Abstract
Background
Due to the high rate of febrile neutropenia (FN) with docetaxel–cyclophosphamide (DC) chemotherapy, primary FN prophylaxis is recommended. However, the optimal choice of prophylaxis [i.e., granulocyte-colony stimulating factors (G-CSF) or antibiotics] is unknown. A systematic review was performed to address this knowledge gap.
Methods
Embase, Ovid Medline, Pubmed, the Cochrane database of systematic reviews, and Cochrane register of controlled trials were searched from 1946 to April 2016 for studies evaluating primary prophylactic FN treatments in breast cancer patients receiving DC chemotherapy. Outcome measures evaluated included: incidence of FN and treatment-related hospitalizations, chemotherapy dose reduction/delays/discontinuations, and adverse events. Screening and data collection were performed by two independent reviewers.
Results
Of 2105 identified records, 7 studies (n = 2535) met the pre-specified eligibility criteria. Seven additional studies (n = 621) were identified from prior systematic reviews. There were 3 randomized controlled trials (RCTs) (n = 2256) and 11 retrospective studies (n = 900). Study sample sizes ranged from 30 to 982 patients (median 99.5), evaluating pegfilgrastim (n = 1274), filgrastim (n = 1758), and oral ciprofloxacin (n = 108). Given the heterogeneity of patients and study design, a narrative synthesis of results was performed. Median FN rates with and without primary prophylaxis were 6.6 % (IQR 3.9–10.6 %) and 31.3 % (IQR 25–33 %), respectively. No FN-related deaths were reported. No RCT directly compared G-CSF with antibiotic interventions.
Conclusions
Primary FN prophylaxis reduces the incidence of FN. Despite considerable cost and toxicity differences between G-CSF and antibiotics, there is insufficient data to make a recommendation of one strategy over another.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Docetaxel–cyclophosphamide (DC) is an effective and commonly used chemotherapy regimen in the adjuvant and neoadjuvant settings for patients with early-stage breast cancer [1–5]. DC is also associated with febrile neutropenia (FN), treatment-related hospitalisations as well as chemotherapy dose reductions/delays and discontinuations. These events can affect both treatment efficacy and patient quality of life [6–8]. While the initial randomized study of DC versus doxorubicin-cyclophosphamide (AC) chemotherapy reported FN rates of 5 % with DC, there was no mention of the use of primary FN prophylaxis [5]. Once the use of DC was adopted into broader clinical practice, higher rates of FN were reported [9, 10]. A meta-analysis of non-randomized trials identified that in routine clinical practice, DC chemotherapy without primary FN prophylaxis was associated with FN rates of 29.1 % (95 % CI 23.8–35.2 %), in contrast to 6.8 % (95 % CI 4.6–9.9 %) with primary prophylaxis [10].
As most local [11], national [12], and international [13–15] guideline groups recommend the use of primary FN prophylaxis when studies reported FN rates of >20 %, it is not surprising that most patients now receive some form of primary prophylaxis with DC chemotherapy. The most commonly recommended prophylactic agents are granulocyte-colony stimulating factors (G-CSF) such as filgrastim (Neupogen), PegFilgrastim (Neulasta), or other biosimilars. Despite the authors of the initial randomized study that compared DC with AC having subsequently reported that primary prophylaxis with oral antibiotics had been used by most patients in the trial [4], only one guideline clearly states the use of oral antibiotics as an alternative to G-CSF [11].
Despite the considerable differences in both cost (direct and indirect) and toxicity between subcutaneous G-CSF and oral antibiotics, we are not aware of published high-quality evidence that directs decisions on optimal patient care. In order to establish the strength of evidence underlying current recommendations for primary FN prophylaxis for patients receiving DC chemotherapy, a systematic review was performed.
