Abstract
We suggest novel experimental model of nerve injury—bilaterally axotomized ganglia of the crayfish ventral nerve cord (VNC). Using proteomic antibody microarrays, we showed upregulation of apoptosis execution proteins (Bcl-10, caspases 3, 6, and 7, SMAC/DIABLO, AIF), proapoptotic signaling proteins and transcription factors (c-Myc, p38, E2F1, p53, GADD153), and multifunctional proteins capable of initiating apoptosis in specific situations (p75, NMDAR2a) in the axotomized VNC ganglia. Simultaneously, anti-apoptotic proteins (p21WAF-1, MDM2, Bcl-x, Mcl-1, MKP1, MAKAPK2, ERK5, APP, calmodulin, estrogen receptor) were overexpressed. Some proteins associated with actin cytoskeleton (α-catenin, catenin p120CTN, cofilin, p35, myosin Vα) were upregulated, whereas other actin-associated proteins (ezrin, distrophin, tropomyosin, spectrin (α + β), phosphorylated Pyk2) were downregulated. Various cytokeratins and βIV-tubulin, components of intermediate filament and microtubule cytoskeletons, were also downregulated that could be the result of tissue destruction. Downregulation of proteins involved in clathrin vesicle formation (AP2α and AP2γ, adaptin (β1 + β2), and syntaxin) indicated impairment of vesicular transport and synaptic processes. The levels of L-DOPA decarboxylase, tyrosine, and tryptophan hydroxylases that mediate synthesis of serotonin, dopamine, norepinephrine, and epinephrine decreased. Overexpression of histone deacetylases HDAC1, HDAC2, and HDAC4 contributed to suppression of transcription and protein synthesis. So, the balance of multidirectional processes aimed either at cell death, or to repair and recovery, determines the cell fate. Present data provide integral, albeit incomplete, view on the nervous tissue response to axotomy. Some of these proteins can be probably potential markers of nerve injury and targets for neuroprotective therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Traumatic brain or spinal cord injury is among main causes of mortality and disability of the population. The death rate of 20–50 year old people from neurotrauma is higher than death from cancer and vascular pathologies. Annually craniocerebral trauma (TBI) kills 1.5 million people in the world, and 2.5 million became disabled. Peripheral nerve injuries account for up to 10% of neurotrauma (Kobeissy 2015; Laskowitz and Grant 2016; Rabinstein 2018; Witiw and Fehlings 2015).
Neurotrauma are very diverse. Various in vitro models including cell cultures and brain sections that display different features of mechanical neuron injury were used in neuropathologic studies. They included nerve stretching, mechanical or hydrostatic compression, hydrodynamic shock, impact of falling weight, or axotomy. On the base of the obtained data, various medications for treatment of acute neurotrauma such as glutamate antagonists, antioxidants, and anti-apoptotic agents were tested, but none of them was approved. Lack of success in this direction indicates the necessity of deeper understanding the cellular and molecular mechanisms of nerve damage and subsequent neuron death, survival, or regeneration (Lichterman 2014; Kobeissy 2015; Laskowitz and Grant 2016; Rabinstein 2018; Witiw and Fehlings 2015).
Nerve injury, in particular its complete transection (axotomy), impair ionic gradients and induces influx of Na+ and Ca2+ and efflux of K+. The signals about the axon damage (Ca2+, some signaling proteins) are retrogradely transported to the neuronal body and nucleus. This stimulates gene expression, synthesis of necessary proteins, and their backward transport to the damaged site. Following recovery processes include restoration of the plasma membrane, organelles and cytoskeleton, nerve regeneration, and restoration of the lost nerve connections with target cells (Rishal and Fainzilber 2014). However, at severe damage, such as axotomy, these measures may be insufficient, and the neuron degenerates (Hill et al. 2016). Axotomy induces death not only neurons and glial cells in the transection site but also satellite glial cells remote from the place of injury. These processes are regulated by a complex and incompletely known signaling system (Hill et al. 2016; Rishal and Fainzilber 2014).
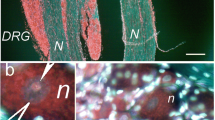
In order to study the signaling and epigenetic mechanisms that regulate neurodegeneration and neuroprotection after nerve damage, we introduce the novel model object—the bilaterally axotomized abdominal ganglion of the crayfish. Its ventral nerve cord (VNC) contains six ganglia (~ 103 neurons each) connected by nerves. The transection of the connectives gives six bilaterally axotomized ganglia. This model is relevant for spinal nerve cord injury. Using the antibody microarrays, we studied the changes in expression of more than 200 signaling and epigenetic proteins in the bilaterally axotomized VNC ganglia at 4 h after axotomy. The results of this proteomic study identified the most reactive proteins.
Materials and Methods
Object
The crayfishes Astacus leptodactilus from the Don River affluents were purchased at the local market. After cutting off the crayfish tail and removal of chitin from its ventral side, the ventral nerve cord was quickly isolated and transferred to the chamber filled with van Harreveld’s saline for cold-blooded animals (mM: NaCl 205; KCl 5.4; NaHCO3 0.24; MgCl2 5.4; CaCl2 13.5; pH 7.2–7.4). In the isolation process control VNCs were transected twice by thin ophthalmic scissors at the anterior and posterior margins before first and after sixth abdominal ganglion, respectively. The experimental VNCs were transected seven times: at the margins and between abdominal ganglia so that six bilaterally transected ganglia were obtained (Fig. 1). Control and experimental (axotomized) VNC samples obtained from 8 to 10 animals each were united and incubated in the dark at room temperature (about 22–25 °C) for 4 h.
Proteomic Study
The “Panorama® Antibody Microarray - Cell Signaling” kits (CSAA1), primary rabbit antibodies anti-HDAC1 (H3284) and anti-caspase 3 (C9598), anti-β-actin (A5441), and the secondary anti-Rabbit IgG peroxidase antibody (A6154) were obtained from Sigma-Aldrich-Rus (Moscow).
