radiation dose. Therefore, with increasing dose as can be seen in stereotactic techniques, the incidence of necrosis and inflammation also increases. This is very crucial for some organs such as lung, heart, brain, skin, gastrointestinal and vascular. Melatonin is able to reduce the incidence of necrosis and subsequent inflammatory responses following exposure to IR. Administering melatonin to rats has shown amelioration of neuronal necrosis and degeneration, leading to reduction of edema and histopathological changes in the brain [74]. NF-κB/NLRP3 inflammasome pathway is an important signaling pathway involved in the secretion of IL-1 and inflammatory mediators following radiotherapy. Animal studies have shown that melatonin through blunting of this pathway alleviates mucositis, attenuates accumulation of inflammatory cells, and reduces bleeding in the intestine and tongue [75, 76]. The protective effect of melatonin on radiotherapy induced mucositis has been confirmed for head and neck cancer patients without any interruption on treatment outcomes [77]. It has also been confirmed that melatonin can alleviate radiation-induced inflammatory and fibrosis markers in the lung, heart, skin, and brain [78,79,80,81] (Table 1; Fig. 1).
Mechanisms of radioprotection by melatonin. Melatonin is able to neutralize-free radicals that are produced by IR or pro-oxidant enzymes. Furthermore, it suppresses inflammatory signaling pathways in different levels, leading to alleviation of long lasting effects of radiation. Melatonin via inhibition of chronic oxidative and nitrative DNA damage improves genomic stability, thereby reducing the risk of carcinogenesis
Melatonin as a radiosensitizer
In addition to the potent radioprotective effects of melatonin, some studies in recent years have proposed that melatonin is able to increase the therapeutic effects of radiotherapy as well as chemotherapy. The most important concern for clinical application of radioprotectors is the possible protection of tumor cells, although melatonin has proved differently and, hence, facilitating its clinical use for cancer patients undergoing radiotherapy. Although, studies involving the use of melatonin as a radiosensitizer are very limited, some experiments have shown promising results.
Escames et al. showed that melatonin has a synergic effect on the therapeutic outcomes of chemotherapy and radiotherapy on head and neck cancer cells. They used Cal-27 cells to induce tumor xenografts in nude mice. The tumor xenografts were irradiated with 8 Gy followed by apoptosis and proliferation evaluation. Results showed the inhibition of tumor growth in this in vivo tumor xenograft model. Moreover, this study showed that in in vitro models of Cal-27 and SCC-9 tongue cell lines, treatment with melatonin had a synergic effect on the outcomes of chemotherapy and radiotherapy [82]. Although studies on the synergic effect of melatonin on the responses of cancer cells to IR are very limited, various studies have shown that melatonin can improve tumor response to therapy. The best example is therapeutic effects of melatonin on breast and prostate cancer cells.
Evidences on synergic effects of melatonin with radiotherapy
Reduction of DNA repair capacity in tumor cells
Several evidences have revealed that DNA damage responses in tumor cells potently impact therapeutic efficacy. Studies have been conducted to target DNA repair mechanisms in cancer cells, thus improving the therapeutic response to radiotherapy or chemotherapy [83]. Gonzalez et al. showed that pre-treatment with melatonin attenuates DNA repair response following exposure to radiation in MCF-7 cancer cells. In this study, MCF-7 human breast cancer cells were incubated in different concentrations of melatonin for 1 week. After irradiation, cells were cultured in same concentrations for 3 or 6 days, followed by evaluation of proliferation and DNA damage response. Results showed that pre-treatment with melatonin reduces the proliferation of MCF-7 cells, while post-treatment had no effect. The most potent effect for different doses of melatonin was found in 1 nM. Further analysis showed that pre-treatment with melatonin led to a decrease in the number of cells in S phase. In addition, treatment with melatonin inhibited the expression of RAD51 and DNA-PKcs compared to irradiation of cells only. Similar to cell proliferation, 1 nM was more effective for the suppression of DNA repair enzymes [84].
Switch from glycolysis to oxidative phosphorylation
One of the most important differences between normal and tumor cells is the difference in metabolism. Energy production by normal cells is mainly due to cancer cells’ dependence on oxidative phosphorylation, while glycolysis is the main source of ATP production in cancer cells to maintain proliferation and survival. Some studies proposed that abnormal increase in glycolysis is as a result of mutations in mitochondrial DNA (mtDNA), hypoxia in tumor cells as well as oncogenes [85, 86]. Increased glycolysis to oxidative phosphorylation ratio in tumor cells is associated with tumor resistance to therapy procedures such as radiotherapy [87]. On the other hand, some studies have shown that a switch from glycolysis to oxidative phosphorylation can increase therapeutic ratio [88]. For example, increased oxidative phosphorylation augments apoptosis through TRAIL (TNF-related apoptosis-inducing ligand) in mantle cell lymphoma cells [89]. Melanoma cells have also shown similar results [90]. A study by Escames et al. showed that melatonin is able to stimulate oxidative phosphorylation in Cal-27 cell lines. They evaluated the treatment of head and neck cancer cells and also treated tumor-bearing mice with melatonin. Results of this study showed that treatment with melatonin induces aerobic metabolism and reduces glycolysis, leading to elevated mitochondrial ROS production and decreased proliferation [91].
Modulation of estrogen biosynthesis
Biosynthesis of estrogen has a key role in the development of breast cancer. Estrogen is able to stimulate cell proliferation via their receptor and also provokes genomic instability through stimulation of metabolism [92]. These changes are associated with tumorigenesis in breast epithelial cells [93]. On the other hand, some reports suggest that the low level of melatonin in women is related to higher risk of breast cancer incidence [94]. Moreover, melatonin has been proposed as a potent agent against initiation and development of breast cancer [95]. Studies have reported that melatonin through its MT1 receptor inhibits estrogen receptor alpha (ERα) in human breast cancer cells [96, 97]. In addition, melatonin disrupts the binding of ERα-calmodulin (CaM) complex to DNA as well as preventing the transcription of ERα in MCF-7 cells [98, 99].