Methods
Study objective and eligibility criteria
Our systematic review was performed to identify and evaluate G-CSF and antibiotics use for primary FN prophylaxis in patients receiving DC chemotherapy (4–6 cycles of 3-weekly docetaxel 75 mg/m2 and cyclophosphamide 600 mg/m2) for breast cancer. The Population–Intervention–Comparator–Outcome–Study Design (PICOS) framework was used to structure the research question for the review and its corresponding literature search. The population of interest was patients diagnosed with breast cancer receiving DC chemotherapy in the adjuvant or neoadjuvant settings, with or without trastuzumab. Interventions were use of G-CSF, biosimilars, and antibiotics, at any dose or treatment duration. Comparators of relevance included best supportive care or prophylactic antibiotics. Outcome measures of interest included the incidence of FN (defined as an absolute neutrophil count <0.5 × 109/L with oral temperature >38.5 °C or two consecutive readings >38.0 °C for 2 h), treatment-related hospitalizations, chemotherapy dose reductions/delays/discontinuations, FN risk factors, and the frequency of adverse events from primary FN prophylaxis. Randomized and non-randomized, retrospective or prospective studies published in English were included. Animal studies, studies in the metastatic setting, and studies enrolling non-chemotherapy naïve patients were excluded.
Literature search
An information specialist (RS) designed and executed an electronic literature search to identify relevant citations from Embase, Ovid Medline, Pubmed (including in-process and other non-indexed citations), the Cochrane database of systematic reviews, and the Cochrane register of controlled trials from 1946 to April 26, 2016. Search terms and their medical subject heading (MeSH) equivalents are shown in Appendix 1, where the Medline search strategy is provided. Previous systematic reviews were also screened to identify additional studies, as were the bibliographies of included studies.
Study screening and selection
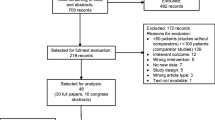
Stage 1 screening consisted of titles and abstracts identified from the literature search by two independent reviewers amongst a participating group of eight (RF, SM, MI, CS, KP, SD, HM, and MC). Disagreements were discussed and resolved between reviewers. Stage 2 screening consisted of a full-text review of all potentially relevant citations identified during stage 1 screening by two independent reviewers (RF, SM, CS, LV, SD, MI, and MC). Results from the screening process are presented in a PRISMA flow diagram (Fig. 1).
Data collection
Data from all included studies were collected using a pre-designed data collection form in Microsoft Excel. All data were extracted independently and subsequently compared for discrepancies, which were resolved by discussion. Data were collected on publication characteristics (year, journal, and authors), patient characteristics (including performance status, median age, disease stage), intervention characteristics (including chemotherapy regimen, neoadjuvant versus adjuvant setting, type, and frequency of G-CSF and antibiotics used) and outcomes of interest (namely the incidence of febrile neutropenia, treatment-related hospitalisations, chemotherapy dose reductions/delays/discontinuation, FN risk factors as well as the frequency of adverse events from primary FN prophylaxis).
Risk of bias assessment
Studies were also independently assessed for risk of bias by two reviewers (RF and SM) using the Cochrane Collaboration’s risk of bias tool for randomized trials [16] which assesses for sources of selection, performance, detection, attrition, reporting, and other sources of bias. Risk of bias was high for one of the included trials due to random sequence generation and allocation concealment [18]. Two studies [19, 24] were judged to have unclear risk of performance and detection bias with blinding outcomes and selective reporting (Appendix 2).
Data analysis
If deemed appropriate, following exploration of study and patient characteristics to ensure sufficient clinical and methodological homogeneities across studies, we planned to conduct meta-analyses using random effects models to combine FN incidence with and without primary prophylaxis data across studies, as described in the Cochrane Handbook [16]. Following inspection of the characteristics of included studies, the research team felt there were considerable clinical and methodological heterogeneities between studies in particular due to patient populations and study design. Meta-analyses were thus not considered appropriate and a narrative summary of each study as well as a descriptive synthesis of common results across studies was developed. Reporting of the review was structured according to recommendations of the PRISMA Statement [17], and a PRISMA checklist was completed (Appendix 3).
Results
Quantity of evidence identified
From 2205 unique citations identified by the electronic search, 77 potentially relevant studies were identified during the first-stage screening of titles and abstracts. The full texts of these 77 studies were subsequently reviewed for the second stage of screening, and 7 were judged to meet the eligibility criteria. In addition, 7 other studies were also included from the inspection of the included study listings from two previous systematic reviews [9, 10] (Table 1). Reasons for study exclusion were: absence of DC chemotherapy (n = 37), lack of breast cancer patient specific data (n = 11), systematic review/review article (n = 5), absence of FN data reported (n = 5), metastatic breast cancer (n = 3), economic analysis (n = 2), multiple chemotherapy regimens used (n = 2), secondary FN prophylaxis (n = 2), no mention of primary prophylaxis with G-CSF or antibiotics (n = 1), duplicate publication (n = 1), and non-breast cancer study (n = 1). Of the 14 included studies, 11 were available as peer-reviewed full journal articles [19–21, 23, 25–31] and 3 were available as meeting abstracts only [18, 22, 24]. The studies were published in 2005 [24], 2009 [23], 2010 [22, 28, 29], 2011 [25–27, 30, 31], 2014 [18, 21], and 2015 [19, 20].