The changes in the expression of proteins in the axotomized VNC ganglia were estimated using the “Panorama® Antibody Microarray - Cell Signaling Kit,” which consists of two identical microarrays containing microdroplets with immobilized antibodies against 224 signaling and epigenetic proteins (in duplicate). The spots with immobilized non-labeled bovine serum albumin were used as a negative control, whereas the spots with Cy3 and Cy5-conjugated BSA were used as a positive control.
At 4 h after axotomy, the control and experimental samples were weighted, homogenized by ultrasound on ice, and lyzed in the Extraction/Labeling Buffer supplemented with protease and phosphatase inhibitor, and nuclease bensonase (CSAA1 components). After centrifugation (10,000 rpm, 4 min, 4 °C), protein content in experimental and control supernatants was determined using Bradford reagent. Then, the samples were diluted to 1 mg/ml protein content and incubated 30 min in the dark with fluorochromes Cy3 or Cy5, respectively. The unbound dye was removed by centrifugation (4000 rpm; 4 min) of the SigmaSpin Columns (CSAA1 components) filled with 200 μl of the labeled protein samples. One microarray was incubated (40 min, room temperature) on a rocking shaker in 5 ml of the mixture of the control and experimental samples (10 μg/ml each) labeled with Cy3 and Cy5, respectively. Another microarray was incubated with the oppositely labeled samples: Cy5 and Cy3, respectively. Such swapped staining provided verification of results and compensation of a potential bias in binding of Cy3 or Cy5 dyes to protein samples. This ensures the double test and full control of the experiment. After following triple washing in the washing buffer (CSAA1 component) and triple washing in pure water, the microarray slides were air dried overnight in the dark.
The microarrays were scanned on the GenePix 4100A Microarray Scanner (Molecular Devices, USA) at 532 and 635 nm (fluorescence maximums of Cy3 and Cy5, respectively). The fluorescence images of the antibody microarrays were analyzed and normalized (ratio-based normalization) using the software GenePix Pro 6.0. The fluorescence values in the rings around each spot served as a background. The median fluorescence value over all spot pixels was proportional to the protein content. The ratios of the experimental to control values characterized the difference in the protein level between axotomized and control VNC ganglia (Demyanenko and Uzdensky 2017). Two samples, labeled independently and reversely in duplicate, provided four experimental values for each protein. Each experiment was repeated twice, and the experiment/control ratios were averaged (n = 8). The Student’s t test and 95% significance level were used. The data are presented as means ± SD. Only proteins whose expression differed more than 40% from control are shown in Table 1 and discussed.
Western Blot
After isolation, transection, and 4-h incubation in the dark, three experimental and three control VNCs were homogenized by ultrasound on ice in the Extraction/Labeling Buffer supplemented with nuclease benzonase and inhibitors of proteases and phosphatases (CSAA1 components). The homogenates were centrifuged 10 min at 10,000g and 4 °C. The samples containing 10–20 μg of protein were subjected to electrophoretic separation in polyacrylamide gel (7–10%) in the mini-PROTEAN Tetra cell (Bio-Rad). The protein standard was ColorBurst Electrophoresis Marker (C1992, Sigma-Aldrich). After separation, proteins were electro-transferred on the Immuno-Blot PVDF Membrane (Bio-Rad) using a Trans-Blot® Turbo Transfer System (Bio-Rad, USA). After washing by PBS, the membrane was consecutively incubated 1 h in blocking buffer (TBS 1% Casein Blocker, Bio-Rad) and incubated overnight with primary antibodies at 4 °C. The following primary rabbit antibodies, the same as in CSAA1—anti-HDAC1 (H3284, 1:1000) and anti-caspase 3 (C9598, 1:500)—were used. Anti-β-actin (A5441, 1:5000) served as a secondary antibody. The membranes were washed by TTBS (10 mM Tris-buffer, pH 8, plus 0.1% Tween-20), incubated 1 h with the secondary anti-Rabbit IgG peroxidase antibody (1:1000) at room temperature, again washed in TTBS and incubated with Сlarity Western ECL. The chemiluminescence was registered using the Gel Documentation system Fusion SL (Vilber Lourmat, France) using the software Vision Capt. Statistical analysis was performed using one-way ANOVA RM. The difference were considered to be reliable at р < 0.05.
Results
The changes in the protein profile in VNC ganglia at 4 h after bilateral axotomy included upregulation of 36 proteins, and downregulation of 35 proteins (Table 1). These proteins perform a variety of functions such as intracellular signaling, epigenetic processes, regulation of apoptosis, remodeling and destruction of the cytoskeleton, deterioration of vesicular transport, and synaptic processes.
The expression of various signaling proteins such as transcription factor c-Myc, protein kinases p38, PKCα, ERK1 and ERK2, MAPKAPK2 (MAPK-activated protein kinase-2), protein phosphatase MKP-1 (MAP kinase phosphatase-1), calmodulin, estrogen receptor, and APP (β-amyloid precursor protein) increased in VNC ganglia more than 1.4 times after axotomy. Simultaneously, other signaling proteins—NAK (kinase activating the transcription factor NF-κB), GAPDH (glyceraldehyde-3-phosphate dehydrogenase), EGF receptor, and protein S-100—were downregulated by 1.6–2.2-fold (Table 1).
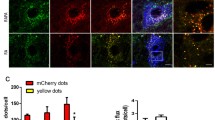
Axotomy caused significant upregulation of a number of proapoptotic proteins in the VNC ganglia (Table 1) that include (a) apoptosis execution proteins: Bcl-10, SMAC/DIABLO, AIF, caspases 6, 7, 3, and the active form of caspase 3, and phosphoserine receptor PSR, which marks the apoptotic cells; (b) transcription factors that regulate expression of various apoptotic proteins: E2F1, p53, and GADD153 (growth arrest- and DNA damage-inducible gene 153); and (c) proteins that perform various cellular functions, but can initiate apoptosis in specific conditions: NGF receptor p75 and glutamate receptor NMDAR2a. The upregulation of caspase 3 at 4 but not 1 h after axotomy was confirmed by western blot (Fig. 2).