González et al. showed that melatonin through changes in estrogen biosynthesis can sensitize human MCF-7 cells to ionizing radiation. At first, MCF-7 cells were incubated for 7 days in different concentrations of melatonin. After irradiation, cells were cultured for 1 week and then the effect of melatonin on aromatase (an enzyme responsible for biosynthesis of estrogen) regulation was evaluated. Results indicated that after irradiating the cells, the activity of aromatase was suppressed by 40%. Interestingly, treatment of MCF-7 cells with melatonin had a synergic effect on the inhibition of aromatase activity. They showed that treatment with 1 mM or 1 nM before exposure to radiation can suppress aromatase activity up to 70%. However, treatment of cells with 10 μM of melatonin also had synergic effect. However, it was less effective compared to 1 mM or 1 nM. This study showed the ability of melatonin to suppress other major sources of estrogen in breast cancer such as estrone sulfatase and 17β-Hydroxysteroid dehydrogenase 1. Results showed that increased inhibition of these enzymes was associated with decreased cell survival [100]. A possible pathway for the suppression of aromatase by melatonin is cyclooxygenase-2 (COX-2) [101]. Targeting COX-2 has been proposed for sensitization of various types of cancers, as well as mitigation of normal tissues [102].
Stimulation of p53 and apoptosis
In addition to the protection of normal cells against cell death, some studies have shown that melatonin has ability to induce apoptosis in cancer cells. p53 plays a pivotal role in inducing apoptosis in tumor cells. Due to the mutated form of p53 in most cancers, these cells can escape from apoptosis, leading to tumorigenesis. Stimulation of p53 gene regulation has been proposed as a strategy for sensitization of cancer cells to apoptosis. Melatonin stimulates apoptosis via upregulation of p53. This is mainly mediated by inhibition of MDM2. In addition, suppression of sirt1 by melatonin is another pathway for inducing apoptosis in cancer cells [103, 104]. Cucina et al. showed that there are two different pathways for apoptosis in MCF-7 cells, including early p53/MDM2 dependent and late TGF-β dependent pathways. Furthermore, they showed that melatonin can induce apoptosis through both mentioned pathways [105]. Melatonin can augment the induction of apoptosis in cancer cells by doxorubicin or other chemotherapy agents such as docetaxel 5-fluorouracil (5-FU) and cisplatin [106,107,108].
González et al. evaluated the effect of 1 mM, 10 μM or 1 nM of melatonin on p53 gene expression in MCF-7 cells following exposure to radiation. Cells were treated with melatonin for 7 days and then irradiated on the 8th day. Six hours after irradiation, cells were cultured and the expression of p53 was detected. Results showed that irradiation alone caused twofold increase in p53 gene expression. Furthermore, all used melatonin doses caused more upregulation of p53 compared to irradiation alone. Interestingly, the physiological concentration of 1 nM melatonin was more effective in the upregulation of p53 compared to 1 mM or 10 μM. These changes were associated with decreased cell survival [100]. An in vitro study by Jang et al. revealed the effect of melatonin treatment on apoptosis induction in two normal and tumoral cell lines. They revealed that the addition of melatonin to mice splenocyte cells reduced the induction of apoptosis due to increased Bcl-2 and reduced p53 and Bax expression. By contrast, when Jurkat leukemia cells were treated with melatonin before irradiation, the induction of apoptosis and p53 increased [72].
In addition to p53, COX-2 inhibition by melatonin is involved in the stimulation of apoptosis by melatonin [19]. In inflammatory conditions, due to radiation exposure, the expression of COX-2 as well as other anti-apoptotic genes like iNOS and NF-κB increases [75]. Inhibition of these inflammatory genes has been shown to sensitize tumor cells to therapeutic strategies such as radiotherapy and chemotherapy [109,110,111,112,113]. COX-2 pathway inhibition by melatonin has a synergic effect on the therapeutic effects of some other agents such as curcumin, fisetin, berberine and ursolic acid [114,115,116,117]. As a result of melatonin been a potent inhibitor of COX-2 and other related genes, it can sensitize COX-2 positive cancer cells through inhibition of COX-2 [118].
Other possible mechanisms
Stimulation of cytotoxic immune cells against tumor
Studies have shown that melatonin has the ability to enhance the activities of immune cells, including lymphocyte T and B, and natural killer (NK) cells. It has been shown that melatonin through various mechanisms such as stimulation of proliferation of lymphocyte T, prolonged survival and increase in antigen presentation by macrophages boosts both innate and adaptive immunity [119,120,121]. An in vivo study has shown that treatment of tumor-bearing mice with melatonin led to a rise in NK cell numbers and consequently increased survival [122]. The release of IL-2 by NK cells plays a key role in anti-tumor activities of these cells [123]. There is a need to study the possible synergic effect of melatonin and radiotherapy through stimulation of cytotoxic T and NK cells.
Suppression of inflammation and angiogenesis in tumor
Inflammation plays a key role in tumor resistance via stimulation of pro-angiogenesis factors such as COX-2, growth factors such as vascular endothelial growth factor (VEGF), and increasing number of macrophages and T regulatory (Tregs) cells [79]. Both in vitro and in vivo studies have shown that melatonin decreases the growth of tumor cells via inhibition of VEGF and other growth factors like endothelin-1, hypoxia-inducible factor-1α (HIF-1α), epidermal growth factor receptor (EGFR), and insulin-like growth factor 1 (IGF-1) [124,125,126,127]. As exposure to a high dose of IR is associated with an increase in inflammatory cells and angiogenesis factors, modulation of this phenomenon has been proposed as a strategy for tumor sensitization in radiotherapy [128] (Table 2; Fig. 2).