Study characteristics
Overall, a total of 2535 patients were treated with DC (median age 55 years, range 24–74). Characteristics of the individual studies and their results are described in Table 1. Across these studies the median use of primary FN prophylaxis was 48.7 % (range 6–100 %). There were three randomized controlled trials (RCTs) (n = 2256) and 11 retrospective studies (n = 900). Study sample sizes ranged from 30 [28] to 982 [18] patients (median 99.5). The agents investigated were filgrastim (n = 1758), pegfilgrastim (n = 1274), and oral ciprofloxacin (n = 108). Filgrastim was evaluated in 10 studies [18, 20, 21, 25–31], pegfilgrastim in 2 studies [19, 24], and ciprofloxacin in 2 studies [20, 23]. FN risk factors were identified in 6 studies [18, 21, 25, 29–31]. With respect to known risk factors for FN, the proportion of patients >65 years old was 18 % [18], 22 % [30], and 26.8 % [21]. In addition, 28.8, 30, and 37 % of patients were found to have medical comorbidities, such as vascular disease, diabetes, and chronic obstructive pulmonary disease, respectively [25, 29, 31]. None of the included studies reported the use of interim neutrophils counts to guide G-CSF dosing. As there was considerable variability between studies in terms of study design and evaluated outcomes, meta-analysis was considered inappropriate, and a descriptive overview of common results is presented below.
Study-specific overviews and findings
FN rates and primary FN prophylaxis
DC chemotherapy was associated with median FN rates of 6.6 % (IQR 3.9–10.6 %) and 31.3 % (IQR 25–33 %), with and without primary prophylaxis, respectively [18–23, 25–31]. There were no head-to-head randomized studies comparing G-CSF with antibiotic use. One retrospective study compared G-CSF with antibiotic use and found that among the 73 (21 %) patients who did not receive any primary prophylaxis with G-CSF or antibiotics, 23 (32 %) developed FN [20]. Two of 192 patients (1 %; p < 0.0001) who received primary prophylaxis with G-CSF alone, and six of 53 patients (11 %; p < 0.01) who received antibiotic primary prophylaxis alone developed FN. Patients >65 years of age had higher FN rates without primary prophylaxis [18, 21, 30]. As evidence of this, the FN rates without primary prophylaxis were observed in 6.7 % of older patients (>65 years old) versus 4.9 % in the overall population [18]. Similarly, among 26.8 % of older patients, 12.1 % experienced FN versus 10 % of younger patients [21].
Additional endpoints assessed
Data regarding hospital admissions due to FN were available from 5 studies and occurred in 11–31 % (median 13 %) of patients for a median duration of 6 days (range: 2–8) [20–22, 28, 30]. These studies did not include these data separately for patients with and without primary prophylaxis. Four studies provided data on the effects of FN on subsequent chemotherapy administration. As a result of treatment-related FN, 0.6–5 % (median 3.3 %) of patients had delays in subsequent cycles of chemotherapy and 4.6–34 % (median 7.5 %) required dose reductions [20, 21, 30, 31]. Only 2 studies reported chemotherapy discontinuation due to FN, accounting for 0.3 and 25 % of patients, respectively [20, 30]. There were no deaths related to FN. With respect to side effects of primary prophylaxis, only one study reported toxicity profile. Pegfilgrastim was associated with bone pain and back pain in 6.4 and 19.1 % of patients, respectively, and 2.3 and 15 % of patients experienced bone and back pain, respectively, with placebo [19].
Discussion
The current study demonstrates that in the absence of primary FN prophylaxis, DC chemotherapy was associated with median FN rates in excess of 20 %. For this reason, in keeping with most guideline recommendations, most patients will therefore appropriately receive some form of primary FN prophylaxis [13–15]. However, despite oral antibiotics being the recommended primary prophylaxis in the definitive DC versus AC trial, most guidelines tend to recommend the use of G-CSF [4]. This is important due to the considerable cost and toxicity differences between G-CSFs and oral antibiotics.