At the same time, different anti-apoptotic and prosurvival proteins—Bcl-x, Mcl-1, p21WAF-1, ERK5, calmodulin, and estrogen receptor—were upregulated. The anti-apoptotic tendency also included downregulation of mitochondrial antioxidant AOP-1 and proapoptotic proteins FADD (Fas-associated protein with death domain) and p14arf (Table 1).
The increase in the protein levels may be partially associated with 44–48% overexpression of histone acetyltransferases HAT1 and PCAF, because histone acetylation facilitates protein synthesis. However, the acetylation of histone H3 decreased more than four times. Probably, it was associated with 53–84% upregulation of histone deacetylases HDAC1, HDAC2, and HDAC4 (Table 1). The upregulation of HDAC1 in the VNC ganglia at 4 but not 1 h after axotomy was confirmed by western blot (Fig. 3). Possibly, certain signaling cascades and transcription proteins but not epigenetic processes could be responsible for the upregulation of specific proteins in the VNC ganglia after axotomy.
Downregulation of cdc7 kinase, topoisomerase 1, Trf-1 (telomere-binding factor-1), and NTF-2 (nuclear transport factor 2) indicated the deteriorations of the cell cycle.
The expression of various cytoskeleton elements differently changed in the axotomized ganglia. The downregulation of cytokeratins 7, 8.60, and 19 indicated the impairment of intermediate filaments. The microtubule cytoskeleton was also deteriorated. Actually, tubulin βIV and its polyglutamated form were downregulated. This could be associated with the downregulation of MAP1 and phosphorylated tau protein, which bind to the microtubules and regulate their assembly. More complicated changes of the actin cytoskeleton were observed in the axotomized VNC ganglia. Cofilin, protein p35, and catenins alpha and p120CTN and myosin Vα (LE-16) were overexpressed (Table 1). However, a number of proteins such as ezrine, spectrin (α + β), and distrophin that bind actin cytoskeleton to the plasma membrane, tropomyosin, which binds to the actin cytoskeleton, phosphorylated form of focal adhesion kinase Pyk2, and placoglobin that participates in adhesive contacts, were downregulated.
Vesicular transport and synaptic processes were also impaired. The expression of proteins AP2α, AP2γ, and adaptins (β1 + β2), which mediate formation of clathrin vesicles, decreased. Additionally, syntaxin involved in docking of synaptic vesicles to the synaptic membrane, and cytohezines 2 and 3 (ARNO and ARNO3), which control protein sorting and membrane traffic in Golgi complexes, were downregulated. The synthesis of neurotransmitters catecholamines, dopamine, and serotonin was also disrupted, as indicated by the downregulation of DOPA decarboxylase, tyrosine, and tryptophan hydroxylases (Table 1).
Discussion
In the present work, we introduced the novel experimental model for study of the axotomy consequences—the bilaterally axotomized ganglia from the crayfish ventral cord. This object is rather simple, inexpensive, and easily prepared. Control VNCs were also axotomized at their edges, but much less. They consisted of four non-axotomized and two edge ganglia, which were transected unilaterally.
Axotomy disrupts the neuronal communications between the VNC ganglia. Main injurious factors at the axon transection site include impairment of ionic homeostasis with Ca2+ and Na+ penetration and loss of K+ that leads to depolarization, edema, disruption of energy metabolism, and formation of the signaling complex that is transported retrogradely to the neuronal body (Rishal and Fainzilber 2014). The present study demonstrated that axotomy induces up- or downregulation of diverse proteins involved in cell signaling, epigenetic processes, apoptosis regulation, cytoskeleton remodeling, vesicular transport, and synaptic processes. Protein downregulation was apparently the result of tissue destruction and proteolysis, whereas upregulation could be the result of additional synthesis. However, the signaling pathways and transcription factors that regulate expression of specific genes in the injured nervous tissue are still incompletely known.
Protein biosynthesis in the cell is globally regulated by epigenetic processes such as acetylation/deacetylation of histones that increase or decrease the transcriptional activity of the genome. Acetylation of histone H3 loosens DNA packing in the chromatin and facilitates binding of transcription factors and RNA polymerase II to gene promoters. Deacetylation of histone H3, oppositely, leads to chromatin condensation and suppression of protein synthesis. The observed upregulation of histone acetyltransferases HAT1 and PCAF could stimulate protein biosynthesis (Contestabile and Sintoni 2013; Kukucka et al. 2013). However, fourfold decrease in the acetylation of histone H3 that could be performed by histone deacetylases HDAC1, HDAC2, and HDAC4, which were upregulated, suggests that epigenetic processes did not play a significant role in protein upregulation in the VNC ganglia after axotomy. Probably, activation of some signaling cascades and transcription factors was more important.
The proteomic experiments revealed the downregulation of some proteins that regulate the cell cycle in the axotomized ganglia: Cdc7 kinase that initiates and regulates the cell division, topoisomerase-1 that is involved in DNA replication, Trf-1 that regulates the telomere length, and NTF2 that transfers proteins from the cytoplasm into the nucleus.
Pro-apoptotic Proteins
Axotomy induced overexpression of various proteins that execute the apoptotic program in the VNC ganglia: SMAC/DIABLO, AIF, and caspases 3, 6, and 7. In the axotomized VNC ganglia, apoptosis could be initiated by different pathways. Overexpression of glutamate receptors NMDAR2a induces massive Ca2+ influx and excitotoxic cell death (Quillinan et al. 2016). The presence of glutamate NMDA receptors in the crayfish nervous system and their role in neuroglial relationships has been earlier reported (Gafurov et al. 2002). The receptor of neurotrophic factors p75 induces neuronal apoptosis upon binding of neurotrophins (Roux and Barker 2002). Its presence in the crayfish nervous system has been recently reported (Kolosov et al. 2016). Bcl-10, whose level increased twofold after axotomy, interacts with different apoptosis regulators such as NF-κB, TRAF2, IAP, and recruits TRADD and RIP proteins that mediate apoptosis and necroptosis (Thome 2004).