Mechanisms of radiosensitive effect of melatonin on cancer cells. Melatonin at different levels can inhibit angiogenesis and tumor resistance, leading to the inhibition of tumor cell proliferation. Moreover, it is possible that melatonin via activation of NK cells and cytotoxic lymphocytes is involved in the direct actions against cancer
Conclusion
Melatonin is a low toxic antioxidant and anti-inflammatory agent that has shown oncostatic effects in several studies. As mentioned in this review, melatonin is not just a simple antioxidant, it also has both radioprotective and radiosensitive effects. In contrast to other conventional antioxidants, melatonin can modulate radiation responses via several pathways. In addition to direct neutralization of free radicals, melatonin upregulates antioxidant enzymes and suppresses pro-oxidant enzymes. Melatonin is a potent stimulator of DNA repair responses such as BER pathway genes. These properties of melatonin can help alleviate acute reactions during radiotherapy in highly radiosensitive organs such as bone marrow, skin and gastrointestinal system. Melatonin is a potent anti-inflammatory agent that has been proposed for managing late effects of radiotherapy. It can modulate inflammation in different levels. For example, it reduces necrosis and apoptosis induction, suppresses TLRs, inhibits secretion of prostaglandins via COX-2 inhibition, and alleviates fibrosis via suppression of pro-fibrotic enzymes. Moreover, through inhibition of continuous NO production by iNOS, melatonin reduces genomic instability and risk of second primary cancers. In addition to the radioprotective properties of melatonin, some studies have revealed that it has a synergic effect with radiation on tumor cells. The most obvious example for radiosensitive effect of melatonin is its effect on breast cancer cells. Melatonin via suppression of estrogen in this cell can inhibit tumor proliferation and growth. Suppression of glycolysis and increase in oxidative phosphorylation by melatonin further inhibit tumor growth. Melatonin also promotes apoptosis in cancer cells through upregulation of p53 and TRAIL ligand. In addition to these mechanisms, other immune system mechanisms are involved in tumor suppressive property of melatonin. As this review has shown, so far, several studies have been conducted showing the radioprotective effects of melatonin in different cell lines as well as various organs in murine. However, studies evaluating the radiosensitive effects of melatonin are very limited. Hence, further studies are needed to illustrate the radiosensitive effect of melatonin especially through modulation of immune system cells such as NK and T cells. Moreover, it is necessary to define the effect of treatment with melatonin on angiogenesis in both in vitro and in vivo studies. Clinical radioprotective effect of melatonin for amelioration of dermatitis is at the first phase in breast cancer patients. However, so far no study has evaluated its possible radiosensitive effect. In future, evaluating the radiosensitive effect of melatonin on breast cancer may be an interesting idea for clinical applications. The possible radioprotective and radiosensitive effects of melatonin on same tissue/tumor increase therapeutic ratio and clinical justification for its use as an adjuvant in radiotherapy.
References
Liauw SL, Connell PP, Weichselbaum RR. New paradigms and future challenges in radiation oncology: an update of biological targets and technology. Sci Transl Med. 2013;5(173):173sr2-sr2. https://doi.org/10.1126/scitranslmed.3005148.
Allison R, Dicker A. Minimizing morbidity in radiation oncology: a special issue from future oncology. Future Oncol. 2014;10(15):2303–5. https://doi.org/10.2217/fon.14.195.
Narmani A, Farhood B, Haghi-Aminjan H, Mortezazadeh T, Aliasgharzadeh A, Mohseni M, et al. Gadolinium nanoparticles as diagnostic and therapeutic agents: their delivery systems in magnetic resonance imaging and neutron capture therapy. J Drug Deliv Sci Technol. 2018;44:457–66. https://doi.org/10.1016/j.jddst.2018.01.011.
Bagheri H, Rezapour S, Najafi M, Motevaseli E, Shekarchi B, Cheki M et al. Protection against radiation-induced micronuclei in rat bone marrow erythrocytes by Curcumin and selenium l-methionine. Iran J Med Sci. 2018.
Najafi M, Motevaseli E, Shirazi A, Geraily Gh, Rezaeyan A, Norouzi F et al. Mechanisms of inflammatory responses to radiation and normal tissues toxicity: clinical implications. Int J Radiat Biol. 2018;94(4):335–356.
Bourhis J, Rosine D. Radioprotective effect of amifostine in patients with head and neck squamous cell carcinoma. Semin Oncol. 2002;29(6 Suppl 19):61–2. https://doi.org/10.1053/sonc.2002.37349.
Rosenthal DI, Chambers MS, Weber RS, Eisbruch A. A phase II study to assess the efficacy of amifostine for submandibular/sublingual salivary sparing during the treatment of head and neck cancer with intensity modulated radiation therapy for parotid salivary sparing. Semin Oncol. 2004;31(6 Suppl 18):25–8. https://doi.org/10.1053/j.seminoncol.2004.12.008.
Gu J, Zhu S, Li X, Wu H, Li Y, Hua F. Effect of amifostine in head and neck cancer patients treated with radiotherapy: a systematic review and meta-analysis based on randomized controlled trials. PLoS One. 2014;9(5):e95968. https://doi.org/10.1371/journal.pone.0095968.
Buntzel J, Glatzel M, Schuth J, Weinaug R, Kuttner K, Frohlich D. Cytoprotection with amifostine in the framework of radiochemotherapy in previously irradiated head and neck carcinoma. Strahlenther Onkol. 1999;175(Suppl 4):37–40.
Rades D, Fehlauer F, Bajrovic A, Mahlmann B, Richter E, Alberti W. Serious adverse effects of amifostine during radiotherapy in head and neck cancer patients. Radiother Oncol. 2004;70(3):261–4. https://doi.org/10.1016/j.radonc.2003.10.005.
Thorstad WL, Chao KS, Haughey B. Toxicity and compliance of subcutaneous amifostine in patients undergoing postoperative intensity-modulated radiation therapy for head and neck cancer. Semin Oncol. 2004;31(6 Suppl 18):8–12. https://doi.org/10.1053/j.seminoncol.2004.12.005.
Chao KS, Ozyigit G, Thorsdad WL. Toxicity profile of intensity-modulated radiation therapy for head and neck carcinoma and potential role of amifostine. Semin Oncol. 2003;30(6 Suppl 18):101–8.
Singh VK, Fatanmi OO, Wise SY, Newman VL, Romaine PL, Seed TM. The potentiation of the radioprotective efficacy of two medical countermeasures, gamma-tocotrienol and amifostine, by a combination prophylactic modality. Radiat Prot Dosim. 2016;172(1–3):302–10.
Ghobadi A, Shirazi A, Najafi M, Kahkesh MH, Rezapoor S. Melatonin ameliorates radiation-induced oxidative stress at targeted and nontargeted lung tissue. J Med Phys. 2017;42(4):241.
Martínez-Campa C, Menéndez-Menéndez J, Alonso-González C, González A, Álvarez-García V, Cos S. What is known about melatonin, chemotherapy and altered gene expression in breast cancer. Oncol Lett. 2017;13(4):2003–14. https://doi.org/10.3892/ol.2017.5712.
Lissoni P. Biochemotherapy with immunomodulating pineal hormones other than melatonin: 5-methoxytryptamine as a new oncostatic pineal agent. Pathol Biol (Paris). 2007;55(3–4):198–200. https://doi.org/10.1016/j.patbio.2006.12.008.
Yahyapour R, Shabeeb D, Cheki M, Musa AE, Farhood B, Rezaeyan A et al. Radiation protection and mitigation by natural antioxidants and flavonoids; implications to radiotherapy and radiation disasters. Curr Mol Pharmacol. 2018. https://doi.org/10.2174/1874467211666180619125653.