The cost differences are important from a global healthcare standpoint, with 4 cycles of DC being associated with direct drug costs of $CAD1740 for 10 days of filgrastim (300 mcg vials), $CAD2422 for pegfilgrastim and $35CAD for a 14-day treatment of ciprofloxacin [11]. These costs do not include the charges for a healthcare professional to administer the G-CSF injections. In addition, there are important side effects with these agents. For example, possible side effects of ciprofloxacin include nausea (>2 %), and less common side effects (<1 %) are diarrhea and vomiting [32]. Possible side effects (>10 %) of G-CSF are bone pain, headaches, irritation at injection site, and diarrhea [33]. Of particular note is the potential risk for Clostridium difficile infection with recurrent hospitalisations, immunosuppression, chemotherapy use, enteral or parenteral nutrition, and antibiotic use [34, 35]. However, there are no data for breast cancer patients receiving primary FN prophylaxis for modern day adjuvant chemotherapy regimens.
This is the first systematic review we are aware of to compare G-CSF and antibiotic use in breast cancer patients undergoing DC chemotherapy. Our analysis demonstrated that the DC regimen was associated with median FN rates of 31.3 % (IQR 25–33 %) when primary prophylaxis was not given, in contrast to 6.6 % (IQR 3.9–10.6 %) with the addition of primary prophylaxis. We did not identify any head-to-head prospective, randomized studies comparing G-CSF with antibiotic use. The only identified study that compared the two options was a retrospective study which demonstrated that only two of 192 patients (1 %; p < 0.0001) who received primary prophylaxis with G-CSF alone and six of 53 patients (11 %; p < 0.01) who received antibiotic primary prophylaxis alone developed FN [20].
There are a number of limitations to be noted regarding this systematic review. First and rather surprising is that despite the widespread global use of DC for a decade, there appears to be a paucity of high-quality literature on the incidence, measurement, and treatment of FN with different primary prophylactic regimens. The identified studies also lacked detailed, consistent outcome data, and three were published only in abstract form, which leads to a risk of bias in these trials. In addition, there was no consistent reporting of G-CSF or antibiotic dose or duration. Although our study aimed to compare two standards of care FN primary prophylaxis options (G-CSF and antibiotics), we were unable to find any such trial conducted prospectively. Another interesting limitation is that the definition of FN can vary. For example, In Japan, febrile neutropenia is defined as having an axillary temperature of >37.5 °C and neutropenia showing an absolute neutrophil count (ANC) of <500 cells/mL or an ANC that is expected to reduce to <500 cells/mL during the next 48 h [36]. We are unsure as to whether or these differences on definition would have any significant impact on reported FN rates. Lastly, 7 studies were not identified from the first- and second-pass screening by 2 independent reviewers. These 7 were identified from previous review articles. We are unsure as to the cause of this error in our screening process subsequent review of the screening lists identified 5 of these articles as being present.
Further studies are warranted to determine optimal prophylaxis strategies to guide appropriate patient and drug selection, as well as to prevent and reduce treatment-related toxicities. This is important as oral ciprofloxacin is considerably cheaper than G-CSF. There is currently one clinical trial prospectively looking at these two standards of care regimens for FN primary prophylaxis, specifically in early-stage breast cancer patients undergoing adjuvant DC chemotherapy [37, 38]. With enhanced datasets, it may be a better strategy to identify those patients at greatest risk of FN upfront (e.g., those patients 65 years or older, and those with comorbid conditions) to treat with primary prophylaxis. One study evaluated whether it might be more fiscally responsible to use oral antibiotics as primary prophylaxis and restricting G-CSF use to those patients that develop FN despite oral antibiotics [20]. For example, while some groups suggest primary G-CSF, others note that secondary G-CSF is more effective and less costly than a no G-CSF strategy [12].
Conclusion
This analysis shows that the use of primary FN prophylaxis is associated with a significant reduction in FN rates for patients receiving DC chemotherapy. However, there was an absence of high-quality data supporting an optimal prophylaxis strategy of G-CSF versus oral antibiotics. In the current era of oncology that demands cost-effective evidence-based treatments as well as an increasing emphasis on patient quality of life, it is imperative that more studies are performed to achieve this standard.