Transcription factor GADD153 is usually weakly expressed in normal cells. However, DNA damage, or inhibition of proliferation, or other damage induce its upregulation and following expression of the downstream genes. Its activation leads to ROS production, glutathione depletion, downregulation of Bcl-2, and finally to apoptosis (Onoue et al. 2005; Oyadomari and Mori 2004). Probably, overexpression of GADD153 in the axotomized VNC ganglia could induce apoptosis.
More than twofold overexpression of the ubiquitous transcription factor c-Myc in the axotomized VNC ganglia was the greatest among studied proteins. c-Myc is a multifunctional master regulator of diverse cellular processes such as anabolic and energy metabolism, cell growth, proliferation, and apoptosis depending on the cell context (Dang 2012). The default function of c-Myc is suggested to be at the head of cascades that drive apoptosis rather than cell cycle progression (McMahon 2014). C-Myc controls the expression of 10–15% of all genes due to regulation of global chromatin structure. In the complex with proteins Max and TRRAP, it attracts histone acetyltransferases GCN5 and TIP60, which maintain euchromatin in an open, accessible state thereby facilitating protein synthesis (Eilersm and Eisenman 2008; Knoepfler 2007). Belin et al. (2015) reported that c-Myc mRNA was overexpressed in the mouse dorsal root ganglion at 1 day after sciatic nerve injury. However, its level decreased 3 days later. This could lead to survival and regeneration of peripheral sensory neurons. The expression of c-Myc at shorter intervals after nerve injury was less studied. One can suggest that the upregulation of c-Myc in VNC ganglia at 4 h after axotomy was proapoptotic.
Overexpression of stress-activated MAP kinase p38 played apparently a proapoptotic role in the axotomized VNC ganglia. Similarly, the damage to the optic nerve induced NMDA receptor-mediated glutamate neurotoxicity, in which p38 mediated apoptosis of retinal ganglion cells (Kikuchi et al. 2000). Oppositely, inhibition of p38 reduced loss of primary sensory neurons in the rat dorsal root ganglia after sciatic nerve transection (Agthong et al. 2012).
Another master regulator in the cell is the proapoptotic protein p53 (Culmsee and Mattson 2005; Morrison et al. 2003). It is a universal transcription factor for hundreds or even thousands genes (Fischer 2017; Sullivan et al. 2018). It stimulates the expression of various pro- and anti-apoptotic proteins such as caspase 6 and p21Waf-1, which were also overexpressed in the axotomized VNC. The early induction of p53 and pro-apoptotic Bcl-2 family proteins, which mediate mitochondrial dysfunction and the release of proapoptotic proteins, was suggested to be the leading mechanism of apoptosis induction in the ischemic brain (Culmsee and Landshamer 2006; Culmsee and Mattson 2005). Perhaps, axotomy induced the similar proapoptotic mechanism in the VNC ganglia.
The pro-apoptotic activity of p53 is associated with activation of E2F1 that transcriptionally controls the expression of this protein (Hou et al. 2002). E2F1 was also upregulated in the crayfish VNC ganglia after axotomy. It is a master regulator of a variety of cellular processes including the cell cycle, DNA repair, and apoptosis (Raimundo et al. 2012). It controls the expression of a variety of proapoptotic proteins such as caspases 3, 7, 8, and 9, p53, SMAC/DIABLO, Apaf-1, and Bcl-2 family pro-apoptotic proteins (Engelmann and Pützer 2010; Pelengaris et al. 2002). In turn, its expression is controlled by p38 and c-Myc (Bretones et al. 2015; de Olano et al. 2012).
The decrease of the viability of VNC ganglia cells after axotomy was also associated with downregulation of some prosurvival proteins. The decrease in the level of mitochondrial antioxidant AOP-1 could lead to oxidative cell damage. Axotomy also decreased the level of NAK, a protein kinase that activates the prosurvival transcription factor NF-κB (Häcker and Karin 2006). Another downregulated protein in the axotomized ganglia is S-100. It is a Ca2+ sensor in the cell. After Ca2+ binding, it regulates Ca2+ homeostasis and activity of various enzymes and transcription factors. S-100 is the biochemical marker of brain injury (Korfias et al. 2006). EGF receptor stimulates DNA reparation, proliferation, and differentiation upon EGF binding (Scafidi et al. 2014). Its downregulation in the axotomized VNC ganglia also decreased the prosurvival capability of neuronal and glial cells.
Exposure to the outside of membrane phosphatidylserine is a signal “eat me,” which allows to recognize apoptotic cells and their fragments and stimulate their phagocytic removal (Li et al. 2003). Upregulation of the phosphatidylserine receptor (PSR) indicated the apoptotic process in the axotomized VNC ganglia.
Anti-apoptotic Processes
The overexpression of diverse anti-apoptotic Bcl-2 family proteins such as Bcl-x and Mcl-1 occurred along with upregulation of pro-apoptotic proteins in the axotomized VNC ganglia. Axotomy also induced the upregulation of p21Waf that suppresses p53-mediated apoptosis. Similarly, the upregulation of p21 after axotomy was involved in proliferation of microglia in the transected rat facial nucleus (Yamamoto et al. 2012).
MAP kinases ERK1 and ERK2 phosphorylate and activate various transcription factors and cytoskeleton proteins, and thus regulate cell proliferation, mobility, and survival. In our experiments, the augment of ERK1 was approximately the same as that of ERK1 and ERK2—about 70%, i.e., ERK1 almost entirely determined the reaction of the crayfish neural tissue to axotomy. The upregulation of MAPK-activated protein kinase-2 (MAPKAPK2) after axotomy could be also neuroprotective. Under stress, MAPKAPK2 is phosphorylated by p38, and then it phosphorylates chaperones HSP25 and HSP27, which stabilize protein structure and prevent apoptosis (Piao et al. 2005).
The overexpression of estrogen receptors, which exert a potent neuroprotective and neurotrophic effect, could play a neuroprotective role in the axotomized ganglia. Similarly, estrogens attenuated cell death after cerebrovascular stroke or neurotrauma (Wise et al. 2001). The presence of estrogen receptors in the freshwater crayfish was reported by Paolucci et al. (2002).