Chen C-Q, Fichna J, Bashashati M, Li Y-Y, Storr M. Distribution, function and physiological role of melatonin in the lower gut. World J Gastroenterol WJG. 2011;17(34):3888–98. https://doi.org/10.3748/wjg.v17.i34.3888.
Talib WH. Melatonin and cancer hallmarks. Molecules. 2018. https://doi.org/10.3390/molecules23030518.
Yu G-M, Kubota H, Okita M, Maeda T. The anti-inflammatory and antioxidant effects of melatonin on LPS-stimulated bovine mammary epithelial cells. PLoS One. 2017;12(5):e0178525. https://doi.org/10.1371/journal.pone.0178525.
Favero G, Franceschetti L, Bonomini F, Rodella LF, Rezzani R. Melatonin as an anti-inflammatory agent modulating inflammasome activation. Int J Endocrinol. 2017;2017:1835195. https://doi.org/10.1155/2017/1835195.
Volt H, Garcia JA, Doerrier C, Diaz-Casado ME, Guerra-Librero A, Lopez LC, et al. Same molecule but different expression: aging and sepsis trigger NLRP3 inflammasome activation, a target of melatonin. J Pineal Res. 2016;60(2):193–205. https://doi.org/10.1111/jpi.12303.
Yu H, Dickson EJ, Jung S-R, Koh D-S, Hille B. High membrane permeability for melatonin. J Gen Physiol. 2016;147(1):63–76. https://doi.org/10.1085/jgp.201511526.
Vasin MV, Ushakov IB. Comparative efficacy and the window of radioprotection for adrenergic and serotoninergic agents and aminothiols in experiments with small and large animals. J Radiat Res. 2015;56(1):1–10. https://doi.org/10.1093/jrr/rru087.
Mozdarani H. Biological complexities in radiation carcinogenesis and cancer radiotherapy: impact of new biological paradigms. Genes (Basel). 2012;3(1):90–114. https://doi.org/10.3390/genes3010090.
Najafi M, Cheki M, Rezapoor S, Geraily G, Motevaseli E, Carnovale C, et al. Metformin: prevention of genomic instability and cancer: a review. Mutat Res. 2018;827:1–8. https://doi.org/10.1016/j.mrgentox.2018.01.007.
Manda K, Glasow A, Paape D, Hildebrandt G. Effects of ionizing radiation on the immune system with special emphasis on the interaction of dendritic and T cells. Front Oncol. 2012;2:102. https://doi.org/10.3389/fonc.2012.00102.
Hekim N, Cetin Z, Nikitaki Z, Cort A, Saygili EI. Radiation triggering immune response and inflammation. Cancer Lett. 2015;368(2):156–63. https://doi.org/10.1016/j.canlet.2015.04.016.
Rodel F, Frey B, Multhoff G, Gaipl U. Contribution of the immune system to bystander and non-targeted effects of ionizing radiation. Cancer Lett. 2015;356(1):105–13. https://doi.org/10.1016/j.canlet.2013.09.015.
Yahyapour R, Amini P, Rezapour S, Cheki M, Rezaeyan A, Farhood B, et al. Radiation-induced inflammation and autoimmune diseases. Mil Med Res. 2018;5(1):9. https://doi.org/10.1186/s40779-018-0156-7.
Yahyapour R, Motevaseli E, Rezaeyan A, Abdollahi H, Farhood B, Cheki M, et al. Mechanisms of radiation bystander and non-targeted effects: implications to radiation carcinogenesis and radiotherapy. Curr Radiopharm. 2018;11(1):34–45. https://doi.org/10.2174/1874471011666171229123130.
Yahyapour R, Motevaseli E, Rezaeyan A, Abdollahi H, Farhood B, Cheki M et al. Reduction–oxidation (redox) system in radiation-induced normal tissue injury: molecular mechanisms and implications in radiation therapeutics. Clin Transl Oncol. 2018;20(8):975–988. https://doi.org/10.1007/s12094-017-1828-6.
Yahyapour R, Amini P, Rezapoor S, Rezaeyan A, Farhood B, Cheki M et al. Targeting of inflammation for radiation protection and mitigation. Curr Mol Pharmacol. 2018;11(3):203–210
Holley AK, Miao L, St. Clair DK, St. Clair WH. Redox-modulated phenomena and radiation therapy: the central role of superoxide dismutases. Antioxid Redox Signal. 2014;20(10):1567–89. https://doi.org/10.1089/ars.2012.5000.
Miao L, Holley AK, Zhao Y, St. Clair DK, St. Clair WH. Redox-mediated and ionizing-radiation-induced inflammatory mediators in prostate cancer development and treatment. Antioxid Redox Signal. 2014;20(9):1481–500. https://doi.org/10.1089/ars.2013.5637.
Castellani P, Balza E, Rubartelli A. Inflammation, DAMPs, tumor development, and progression: a vicious circle orchestrated by redox signaling. Antioxid Redox Signal. 2014;20(7):1086–97. https://doi.org/10.1089/ars.2012.5164.
Vijayalaxmi, Reiter RJ, Herman TS, Meltz ML. Melatonin reduces gamma radiation-induced primary DNA damage in human blood lymphocytes. Mutat Res. 1998;397(2):203–8.
Vijayalaxmi, Reiter RJ, Meltz ML, Herman TS. Melatonin: possible mechanisms involved in its ‘radioprotective’ effect. Mutat Res Fundam Mol Mech Mutagen. 1998;404(1):187–9. https://doi.org/10.1016/S0027-5107(98)00112-2.
Vijayalaxmi, Reiter RJ, Tan DX, Herman TS, Thomas CR Jr. Melatonin as a radioprotective agent: a review. Int J Radiat Oncol Biol Phys. 2004;59(3):639–53. https://doi.org/10.1016/j.ijrobp.2004.02.006.
Galano A, Tan D-X, Reiter RJ. Melatonin: a versatile protector against oxidative DNA damage. Molecules. 2018;23(3):530.
Reiter RJ, Tan DX, Manchester LC, Qi W. Biochemical reactivity of melatonin with reactive oxygen and nitrogen species: a review of the evidence. Cell Biochem Biophys. 2001;34(2):237–56. https://doi.org/10.1385/cbb:34:2:237.