References
Fishe B, Jeong JH, Dignam J et al (2001) Findings from recent National Surgical Adjuvant Breast and Bowel Project (NSABP) adjuvant studies in stage I breast cancer. J Natl Cancer Inst Monogr 30:62–66
National Comprehensive Cancer Network. Clinical Practice guidelines in oncology: breast cancer. V2.2013. http://www.nccn.org. Accessed 1 Sept 2016
Rastogi P, Anderson SJ, Bear HD et al (2008) Preoperative chemotherapy: updates of National surgical adjuvant breast and bowel protocols B-18 and B-27. J Clin Oncol 26(5):778–785
Jones S, Holmes FA, O’Shaughnessy J et al (2009) Docetaxel with cyclophosphamide is associated with an overall survival benefit compared with doxorubicin and cyclophosphamide: 7-year follow-up of US Oncology Research Trial 9735. J Clin Oncol 27(8):1177–1183
Jones SE, Savin MA, Holmes FA et al (2006) Phase III trial comparing doxorubicin plus cyclophosphamide with docetaxel plus cyclophosphamide as adjuvant therapy for operable breast cancer. J Clin Oncol 24(34):5381–5387
Culakova E, Thota R, Poniewierski MS et al (2014) Patterns of chemotherapy-associated toxicity and supportive care in US oncology practice: a nationwide prospective cohort study. Cancer Med 2:434–444
Lathia N, Mittmann N, De Angelis C et al (2010) Evaluation of direct medical costs of hospitalization for febrile neutropenia. Cancer 116:742–748
Dinan MA, Hirsch BR, Lyman GH (2015) Management of chemotherapy-induced neutropenia: measuring quality, cost, and value. J Natl Compr Cancer Netw 13:e1–e7
Do T, Medhekar R, Bhat R et al (2015) The risk of febrile neutropenia and need for G-CSF primary prophylaxis with the docetaxel and cyclophosphamide regimen in early-stage breast cancer patients: a meta-analysis. Breast Cancer Res Treat 153(3):591–597
Younis T, Rayson D, Thompson K (2012) Primary G-CSF prophylaxis for adjuvant TC or FEC-D chemotherapy outside of clinical trial settings: a systematic review and meta-analysis. Support Care Cancer 20(10):2523–2530
Cancer Care Ontario (2016) Cancer Canre Ontario GCSF Recommendations 2016. Version March 21, 2016. https://www.cancercare.on.ca/common/pages/UserFile.aspx?fileId=352101. Accessed 5 Aug 2016
Crawford J, Allen J, Armitage J et al (2012) NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) Myeloid Growth Factors. Version 1.2012. https://www.tri-kobe.org/nccn/guideline/hematologic/english/myeloid_growth.pdf. Accessed 10 Aug 2016
Aapro M, Bohlius J, Cameron D et al (2011) 2010 update of EORTC guidelines for the use of granulocyte-colony stimulating factor to reduce the incidence of chemotherapy-induced febrile neutropenia in adult patients with lymphoproliferative disorders and solid tumors. Eur J Cancer 47:8–32
Smith TJ, Bohlke K, Lyman GH et al (2015) Recommendations for the use of WBC growth factors: American Society of Clinical Oncology Clinical Practice Guideline Update. J Clin Oncol 33(28):3199–3212
Skedgel C, Rayson D, Younis T (2016) Is febrile neutropenia prophylaxis with granulocyte-colony stimulating factors economically justified for adjuvant TC chemotherapy in breast cancer? Support Care Cancer 24(1):387–394
Higgins JPT, Green S (ed) (2011) Cochrane handbook for systematic reviews of interventions Version 5.1.0. The Cochrane Collaboration
Moher D, Liberati A, Tetzlaff J et al (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 6(7):e1000097
Gluz O, Hofmann D, Von SR et al (2014) Febrile neutropenia (FN) and infections under adjuvant chemotherapy of breast cancer with 6 × TC vs. 4 × EC-4 × Doc: toxicity data of the WSG planB trial. Oncol Res Treat 37:14–15
Kosaka Y, Rai Y, Masuda N et al (2015) Phase III placebo-controlled, double-blind, randomized trial of pegfilgrastim to reduce the risk of febrile neutropenia in breast cancer patients receiving docetaxel/cyclophosphamide chemotherapy. Support Care Cancer 23(4):1137–1143
Yu JL, Chan K, Kurin M et al (2015) Clinical outcomes and cost-effectiveness of primary prophylaxis of febrile neutropenia during adjuvant docetaxel and cyclophosphamide chemotherapy for breast cancer. Breast J 21(6):658–664