Protein phosphatase MKP-1 dephosphorylates proteins and stops their activation. It is known to abolish the proapoptotic activity of MAP kinases JNK and p38 in the ischemic brain, thus participating in the endogenous neuroprotective mechanism (Liu et al. 2014). So, overexpression of MKP-1 in the axotomized VNC ganglia could be neuroprotective.
Calmodulin is a major Ca2+-binding protein in the brain. Ca2+/calmodulin-dependent signaling pathways regulate gene expression, cell survival, production and secretion of neurotransmitters, cytoskeleton remodeling, axonal transport, and other physiological processes. Calmodulin and calmodulin-dependent kinases CaMKIIα and CaMKIV are abundant in the brain (Solà et al. 1999). Despite abundance, the calmodulin level increased even more (+ 59%) in the axotomized VNC ganglia that could activate physiological and protective processes in the injured nervous tissue.
Upregulation of protein kinase Cα (PKCα) and ERK5, which are activated by Ca2+, could be also associated with physiological and protective processes in the axotomized VNC ganglia (Su et al. 2014). Likewise, upregulation of PKC-α after axon injury in the retinal ganglion cells participated in neurite outgrowth in mice (Wu et al. 2003).
Interesting, amyloid precursor protein (APP) was overexpressed in the axotomized crayfish ganglia. APP is involved in the pathogenesis of Alzheimer’s disease. It also accumulates in injured nerves (Ahlgren et al. 1996). APP is involved in neuronal differentiation, neuritic outgrowth, synapse formation, long-term memory, and maintaining brain integrity. It is present in the nervous system of various invertebrates (Ewald and Li 2012; Guo et al. 2012; Poeck et al. 2012). APP overexpression in the ischemic brain was considered to play the neuroprotective role (Nalivaeva and Turner 2013). Possibly, it participated in the protection processes in the axotomized VNC ganglia.
The glycolysis enzyme GAPDH could be involved in apoptosis in the axotomized ganglia. The expression of the GAPDH gene remains stable under changing cellular conditions. However, it was shown to contribute to diverse cellular functions unrelated to glycolysis including DNA replication, DNA repair, nuclear RNA export, membrane fusion, and cytoskeletal organization. Pathologically, GAPDH was involved in regulation of apoptosis and neurodegenerative disorders (Berry and Boulton 2000; Tatton et al. 2000).
The downregulation of FADD, which initiates apoptosis upon binding of the extracellular Fas ligand to Fas receptor, and p14arf, which initiates p53-dependent cell cycle arrest and apoptosis (Fadeel and Orrenius 2005), also contributed to anti-apoptotic tendencies in the axotomized VNC ganglia.
Cytoskeleton
An important result of traumatic nerve damage is the changes in expression of cytoskeleton proteins and proteins involved in intercellular interactions. Actin cytoskeleton is responsible for maintaining the cell shape, its remodeling, cell migration, and intracellular movements. In the axotomized VNC ganglia, α-catenin and catenin p120CTN, which bind the intracellular domains of N-cadherin, a component of the cell-cell adhesion complex, to the actin cytoskeletons (Ishiyama and Ikura 2012), were over-expressed. These catenins regulate the cytoskeleton architecture and dynamics. On the contrary, cofilin depolymerizes fibrillar actin that is necessary for cytoskeleton rearrangement and cell motility. Under stress conditions, cofilin forms rods that impede axonal transport and contribute to synaptic deficit (Maloney and Bamburg 2007). The functional role of its overexpression in the axotomized VNC ganglia is not clear. Protein p35, a component of almost all neurons, forms a complex with Cdk5 kinase that mediates binding of the β-catenin-associated adhesion complex to the actin cytoskeleton. Upregulation of p35 in the VNC ganglia after axotomy may be associated with the cytoskeleton depolymerization and neuronal death, as in the case of cerebral ischemia (Hayashi et al. 1999).
Myosin Vα is highly expressed in the brain. It participates in the transport of vesicles, organelles, and mRNA along actin fibers, in exocytosis of secretory vesicles and recycling endosomes in neurons and glial cells. Myosin Vα is upregulated in the proximal stump of severed sciatic nerve. It also transfers mRNA from Schwann cells to axons of injured neurons in both vertebrates and invertebrates (Calliari et al. 2002; Sotelo et al. 2013). Its upregulation in the VNC ganglia after axotomy was apparently associated with recovery processes.
Some proteins associated with actin cytoskeleton such as ezrin, distrophin, placoglobin, spectrin (α + β), and tropomyosin were downregulated in the axotomized VNC ganglia. Ezrin mediates linking the actin cytoskeleton to the plasma membrane. It regulates adhesion, cytoskeleton rearrangements, shape, and cell migration, and participates in intracellular signaling and apoptosis. Distrophin is a component of the scaffold, which binds actin filaments to the plasma membrane. Placoglobin is a homolog of β-catenin. It is a component of desmosomes and adhesive contacts. Spectrin (α + β) that is located on the intracellular side of the plasma membrane maintains its integrity and forms a framework for the actin cytoskeleton. Tropomyosin stabilizes fibrillar actin and mediates the formation of a complex actin network in cells. It controls the dynamics of actin filaments and the cell shape during migration and cytokinesis. The downregulation of these proteins was apparently the result of the nervous tissue destruction after axotomy.
After formation of adhesion contacts, the focal adhesion kinase Pyk2, specific for the nervous system, is phosphorylated and stimulates MAP kinases ERK, JNK, and p38. This modulates the architecture of the cytoskeleton, cell morphology, neuronal activity, cell division, and death. The reduced phosphorylation of Pyk2 after axotomy could be associated with a destruction of the ganglion tissue.
The downregulation of elements of the microtubular cytoskeleton — βIV-tubulin and its polyglutamated form, proteins MAP1 (microtubule associated protein 1) and tau linked to microtubules and regulating their assembly, stability, and connections to other proteins — was also the result of destructive processes in the axotomized ganglia. Likewise, downregulation of these proteins was observed in the isolated chick cortical neurons in the presence of NaCN and calcium ionophore ionomycin (Hutter-Paier et al. 2000). Similarly, the decrease in the levels of cytokeratins 7, 19, and 8.60, elements of the intermediate filaments that form the third cytoskeleton in the cell, could be associated with destruction of the nervous tissue.