Karbownik M, Reiter RJ. Antioxidative effects of melatonin in protection against cellular damage caused by ionizing radiation. Proc Soc Exp Biol Med. 2000;225(1):9–22.
Tan DX, Reiter RJ, Manchester LC, Yan MT, El-Sawi M, Sainz RM, et al. Chemical and physical properties and potential mechanisms: melatonin as a broad spectrum antioxidant and free radical scavenger. Curr Top Med Chem. 2002;2(2):181–97.
Karslioglu I, Ertekin MV, Taysi S, Kocer I, Sezen O, Gepdiremen A, et al. Radioprotective effects of melatonin on radiation-induced cataract. J Radiat Res. 2005;46(2):277–82.
Cakmak Karaer I, Simsek G, Yildiz A, Vardi N, Polat A, Tanbek K, et al. Melatonin’s protective effect on the salivary gland against ionized radiation damage in rats. J Oral Pathol Med. 2016;45(6):444–9. https://doi.org/10.1111/jop.12386.
Koc M, Taysi S, Buyukokuroglu ME, Bakan N. Melatonin protects rat liver against irradiation-induced oxidative injury. J Radiat Res. 2003;44(3):211–5.
Sener G, Jahovic N, Tosun O, Atasoy BM, Yegen BC. Melatonin ameliorates ionizing radiation-induced oxidative organ damage in rats. Life Sci. 2003;74(5):563–72.
Sener G, Atasoy BM, Ersoy Y, Arbak S, Sengoz M, Yegen BC. Melatonin protects against ionizing radiation-induced oxidative damage in corpus cavernosum and urinary bladder in rats. J Pineal Res. 2004;37(4):241–6. https://doi.org/10.1111/j.1600-079X.2004.00161.x.
Koc M, Taysi S, Emin Buyukokuroglu M, Bakan N. The effect of melatonin against oxidative damage during total-body irradiation in rats. Radiat Res. 2003;160(2):251–5.
Barlas AM, Sadic M, Atilgan HI, Bag YM, Onalan AK, Yumusak N, et al. Melatonin: a hepatoprotective agent against radioiodine toxicity in rats. Bratisl Lek Listy. 2017;118(2):95–100. https://doi.org/10.4149/bll_2017_020.
McDonald JT, Kim K, Norris AJ, Vlashi E, Phillips TM, Lagadec C, et al. Ionizing radiation activates the Nrf2 antioxidant response. Cancer Res. 2010;70(21):8886–95. https://doi.org/10.1158/0008-5472.can-10-0171.
Jiang Y, Chen X, Tian W, Yin X, Wang J, Yang H. The role of TGF-beta1-miR-21-ROS pathway in bystander responses induced by irradiated non-small-cell lung cancer cells. Br J Cancer. 2014;111(4):772–80. https://doi.org/10.1038/bjc.2014.368.
Tian W, Yin X, Wang L, Wang J, Zhu W, Cao J, et al. The key role of miR-21-regulated SOD2 in the medium-mediated bystander responses in human fibroblasts induced by alpha-irradiated keratinocytes. Mutat Res. 2015;780:77–85. https://doi.org/10.1016/j.mrfmmm.2015.08.003.
Janjetovic Z, Jarrett SG, Lee EF, Duprey C, Reiter RJ, Slominski AT. Melatonin and its metabolites protect human melanocytes against UVB-induced damage: involvement of NRF2-mediated pathways. Sci Rep. 2017;7(1):1274. https://doi.org/10.1038/s41598-017-01305-2.
Guo Y, Sun J, Li T, Zhang Q, Bu S, Wang Q, et al. Melatonin ameliorates restraint stress-induced oxidative stress and apoptosis in testicular cells via NF-κB/iNOS and Nrf2/HO-1 signaling pathway. Sci Rep. 2017;7:9599. https://doi.org/10.1038/s41598-017-09943-2.
Amini P, Mirtavoos-Mahyari H, Motevaseli E, Shabeeb D, Musa AE, Cheki M et al. Mechanisms for radioprotection by melatonin; can it be used as a radiation countermeasure?. Curr Mol Pharmacol. 2018. https://doi.org/10.2174/1874467211666180802164449.
Brand RM, Epperly MW, Stottlemyer JM, Skoda EM, Gao X, Li S, et al. A topical mitochondria-targeted redox-cycling nitroxide mitigates oxidative stress-induced skin damage. J Investig Dermatol. 2017;137(3):576–86. https://doi.org/10.1016/j.jid.2016.09.033.
Kam WW, Banati RB. Effects of ionizing radiation on mitochondria. Free Radic Biol Med. 2013;65:607–19. https://doi.org/10.1016/j.freeradbiomed.2013.07.024.
Farhood B, Goradel NH, Mortezaee K, Khanlarkhani N, Salehi E, Nashtaei MS, et al. Intercellular communications-redox interactions in radiation toxicity; potential targets for radiation mitigation. J Cell Commun Signal. 2018. https://doi.org/10.1007/s12079-018-0473-3.
Atkinson J, Kapralov AA, Yanamala N, Tyurina YY, Amoscato AA, Pearce L, et al. A mitochondria-targeted inhibitor of cytochrome c peroxidase mitigates radiation-induced death. Nat Commun. 2011;2:497. https://doi.org/10.1038/ncomms1499.
Rwigema J-CM, Beck B, Wang W, Doemling A, Epperly MW, Shields D, et al. Two strategies for the development of mitochondrial-targeted small molecule radiation damage mitigators. Int J Radiat Oncol Biol Phys. 2011;80(3):860–8. https://doi.org/10.1016/j.ijrobp.2011.01.059.
Reiter RJ, Tan DX, Mayo JC, Sainz RM, Leon J, Czarnocki Z. Melatonin as an antioxidant: biochemical mechanisms and pathophysiological implications in humans. Acta Biochim Pol. 2003;50(4):1129–46 (0350041129).
Najafi M, Shirazi A, Motevaseli E, Rezaeyan AH, Salajegheh A, Rezapoor S. Melatonin as an anti-inflammatory agent in radiotherapy. Inflammopharmacology. 2017;25(4):403–13. https://doi.org/10.1007/s10787-017-0332-5.
Vijayalaxmi, Reiter RJ, Herman TS, Meltz ML. Melatonin and radioprotection from genetic damage: in vivo/in vitro studies with human volunteers. Mutat Res. 1996;371(3–4):221–8.
Rostami A, Moosavi SA, Dianat Moghadam H, Bolookat ER. Micronuclei assessment of the radioprotective effects of melatonin and vitamin C in human lymphocytes. Cell J. 2016;18(1):46–51.