Yerushalmi R, Goldvaser H, Sulkes A et al (2014) Adjuvant docetaxel and cyclophosphamide (DC) with prophylactic granulocyte colony-stimulating factor (G-CSF) on days 8 & 12 in breast cancer patients: a retrospective analysis. PLoS ONE 9(10):e107273
Caley A, Bertelli G, Rolles M et al (2010) Adjuvant taxane chemotherapy is associated with a significant risk of febrile neutropenia. Eur J Cancer Suppl 8(3):70
Yau T-K, Ng T-Y, Soong IS et al (2009) Toxicity of docetaxel plus cyclophosphamide as adjuvant therapy for breast cancer in Chinese patients - The Hong Kong experience. Asia Pac J Clin Oncol 5(2):123–128
Vogel C, Rader M, Tyulandin S et al (2005) Pegfilgrastim nearly abrogates occurrence of neutropenic events early in the course of chemotherapy: results of a phase III, randomized, double-blind, placebo-controlled study of patients with breast cancer receiving docetaxel. J Support Oncol 3(2 SUPPL. 1):58–59
Marinho FDS, Lopes MDS, Monteiro MMF et al (2011) Incidence of febrile neutropenia with adjuvant docetaxel and cyclophosphamide in patients with early breast cancer. J Clin Oncol 29(suppl):e11501
Soni A, Brufsky A, Jankowitz RC et al (2011) Incidence of febrile neutropenia with docetaxel plus cyclophosphamide in a university-based breast oncology clinic. J Clin Oncol 29(suppl):9061
Kotasek D (2011) Febrile neutropenia rates during docetaxel and cyclophosphamide (TC) adjuvant therapy in early breast cancer (EBC). J Clin Oncol 29(suppl):1101
Santos FN, Cruz MR, Cezana L et al (2010) Hematologic toxicity with adjuvant docetaxel and cyclophosphamide in early breast cancer. J Clin Oncol 28(suppl)(15):e11081
Vandenberg T, Younus J, Al-Khayyat S (2010) Febrile neutropenia rates with adjuvant docetaxel and cyclophosphamide chemotherapy in early breast cancer: discrepancy between published reports and community practice—a retrospective analysis. Curr Oncol 17(2):2–3
Ngamphaiboon N, Advani PP, O’Connor TL et al (2011) Febrile neutropenia in adjuvant docetaxel and cyclophosphamide (TC) with prophylactic pegfilgrastim in patients with breast cancer: a retrospective analysis. J Clin Oncol 29(suppl):1134
Chan KK, Trudeau ME, Eisen A et al (2011) The cost-effectiveness of primary prophylaxis with granulocyte colony-stimulating factor in docetaxel-containing adjuvant chemotherapy in early breast cancer: the impact of risk of febrile neutropenia and its mortality. J Clin Oncol 29(suppl)(15):6086
Bayer Inc. (2016) Product Monograph Cipro XL. Version March 9, 2015. http://www.bayer.ca/omr/online/cipro-xl-pm-eng-9mar2015.pdf. Accessed 6 Sept 2016
Amgen Canada Inc. (2016) Product Monograph Neupogen (filgrastim). Version May 26, 2015. https://www.amgen.ca/Neupogen_PM.pdf. Accessed 6 Sept 6 2016
Hautmann MG, Hipp M, Kölbl O (2011) Clostridium difficile-associated diarrhea in radiooncology: an underestimated problem for the feasibility of the radiooncological treatment? Radiat Oncol 6:89
Bishop KD, Castillo JJ (2012) Risk factors associated with Clostridium difficile infection in adult oncology patients with a history of recent hospitalization for febrile neutropenia. Leuk Lymphoma 53:1617–1619
Masaoka T, Urabe A, Ohno R et al (1998) Evidence-based recommendations on antimicrobial use in febrile neutropenia in Japan. Int J Hematol 68(Suppl 1):S5–S6
Clinicaltrials.gov (2016) REaCT integrated consent model to compare two standard of care regimens NCT02173262. https://clinicaltrials.gov/ct2/show/NCT02173262. Accessed 9 Sept 2016
Hilton J, Mazzarello S, Fergusson D et al (2016) Novel methodology for comparing standard-of-care interventions in patients with cancer. J Oncol Pract. JOPR013474
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Appendices
Appendix 1: Systematic review supplement: electronic literature search strategy
Database: Embase Classic + Embase <1947 to 2016 April 13>, Ovid MEDLINE(R) In-Process & Other Non-Indexed Citations and Ovid MEDLINE(R) <1946 to Present>.