Vesicular Transport and Synaptic Processes
Vesicular transport plays an important role in protein processing and sorting after synthesis, in intracellular traffic between ER, Golgi apparatus and plasma membrane, in endo- and exocytosis, and in synaptic transmission. Our experiments showed that axotomy reduced the levels of some proteins involved in vesicular transport and synaptic processes in the VNC ganglia: AP2α and AP2γ that form the envelope of clathrin vesicles, and adaptin (β1 + β2) that is involved in vesicle formation, and cytohesins 2 and 3 (ARNO) that regulate protein sorting and membrane trafficking, and control Golgi structure and function.
Vesicular transport plays a central role in synaptic transmission. The downregulation of synaptic proteins such as syntaxin involved in docking of synaptic vesicles, fusing with the synaptic membrane, and release of neurotransmitters (Jahn and Fasshauer 2012), led apparently to deterioration of synaptic processes in the axotomized VNC ganglia.
The expression of proteins involved in the metabolism of neurotransmitters was also decreased. In particular, tyrosine hydroxylase that produces L-DOPA from l-tyrosine during biosynthesis of dopamine, norepinephrine, and epinephrine, L-DOPA decarboxylase, which transforms L-DOPA into dopamine, and tryptophan hydroxylase, which synthesizes serotonin, were downregulated.
Conclusion
In the present work, the novel experimental model of nerve injury consequences, the bilaterally axotomized ganglia of the crayfish ventral cord, was suggested. Proteomic antibody microarray study showed the upregulation of 36 proteins involved in signal transduction and epigenetic regulation, pro- and antiapoptotic processes, impairment of actin, microtubule and intermediate filament cytoskeletons, vesicular transport, and synaptic transmission after bilateral transection of the inter-ganglion connectives. Simultaneously 35 proteins were downregulated. Among upregulated proteins were apoptosis execution proteins (Bcl-10, caspases 3, 6, and 7, SMAC/DIABLO, AIF), proapoptotic signaling proteins and transcription factors (E2F1, p53, c-Myc, p38, GADD153), and multifunctional proteins capable of initiating apoptosis in specific situations (p75, NMDAR2a). Simultaneously, overexpression of some anti-apoptotic proteins (Bcl-x, Mcl-1, p21WAF-1, MDM2, MKP1, MAKAPK2, ERK5, APP, calmodulin, estrogen receptor) was observed. Some proteins associated with the actin cytoskeleton and responsible for its stability and rearrangement of the cell shape, migration, intracellular movements, and intercellular interactions (α-catenin, catenin p120CTN, cofilin, p35, myosin Vα) were upregulated, whereas other proteins associated with actin cytoskeleton (ezrin, distrophin, tropomyosin, spectrin (α + β), phosphorylated Pyk2) were downregulated. The components of microtubule and intermediate filament cytoskeletons (βIV-tubulin and various cytokeratins) were downregulated that probably was the result of tissue destruction. Vesicular transport and synaptic processes were impaired. The levels of adaptin (β1 + β2), clathrin vesicles AP2α and AP2γ proteins, and syntaxin decreased. L-DOPA decarboxylase, tyrosine and tryptophan hydroxylases that mediate synthesis of serotonin, dopamine, norepinephrine, and epinephrine were downregulated. Overexpression of histone deacetylases HDAC1, HDAC2, and HDAC4 contributed to suppression of transcription and protein synthesis. The balance of multidirectional processes aimed either at cell death, or repair and recovery, determines the fate of the cells and tissue.
The present data provide the integral, albeit incomplete, view on the nervous tissue response to axotomy. A comparison of the obtained data on axotomy-induced changes in protein composition in the crayfish VNC ganglia with that induced by photothrombotic stroke in the rat brain cortex, which were studied using the same microarrays (Demyanenko and Uzdensky 2017), showed that despite significant differences in the object of study and the method of the nervous tissue damage, many changes in protein profile were similar, both qualitatively and quantitatively. Such similarity suggests some common mechanisms in responses of the nervous system of different animals to different damage modalities. Probably, some of these proteins can be potential markers of nerve injury and targets for neuroprotective therapy.