Rezapoor S, Shirazi A, Abbasi S, Bazzaz JT, Izadi P, Rezaeejam H, et al. Modulation of radiation-induced base excision repair pathway gene expression by melatonin. J Med Phys. 2017;42(4):245–50.
Valizadeh M, Shirazi A, Izadi P, Tavakkoly Bazzaz J, Rezaeejam H. Expression levels of two DNA repair-related genes under 8 Gy ionizing radiation and 100 mg/kg melatonin delivery in rat peripheral blood. J Biomed Phys Eng. 2017;7(1):27–36.
Valizadeh M, Shirazi A, Izadi P, Bazzaz JT, Rezaeejam H, Tabesh GA. Effects of melatonin on repair of DNA double strand breaks caused by ionizing radiation in rat peripheral blood. 2016.
Rezaeejam H, Shirazi A, Izadi P, Bazzaz JT, Ghazi-Khansari M, Valizadeh M, et al. Radioprotective effect of melatonin on expression of Cdkn1a and Rad50 genes in rat peripheral blood. 2018.
Cui YF, Ding YQ, Zhang Y, Xu H, Jin W, Liu XL, et al. Apoptotic characteristics of spleen lymphocyte in mice irradiated by lethal dose and its relationship to the expression of Bax and Bcl-XL proteins. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue. 2005;17(2):109–12.
Mohseni M, Mihandoost E, Shirazi A, Sepehrizadeh Z, Bazzaz JT, Ghazi-khansari M. Melatonin may play a role in modulation of bax and bcl-2 expression levels to protect rat peripheral blood lymphocytes from gamma irradiation-induced apoptosis. Mutat Res. 2012;738–739:19–27. https://doi.org/10.1016/j.mrfmmm.2012.08.006.
Jang SS, Kim WD, Park WY. Melatonin exerts differential actions on X-ray radiation-induced apoptosis in normal mice splenocytes and Jurkat leukemia cells. J Pineal Res. 2009;47(2):147–55. https://doi.org/10.1111/j.1600-079X.2009.00694.x.
Khan S, Adhikari JS, Rizvi MA, Chaudhury NK. Melatonin attenuates (60) Co gamma-ray-induced hematopoietic, immunological and gastrointestinal injuries in C57BL/6 male mice. Environ Toxicol. 2017;32(2):501–18. https://doi.org/10.1002/tox.22254.
Erol FS, Topsakal C, Ozveren MF, Kaplan M, Ilhan N, Ozercan IH, et al. Protective effects of melatonin and vitamin E in brain damage due to gamma radiation: an experimental study. Neurosurg Rev. 2004;27(1):65–9. https://doi.org/10.1007/s10143-003-0291-8.
Fernandez-Gil B, Moneim AE, Ortiz F, Shen YQ, Soto-Mercado V, Mendivil-Perez M, et al. Melatonin protects rats from radiotherapy-induced small intestine toxicity. PLoS One. 2017;12(4):e0174474. https://doi.org/10.1371/journal.pone.0174474.
Ortiz F, Acuna-Castroviejo D, Doerrier C, Dayoub JC, Lopez LC, Venegas C, et al. Melatonin blunts the mitochondrial/NLRP3 connection and protects against radiation-induced oral mucositis. J Pineal Res. 2015;58(1):34–49. https://doi.org/10.1111/jpi.12191.
Onseng K, Johns NP, Khuayjarernpanishk T, Subongkot S, Priprem A, Hurst C, et al. Beneficial effects of adjuvant melatonin in minimizing oral mucositis complications in head and neck cancer patients receiving concurrent chemoradiation. J Altern Complement Med. 2017;23(12):957–63. https://doi.org/10.1089/acm.2017.0081.
Ersahin M, Toklu HZ, Cetinel S, Yuksel M, Yegen BC, Sener G. Melatonin reduces experimental subarachnoid hemorrhage-induced oxidative brain damage and neurological symptoms. J Pineal Res. 2009;46(3):324–32. https://doi.org/10.1111/j.1600-079X.2009.00664.x.
Najafi M, Shirazi A, Motevaseli E, Geraily G, Norouzi F, Heidari M, et al. The melatonin immunomodulatory actions in radiotherapy. Biophys Rev. 2017;9(2):139–48. https://doi.org/10.1007/s12551-017-0256-8.
Gurses I, Ozeren M, Serin M, Yucel N, Erkal HS. Histopathological evaluation of melatonin as a protective agent in heart injury induced by radiation in a rat model. Pathol Res Pract. 2014;210(12):863–71. https://doi.org/10.1016/j.prp.2014.08.006.
Aricigil M, Dundar MA, Yucel A, Eryilmaz MA, Aktan M, Alan MA, et al. Melatonin prevents possible radiotherapy-induced thyroid injury. Int J Radiat Biol. 2017;93(12):1350–6. https://doi.org/10.1080/09553002.2017.1397296.
Escames G, Fernández-Gil BI, Guerra-Librero A, Shen Y, García-López S, Florido J et al. PO-089: Melatonin enhances the toxicity of radio- and chemotherapy in head and neck cancer cells. Radiother Oncol.122:43. https://doi.org/10.1016/s0167-8140(17)30223-2.
Gavande NS, VanderVere-Carozza PS, Hinshaw HD, Jalal SI, Sears CR, Pawelczak KS, et al. DNA repair targeted therapy: the past or future of cancer treatment? Pharmacol Ther. 2016;160:65–83. https://doi.org/10.1016/j.pharmthera.2016.02.003.
Alonso-Gonzalez C, Gonzalez A, Martinez-Campa C, Gomez-Arozamena J, Cos S. Melatonin sensitizes human breast cancer cells to ionizing radiation by downregulating proteins involved in double-strand DNA break repair. J Pineal Res. 2015;58(2):189–97. https://doi.org/10.1111/jpi.12205.
Zheng JIE. Energy metabolism of cancer: glycolysis versus oxidative phosphorylation (Review). Oncol Lett. 2012;4(6):1151–7. https://doi.org/10.3892/ol.2012.928.
Yadav N, Chandra D. Mitochondrial DNA mutations and breast tumorigenesis. Biochim Biophys Acta. 2013;1836(2):336–44. https://doi.org/10.1016/j.bbcan.2013.10.002.