Search Strategy:
1 | exp Breast Neoplasms/ |
2 | (breast adj2 (cancer$ or neoplasm$ or carcinoma$)).tw. |
3 | 1 or 2 |
4 | Granulocyte Colony-Stimulating Factor/ |
5 | Granulocyte-colony stimulating factor$.tw. |
6 | gcsf.tw. |
7 | g csf.tw. |
8 | Neupogen.tw. |
9 | Neulasta.tw. |
10 | PegFilgrastim.tw. |
11 | Filgrastim/ or Filgrastim.tw. |
12 | colony-stimulating factor 3.tw. |
13 | or/4–12 |
14 | 3 and 13 |
15 | exp Anti-Bacterial Agents/ and pc.fs. |
16 | exp Anti-Bacterial Agents/ and (prevention or prophyla$).tw. |
17 | Antibiotic Prophylaxis/ |
18 | (antibiotic$ or antibacterial$) adj5 (prevent$ or prophyla$)).tw. |
19 | (septra or Trimethoprim-Sulfamethoxazole or cipro or Ciprofloxacin or Moxifloxacin) adj5 (prophyla$ or prevent$)).tw. |
20 | or/15–19 |
21 | 3 and 20 |
22 | 14 or 21 |
23 | exp antineoplastic agents/ |
24 | Antineoplastic Combined Chemotherapy Protocols/ |
25 | Neoadjuvant Therapy/ |
26 | Cyclophosphamide.tw. |
27 | Fluorouracil.tw. |
28 | Epirubicin.tw. |
29 | Docetaxel.tw. |
30 | (Paclitaxel or Taxol).tw. |
31 | (Adriamycin or doxorubicin).tw. |
32 | Cytoxan.tw. |
33 | Methotrexate.tw. |
34 | (chemotherap$ or antineoplastic agent$).tw. |
35 | Chemotherapy, Adjuvant/ |
36 | or/23–35 |
37 | 22 and 36 |
PRESS EBC search submission
AMED | □ |
C2-SPCTRE | □ |
CINAHL | □ |
Cochrane Database of Systematic Reviews (CDSR; Cochrane Reviews) | □ |
Cochrane Central Register of Controlled Trials (CENTRAL; Clinical Trials) | □ |
Cochrane Methodology Register (CMR; Methods Studies) | □ |
Cochrane Library (all databases) | □ |
Database of Abstracts of Reviews of Effects (DARE; Other Reviews) | □ |
Embase | □ |
ERIC | □ |
ICTRP (International Clinical Trials Registry Platform) | □ |
LILACS (Latin American and Caribbean Health Sciences Literature) | □ |
MEDLINE | □ |
PreMEDLINE | □ |
PsycINFO | □ |
Other | □ |
Other | □ |
1. Translation of the research question | x |
2. Boolean and proximity operators | x |
3. Subject headings | x |
4. Natural language/free-text | x |
5. Spelling, syntax and line numbers | x |
6. Limits and filters | x |
7. Search strategy adaptations | x |
Appendix 2: Risk of bias assessment of Included Randomized Trials

Trials at low risk of bias (green), high risk of bias (red) or unclear risk of bias (yellow)
Green (−) = low risk bias
Red (+) = high risk bias
Yellow (?) = unclear risk of bias
Appendix 3: PRISMA Checklist
Section/topic | # | Checklist item | Reported on page # |
|---|---|---|---|
Title | |||
Title | 1 | Identify the report as a systematic review, meta-analysis, or both | 1 |
Abstract | |||
Structured summary | 2 | Provide a structured summary including, as applicable: background; objectives; data sources; study eligibility criteria, participants, and interventions; study appraisal and synthesis methods; results; limitations; conclusions and implications of key findings; systematic review registration number | 2,3 |
Introduction | |||
Rationale | 3 | Describe the rationale for the review in the context of what is already known | 4,5 |
Objectives | 4 | Provide an explicit statement of questions being addressed with reference to participants, interventions, comparisons, outcomes, and study design (PICOS) | 5 |
Methods | |||
Protocol and registration | 5 | Indicate if a review protocol exists, if and where it can be accessed (e.g., Web address), and, if available, provide registration information including registration number | N/A |
Eligibility criteria | 6 | Specify study characteristics (e.g., PICOS, length of follow-up) and report characteristics (e.g., years considered, language, publication status) used as criteria for eligibility, giving rationale | 6,7 |
Information sources | 7 | Describe all information sources (e.g., databases with dates of coverage, contact with study authors to identify additional studies) in the search and date last searched | 6,7 |