References
Agthong S, Kaewsema A, Chentanez V (2012) Inhibition of p38 MAPK reduces loss of primary sensory neurons after nerve transection. Neurol Res 34:714–720. https://doi.org/10.1179/1743132812Y.0000000070
Ahlgren S, Li GL, Olsson Y (1996) Accumulation of β-amyloid precursor protein and ubiquitin in axons after spinal cord trauma in humans: immunohistochemical observations on autopsy material. Acta Neuropathol 92:49–55
Belin S, Nawabi H, Wang C, Tang S, Latremoliere A, Warren P, Schorle H, Uncu C, Woolf CJ, He Z, Steen JA (2015) Injury-induced decline of intrinsic regenerative ability revealed by quantitative proteomics. Neuron 86:1000–1014. https://doi.org/10.1016/j.neuron.2015.03.060
Berry MD, Boulton AA (2000) Glyceraldehyde-3-phosphate dehydrogenase and apoptosis. J Neurosci Res 60:150–154
Bretones G, Delgado MD, Leó J (2015) Myc and cell cycle control. Biochim Biophys Acta 1849:506–516. https://doi.org/10.1016/j.bbagrm.2014.03.013
Calliari A, Sotelo-Silveira J, Costa MC, Nogueira J, Cameron L, Kun A, Benech J, Sotelo JR (2002) Myosin Va is locally synthesized following nerve injury. Cell Motil Cytoskeleton 51:169–176
Contestabile A, Sintoni S (2013) Histone acetylation in neurodevelopment. Curr Pharm Des 19:5043–5050
Culmsee C, Landshamer S (2006) Molecular insights into mechanisms of the cell death program: role in the progression of neurodegenerative disorders. Curr Alzheimer Res 3:269–283
Culmsee C, Mattson MP (2005) p53 in neuronal apoptosis. Biochem Biophys Res Commun 331:761–777. https://doi.org/10.1016/j.bbrc.2005.03.149
Dang CV (2012) MYC on the path to cancer. Cell 149:22–35. https://doi.org/10.1016/j.cell.2012.03.003
de Olano N, Koo CY, Monteiro LJ, Pinto PH, Gomes AR, Aligue R, Lam EW (2012) The p38 MAPK-MK2 axis regulates E2F1 and FOXM1 expression after epirubicin treatment. Mol Cancer Res 10:1189–1202. https://doi.org/10.1158/1541-7786.MCR-11-0559
Demyanenko S, Uzdensky A (2017) Profiling of signaling proteins in penumbra after focal Photothrombotic infarct in the rat brain cortex. Mol Neurobiol 54:6839–6856. https://doi.org/10.1007/s12035-016-0191-x
Eilersm M, Eisenman RN (2008) Myc’s broad reach. Genes Dev 22:2755–2766. https://doi.org/10.1101/gad.1712408
Engelmann D, Pützer BM (2010) Translating DNA damage into cancer cell death-a roadmap for E2F1 apoptotic signalling and opportunities for new drug combinations to overcome chemoresistance. Drug Resist Updat 13:119–131. https://doi.org/10.1016/j.drup.2010.06.001
Ewald CY, Li C (2012) Caenorhabditis elegans as a model organism to study APP function. Exp Brain Res 217:397–411. https://doi.org/10.1007/s00221-011-2905-7
Fadeel B, Orrenius S (2005) Apoptosis: a basic biological phenomenon with wide-ranging implications in human disease. J Intern Med 258:479–517. https://doi.org/10.1111/j.1365-2796.2005.01570.x
Fischer M (2017) Census and evaluation of p53 target genes. Oncogene 36:3943–3956. https://doi.org/10.1038/onc.2016.502
Gafurov BS, Urazaev AK, Grossfeld RM, Lieberman EM (2002) Mechanism of NMDA receptor contribution to axon-to-glia signaling in the crayfish medial giant nerve fiber. Glia 38:80–86
Guo Q, Wang Z, Li H, Wiese M, Zheng H (2012) APP physiological and pathophysiological functions: insights from animal models. Cell Res 22:78–89. https://doi.org/10.1038/cr.2011.116
Häcker H, Karin M (2006) Regulation and function of IKK and IKK-related kinases. Sci STKE 2006(357):re13. https://doi.org/10.1126/stke.3572006re13
Hayashi T, Warita H, Abe K, Itoyama Y (1999) Expression of cyclin-dependent kinase 5 and its activator p35 in rat brain after middle cerebral artery occlusion. Neurosci Lett 265:37–40
Hill CS, Coleman MP, Menon DK (2016) Traumatic axon injury: mechanisms and translational opportunities. Trends Neurosci 39:311–324. https://doi.org/10.1016/j.tins.2016.03.002
Hou ST, Xie X, Baggley A, Park DS, Chen G, Walker T (2002) Activation of the Rb/E2F1 pathway by the nonproliferative p38 MAPK during Fas (APO1/CD95)-mediated neuronal apoptosis. J Biol Chem 277:48764–48770
Hutter-Paier B, Grygar E, Loibner M, Skofitsch G, Windisch M (2000) Effects of NaCN and ionomycin on neuronal viability and on the abundance of microtubule-associated proteins MAP1, MAP2, and tau in isolated chick cortical neurons. Cell Tissue Res 302:39–47
Ishiyama N, Ikura M (2012) The three-dimensional structure of the cadherin-catenin complex. Subcell Biochem 60:39–62. https://doi.org/10.1007/978-94-007-4186-7_3
Jahn R, Fasshauer D (2012) Molecular machines governing exocytosis of synaptic vesicles. Nature 490:201–207. https://doi.org/10.1038/nature11320
Kikuchi M, Tenneti L, Lipton SA (2000) Role of p38 mitogen-activated protein kinase in axotomy-induced apoptosis of rat retinal ganglion cells. J Neurosci 20:5037–5044
Knoepfler PS (2007) Myc goes global: new tricks for an old oncogene. Cancer Res 67:5061–5063. https://doi.org/10.1158/0008-5472.CAN-07-0426
Kobeissy FH (ed) (2015) Brain neurotrauma: molecular, neuropsychological, and rehabilitation aspects. CRC Press/Taylor & Francis, Boca Raton, FL
Kolosov MS, Komandirov MA, Terent’ev VV, Shitov AV, Kiroy RI (2016) Immunological study of freshwater crayfish nervous tissue for receptors for neurotrophins and ciliary neurotrophic factor. Neurochem J 10:195–198
Korfias S, Stranjalis G, Papadimitriou A, Psachoulia C, Daskalakis G, Antsaklis A, Sakas DE (2006) Serum S-100B protein as a biochemical marker of brain injury: a review of current concepts. Curr Med Chem 13:3719–3731
Kukucka J, Wyllie T, Read J, Mahoney L, Suphioglu C (2013) Human neuronal cells: epigenetic aspects. Biomol Concepts 4:319–333. https://doi.org/10.1515/bmc-2012-0053