Shimura T, Noma N, Sano Y, Ochiai Y, Oikawa T, Fukumoto M, et al. AKT-mediated enhanced aerobic glycolysis causes acquired radioresistance by human tumor cells. Radiother Oncol. 2014;112(2):302–7. https://doi.org/10.1016/j.radonc.2014.07.015.
Yu L, Chen X, Sun X, Wang L, Chen S. The glycolytic switch in tumors: how many players are involved? J Cancer. 2017;8(17):3430–40. https://doi.org/10.7150/jca.21125.
Robinson GL, Dinsdale D, MacFarlane M, Cain K. Switching from aerobic glycolysis to oxidative phosphorylation modulates the sensitivity of mantle cell lymphoma cells to TRAIL. Oncogene. 2012;31:4996. https://doi.org/10.1038/onc.2012.13.
Cao K, Li J, Chen J, Qian L, Wang A, Chen X, et al. microRNA-33a-5p increases radiosensitivity by inhibiting glycolysis in melanoma. Oncotarget. 2017;8(48):83660–72. https://doi.org/10.18632/oncotarget.19014.
Escames G, Guerra-Librero A, Shen Y, Florido J, Sayed R, Molina-Navarro M et al. PO-090: Oncostatic effect of melatonin in head and neck cancer: role of mitochondrial function. Radiother Oncol. 122:43–4. https://doi.org/10.1016/s0167-8140(17)30224-4.
Yue W, Wang J-P, Li Y, Fan P, Liu G, Zhang N, et al. Effects of estrogen on breast cancer development: role of estrogen receptor independent mechanisms. Int J Cancer J Int Cancer. 2010;127(8):1748–57. https://doi.org/10.1002/ijc.25207.
Russo J, Hasan Lareef M, Balogh G, Guo S, Russo IH. Estrogen and its metabolites are carcinogenic agents in human breast epithelial cells. J Steroid Biochem Mol Biol. 2003;87(1):1–25.
Travis RC, Allen DS, Fentiman IS, Key TJ. Melatonin and breast cancer: a prospective study. JNCI. 2004;96(6):475–82. https://doi.org/10.1093/jnci/djh077.
Sabzichi M, Samadi N, Mohammadian J, Hamishehkar H, Akbarzadeh M, Molavi O. Sustained release of melatonin: a novel approach in elevating efficacy of tamoxifen in breast cancer treatment. Colloids Surf B. 2016;145:64–71. https://doi.org/10.1016/j.colsurfb.2016.04.042.
Kiefer T, Ram PT, Yuan L, Hill SM. Melatonin inhibits estrogen receptor transactivation and cAMP levels in breast cancer cells. Breast Cancer Res Treat. 2002;71(1):37–45.
Lopes J, Arnosti D, Trosko JE, Tai M-H, Zuccari D. Melatonin decreases estrogen receptor binding to estrogen response elements sites on the OCT4 gene in human breast cancer stem cells. Genes Cancer. 2016;7(5–6):209–17. https://doi.org/10.18632/genesandcancer.107.
del Rio B, Garcia Pedrero JM, Martinez-Campa C, Zuazua P, Lazo PS, Ramos S. Melatonin, an endogenous-specific inhibitor of estrogen receptor alpha via calmodulin. J Biol Chem. 2004;279(37):38294–302. https://doi.org/10.1074/jbc.M403140200.
Martinez-Campa C, Alonso-Gonzalez C, Mediavilla MD, Cos S, Gonzalez A, Ramos S, et al. Melatonin inhibits both ER alpha activation and breast cancer cell proliferation induced by a metalloestrogen, cadmium. J Pineal Res. 2006;40(4):291–6. https://doi.org/10.1111/j.1600-079X.2006.00315.x.
Alonso-Gonzalez C, Gonzalez A, Martinez-Campa C, Menendez-Menendez J, Gomez-Arozamena J, Garcia-Vidal A, et al. Melatonin enhancement of the radiosensitivity of human breast cancer cells is associated with the modulation of proteins involved in estrogen biosynthesis. Cancer Lett. 2016;370(1):145–52. https://doi.org/10.1016/j.canlet.2015.10.015.
Martinez-Campa C, Gonzalez A, Mediavilla MD, Alonso-Gonzalez C, Alvarez-Garcia V, Sanchez-Barcelo EJ, et al. Melatonin inhibits aromatase promoter expression by regulating cyclooxygenases expression and activity in breast cancer cells. Br J Cancer. 2009;101(9):1613–9. https://doi.org/10.1038/sj.bjc.6605336.
Cheki M, Yahyapour R, Farhood B, Rezaeyan A, Shabeeb D, Amini P, Rezapoor S, Najafi M. COX-2 in radiotherapy; a potential target for radioprotection and radiosensitization. Curr Mol Pharmacol. 2018;11(3):173–183
Bizzarri M, Proietti S, Cucina A, Reiter RJ. Molecular mechanisms of the pro-apoptotic actions of melatonin in cancer: a review. Expert Opin Ther Targets. 2013;17(12):1483–96. https://doi.org/10.1517/14728222.2013.834890.
Cheng Y, Cai L, Jiang P, Wang J, Gao C, Feng H, et al. SIRT1 inhibition by melatonin exerts antitumor activity in human osteosarcoma cells. Eur J Pharmacol. 2013;715(1–3):219–29. https://doi.org/10.1016/j.ejphar.2013.05.017.
Cucina A, Proietti S, D’Anselmi F, Coluccia P, Dinicola S, Frati L, et al. Evidence for a biphasic apoptotic pathway induced by melatonin in MCF-7 breast cancer cells. J Pineal Res. 2009;46(2):172–80. https://doi.org/10.1111/j.1600-079X.2008.00645.x.
Kosar PA, Naziroglu M, Ovey IS, Cig B. Synergic effects of doxorubicin and melatonin on apoptosis and mitochondrial oxidative stress in MCF-7 breast cancer cells: involvement of TRPV1 channels. J Membr Biol. 2016;249(1–2):129–40. https://doi.org/10.1007/s00232-015-9855-0.
Alonso-Gonzalez C, Menendez-Menendez J, Gonzalez-Gonzalez A, Gonzalez A, Cos S, Martinez-Campa C. Melatonin enhances the apoptotic effects and modulates the changes in gene expression induced by docetaxel in MCF7 human breast cancer cells. Int J Oncol. 2018;52(2):560–70. https://doi.org/10.3892/ijo.2017.4213.