Search | 8 | Present full electronic search strategy for at least one database, including any limits used, such that it could be repeated | 18-22 |
Study selection | 9 | State the process for selecting studies (i.e., screening, eligibility, included in systematic review, and, if applicable, included in the meta-analysis) | 6,7 |
Data collection process | 10 | Describe method of data extraction from reports (e.g., piloted forms, independently, in duplicate) and any processes for obtaining and confirming data from investigators | 6,7 |
Data items | 11 | List and define all variables for which data were sought (e.g., PICOS, funding sources) and any assumptions and simplifications made | 6,7 |
Risk of bias in individual studies | 12 | Describe methods used for assessing risk of bias of individual studies (including specification of whether this was done at the study or outcome level), and how this information is to be used in any data synthesis | 7,28 |
Summary measures | 13 | State the principal summary measures (e.g., risk ratio, difference in means) | N/A |
Synthesis of results | 14 | Describe the methods of handling data and combining results of studies, if done, including measures of consistency (e.g., I2) for each meta-analysis | N/A |
Risk of bias across studies | 15 | Specify any assessment of risk of bias that may affect the cumulative evidence (e.g., publication bias, selective reporting within studies) | 7, 28 |
Additional analyses | 16 | Describe methods of additional analyses (e.g., sensitivity or subgroup analyses, meta-regression), if done, indicating which were pre-specified | N/A |
Results | |||
Study selection | 17 | Give numbers of studies screened, assessed for eligibility, and included in the review, with reasons for exclusions at each stage, ideally with a flow diagram | 6-7,18 |
Study characteristics | 18 | For each study, present characteristics for which data were extracted (e.g., study size, PICOS, follow-up period) and provide the citations | 6-9 |
Risk of bias within studies | 19 | Present data on risk of bias of each study and, if available, any outcome level assessment (see item 12) | 7,28 |
Results of individual studies | 20 | For all outcomes considered (benefits or harms), present, for each study: (a) simple summary data for each intervention group (b) effect estimates and confidence intervals, ideally with a forest plot | 6-110 |
Synthesis of results | 21 | Present results of each meta-analysis done, including confidence intervals and measures of consistency | N/A |
Risk of bias across studies | 22 | Present results of any assessment of risk of bias across studies (see Item 15) | 7,28 |
Additional analysis | 23 | Give results of additional analyses, if done (e.g., sensitivity or subgroup analyses, meta-regression [see Item 16]) | N/A |
Discussion | |||
Summary of evidence | 24 | Summarize the main findings including the strength of evidence for each main outcome; consider their relevance to key groups (e.g., healthcare providers, users, and policy makers) | 6-10 |
Limitations | 25 | Discuss limitations at study and outcome level (e.g., risk of bias), and at review-level (e.g., incomplete retrieval of identified research, reporting bias) | 10-13 |
Conclusions | 26 | Provide a general interpretation of the results in the context of other evidence, and implications for future research | 10-13 |
Funding | |||
Funding | 27 | Describe sources of funding for the systematic review and other support (e.g., supply of data); role of funders for the systematic review | N/A |
Rights and permissions
About this article
Cite this article
Fernandes, R., Mazzarello, S., Stober, C. et al. Optimal primary febrile neutropenia prophylaxis for patients receiving docetaxel–cyclophosphamide chemotherapy for breast cancer: a systematic review. Breast Cancer Res Treat 161, 1–10 (2017). https://doi.org/10.1007/s10549-016-4028-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-016-4028-0