Laskowitz D, Grant G (2016) Translational research in traumatic brain injury. CRC Press/Taylor and Francis Group, Boca Raton, FL
Li MO, Sarkisian MR, Mehal WZ, Rakic P, Flavell RA (2003) Phosphatidylserine receptor is required for clearance of apoptotic cells. Science 302:1560–1563
Lichterman LB (2014) Traumatic brain injury. In: Diagnosis and treatment. GEOTAR-Media, Moscow (in Russian)
Liu L, Doran S, Xu Y, Manwani B, Ritzel R, Benashski S, McCullough L, Li J (2014) Inhibition of mitogen-activated protein kinase phosphatase-1 (MKP-1) increases experimental stroke injury. Exp Neurol 261:404–411. https://doi.org/10.1016/j.expneurol.2014.05.009
Maloney MT, Bamburg JR (2007) Cofilin-mediated neurodegeneration in Alzheimer's disease and other amyloidopathies. Mol Neurobiol 35:21–44
McMahon SB (2014) MYC and the control of apoptosis. Cold Spring Harbor Persp Med 4:a014407. https://doi.org/10.1101/cshperspect.a014407
Morrison RS, Kinoshita Y, Johnson MD, Guo W, Garden GA (2003) p53-dependent cell death signaling in neurons. Neurochem Res 28:15–27
Nalivaeva NN, Turner AJ (2013) The amyloid precursor protein: a biochemical enigma in brain development, function and disease. FEBS Lett 587:2046–2054
Onoue S, Kumon Y, Igase K, Ohnishi T, Sakanaka M (2005) Growth arrest and DNA damage-inducible gene 153 increases transiently in the thalamus following focal cerebral infarction. Brain Res Mol Brain Res 134:189–197
Oyadomari S, Mori M (2004) Roles of CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ 11:381–389
Paolucci M, Di Cristo C, Di Cosmo A (2002) Immunological evidence for progesterone and estradiol receptors in the freshwater crayfish Austropotamobius pallipes. Mol Reprod Dev 63:55–62
Pelengaris S, Khan M, Evan G (2002) c-MYC: more than just a matter of life and death. Nat Rev Cancer 2:764–776. https://doi.org/10.1038/nrc904
Piao CS, Kim SW, Kim JB, Lee JK (2005) Co-induction of αB-crystallin and MAPKAPK-2 in astrocytes in the penumbra after transient focal cerebral ischemia. Exp Brain Res 163:421–429. https://doi.org/10.1007/s00221-004-2197-2
Poeck B, Strauss R, Kretzschmar D (2012) Analysis of amyloid precursor protein function in Drosophila melanogaster. Exp Brain Res 217:413–421. https://doi.org/10.1007/s00221-011-2860-3
Quillinan N, Herson PS, Traystman RJ (2016) Neuropathophysiology of brain injury. Anesthesiol Clin 34:453–464. https://doi.org/10.1016/j.anclin.2016.04.011
Rabinstein AA (2018) Traumatic spinal cord injury. CONTINUUM: Lifelong Learning Neurol 24:551–566. https://doi.org/10.1212/con.0000000000000581
Raimundo N, Song L, Shutt TE, McKay SE, Cotney J, Guan M-X, Gilliland TC, Hohuan D, Santos-Sacchi J, Shadel GS (2012) Mitochondrial stress engages E2F1 apoptotic signaling to cause deafness. Cell 148:716–726. https://doi.org/10.1016/j.cell.2011.12.027
Rishal I, Fainzilber M (2014) Axon-soma communication in neuronal injury. Nat Rev Neurosci 15:З2–З42. https://doi.org/10.1038/nrn3609
Roux PP, Barker PA (2002) Neurotrophin signaling through the p75 neurotrophin receptor. Prog Neurobiol 67:203–233
Scafidi J, Hammond TR, Scafidi S, Ritter J, Jablonska B, Roncal M, Szigeti-Buck K, Coman D, Huang Y, McCarter RJ Jr, Hyder F, Horvath TL, Gallo V (2014) Intranasal epidermal growth factor treatment rescues neonatal brain injury. Nature 506:230–234. https://doi.org/10.1038/nature12880
Solà C, Barrón S, Tusell JM, Serratosa J (1999) The Ca2+/calmodulin signaling system in the neural response to excitability. Involvement of neuronal and glial cells. Prog Neurobiol 58:207–232
Sotelo JR, Canclini L, Kun A, Sotelo-Silveira JR, Xu L, Wallrabe H, Calliari A, Rosso G, Cal K, Mercer JA (2013) Myosin-Va-dependent cell-to-cell transfer of RNA from Schwann cells to axons. PLoS One 8:e61905. https://doi.org/10.1371/journal.pone.0061905
Su C, Sun F, Cunningham RL, Rybalchenko N, Singh M (2014) ERK5/KLF4 signaling as a common mediator of the neuroprotective effects of both nerve growth factor and hydrogen peroxide preconditioning. AGE 36:9685. https://doi.org/10.1007/s11357-014-9685-5
Sullivan KD, Galbraith MD, Andrysik Z, Espinosa JM (2018) Mechanisms of transcriptional regulation by p53. Cell Death Differ 25:133–143. https://doi.org/10.1038/cdd.2017.174
Tatton WG, Chalmers-Redman RM, Elstner M, Leesch W, Jagodzinski FB, Stupak DP, Sugrue MM, Tatton NA (2000) Glyceraldehyde-3-phosphate dehydrogenase in neurodegeneration and apoptosis signaling. J Neural Transm Suppl 60:77–100
Thome M (2004) CARMA1, BCL-10 and MALT1 in lymphocyte development and activation. Nat Rev Immunol 4:348–359
Wise PM, Dubal DB, Wilson ME, Rau SW, Böttner M (2001) Minireview: neuroprotective effects of estrogen-new insights into mechanisms of action. Endocrinol 142:969–973
Witiw CD, Fehlings MG (2015) Acute spinal cord injury. J Spinal Disord Tech 28:202–210. https://doi.org/10.1097/BSD.0000000000000287
Wu DY, Zheng JQ, McDonald MA, Chang B, Twiss JL (2003) PKC isozymes in the enhanced regrowth of retinal neurites after optic nerve injury. Invest Ophthalmol Vis Sci 44:2783–2790
Yamamoto S, Kohsaka S, Nakajima K (2012) Role of cell cycle-associated proteins in microglial proliferation in the axotomized rat facial nucleus. Glia 60(4):570–581. https://doi.org/10.1002/glia.22291
Funding
This study was funded by the Ministry of Education and Science of Russian Federation (grant no. 6.6324.2017/8,9 and no. 6.4851.2017/6,7).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Demyanenko, S., Dzreyan, V. & Uzdensky, A. Axotomy-Induced Changes of the Protein Profile in the Crayfish Ventral Cord Ganglia. J Mol Neurosci 68, 667–678 (2019). https://doi.org/10.1007/s12031-019-01329-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-019-01329-5