Uguz AC, Cig B, Espino J, Bejarano I, Naziroglu M, Rodriguez AB, et al. Melatonin potentiates chemotherapy-induced cytotoxicity and apoptosis in rat pancreatic tumor cells. J Pineal Res. 2012;53(1):91–8. https://doi.org/10.1111/j.1600-079X.2012.00974.x.
Gao Y, Xiao X, Zhang C, Yu W, Guo W, Zhang Z, et al. Melatonin synergizes the chemotherapeutic effect of 5-fluorouracil in colon cancer by suppressing PI3K/AKT and NF-kappaB/iNOS signaling pathways. J Pineal Res. 2017. https://doi.org/10.1111/jpi.12380.
Gore E. Celecoxib and radiation therapy in non-small-cell lung cancer. Oncology (Williston Park). 2004;18(14 Suppl 14):10–4.
Gore E, Bae K, Langer C, Extermann M, Movsas B, Okunieff P, et al. Phase I/II trial of a COX-2 inhibitor with limited field radiation for intermediate prognosis patients who have locally advanced non-small-cell lung cancer: radiation therapy oncology group 0213. Clin Lung Cancer. 2011;12(2):125–30. https://doi.org/10.1016/j.cllc.2011.03.007.
Ganswindt U, Budach W, Jendrossek V, Becker G, Bamberg M, Belka C. Combination of celecoxib with percutaneous radiotherapy in patients with localised prostate cancer—a phase I study. Radiat Oncol (London, England). 2006;1:9. https://doi.org/10.1186/1748-717x-1-9.
Li W, Wang Z, Chen Y, Wang K, Lu T, Ying F, et al. Melatonin treatment induces apoptosis through regulating the nuclear factor-κB and mitogen-activated protein kinase signaling pathways in human gastric cancer SGC7901 cells. Oncol Lett. 2017;13(4):2737–44. https://doi.org/10.3892/ol.2017.5785.
Shrestha S, Zhu J, Wang Q, Du X, Liu F, Jiang J, et al. Melatonin potentiates the antitumor effect of curcumin by inhibiting IKKbeta/NF-kappaB/COX-2 signaling pathway. Int J Oncol. 2017;51(4):1249–60. https://doi.org/10.3892/ijo.2017.4097.
Yi C, Zhang Y, Yu Z, Xiao Y, Wang J, Qiu H, et al. Melatonin enhances the anti-tumor effect of fisetin by inhibiting COX-2/iNOS and NF-kappaB/p300 signaling pathways. PLoS One. 2014;9(7):e99943. https://doi.org/10.1371/journal.pone.0099943.
Wang J, Guo W, Chen W, Yu W, Tian Y, Fu L, et al. Melatonin potentiates the antiproliferative and pro-apoptotic effects of ursolic acid in colon cancer cells by modulating multiple signaling pathways. J Pineal Res. 2013;54(4):406–16. https://doi.org/10.1111/jpi.12035.
Lu JJ, Fu L, Tang Z, Zhang C, Qin L, Wang J, et al. Melatonin inhibits AP-2beta/hTERT, NF-kappaB/COX-2 and Akt/ERK and activates caspase/Cyto C signaling to enhance the antitumor activity of berberine in lung cancer cells. Oncotarget. 2016;7(3):2985–3001. https://doi.org/10.18632/oncotarget.6407.
Woo SM, Min KJ, Kwon TK. Melatonin-mediated Bim up-regulation and cyclooxygenase-2 (COX-2) down-regulation enhances tunicamycin-induced apoptosis in MDA-MB-231 cells. J Pineal Res. 2015;58(3):310–20. https://doi.org/10.1111/jpi.12217.
Pioli C, Caroleo MC, Nistico G, Doria G. Melatonin increases antigen presentation and amplifies specific and non specific signals for T-cell proliferation. Int J Immunopharmacol. 1993;15(4):463–8.
Poon AM, Liu ZM, Pang CS, Brown GM, Pang SF. Evidence for a direct action of melatonin on the immune system. Biol Signals. 1994;3(2):107–17.
Bonilla E, Rodon C, Valero N, Pons H, Chacin-Bonilla L, Garcia Tamayo J, et al. Melatonin prolongs survival of immunodepressed mice infected with the Venezuelan equine encephalomyelitis virus. Trans R Soc Trop Med Hyg. 2001;95(2):207–10.
Currier NL, Miller SC. Echinacea purpurea and melatonin augment natural-killer cells in leukemic mice and prolong life span. J Altern Complement Med. 2001;7(3):241–51. https://doi.org/10.1089/107555301300328115.
Christopher FL, Dussault I, Miller SC. Population dynamics of natural killer cells in the spleen and bone marrow of normal and leukemic mice during in vivo exposure to interleukin-2. Immunobiology. 1991;184(1):37–52. https://doi.org/10.1016/s0171-2985(11)80570-x.
Leon J, Casado J, Jimenez Ruiz SM, Zurita MS, Gonzalez-Puga C, Rejon JD, et al. Melatonin reduces endothelin-1 expression and secretion in colon cancer cells through the inactivation of FoxO-1 and NF-kappabeta. J Pineal Res. 2014;56(4):415–26. https://doi.org/10.1111/jpi.12131.
Cos S, Blask DE. Melatonin modulates growth factor activity in MCF-7 human breast cancer cells. J Pineal Res. 1994;17(1):25–32.
Cos S, Fernandez R, Guezmes A, Sanchez-Barcelo EJ. Influence of melatonin on invasive and metastatic properties of MCF-7 human breast cancer cells. Cancer Res. 1998;58(19):4383–90.
Jardim-Perassi BV, Lourenco MR, Doho GM, Grigolo IH, Gelaleti GB, Ferreira LC, et al. Melatonin regulates angiogenic factors under hypoxia in breast cancer cell lines. Anticancer Agents Med Chem. 2016;16(3):347–58.
Raben D, Helfrich B. Angiogenesis inhibitors: a rational strategy for radiosensitization in the treatment of non-small-cell lung cancer? Clin Lung Cancer. 2004;6(1):48–57. https://doi.org/10.3816/CLC.2004.n.021.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
Informed consent for this article is not required.
Rights and permissions
About this article
Cite this article
Farhood, B., Goradel, N.H., Mortezaee, K. et al. Melatonin as an adjuvant in radiotherapy for radioprotection and radiosensitization. Clin Transl Oncol 21, 268–279 (2019). https://doi.org/10.1007/s12094-018-1934-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-018-1934-0