Abstract
Retinal ganglion cell (RGC) apoptosis is considered an important pathological hallmark of glaucoma. Pituitary adenylate cyclase-activating polypeptide (PACAP) is a pleiotropic peptide with potent neuroprotective properties. In our previous study, we found that the expression of PACAP and its high-affinity receptor PACAP receptor type 1 (PAC1R) increased markedly after optic nerve crush (ONC), and occurred mainly in the ganglion cell layer of the retina. This suggests that the upregulation of PACAP may play a vital role in inhibiting RGC death after ONC. Therefore, in the present study, we investigate the specific effects and underlying mechanism of PACAP in RGC death after ONC. Vehicle (physiological saline) or PACAP (1 nM to 200 nM) solution was injected into the vitreous body. Seven days later, the retinas were harvested, and the surviving RGCs were retrogradely labeled with Fluoro-Gold (FG; Fluorochrome) at different concentrations of PACAP. Immunofluorescence double staining and terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) assay were used to observe the effects of PACAP on RGC apoptosis. Our results showed that PACAP treatment inhibited caspase-3-mediated RGC apoptosis, promoted the phosphorylation of cAMP response element binding protein (CREB), up-regulated the expression of B-cell lymphoma 2 (Bcl-2), and ultimately improved RGC survival. These results suggest that PACAP may prevent RGC apoptosis after ONC via activation of CREB-mediated Bcl-2 transcription. The study thus contributes to a basic understanding of the mechanism by which PACAP decreased RGC apoptosis and provides a theoretical basis for future clinical application of PACAP in the treatment of glaucoma.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Glaucoma, which represents the leading cause of severe visual disability worldwide, is clinically characterized mainly by progressive optic nerve atrophy (Quigley 1996; Tham et al. 2014). Historically, elevation of intraocular pressure (IOP), which is determined by the balance between the rate of aqueous production and its outflow, has been considered the major risk factor for glaucomatous optic neuropathy (Donegan and Lieberman 2016). However, a majority of glaucoma patients present with normal IOP (Prum 2014). In addition, various studies have demonstrated that the development of glaucoma is related to ganglion cell apoptosis (Quigley 2011; Doozandeh and Yazdani 2016). Thus, controlling IOP is not sufficient for glaucoma treatment, and alternative therapies to prevent vision deterioration should be developed, including neuroprotective medications.
Pituitary adenylate cyclase-activating polypeptide (PACAP), which was originally isolated from ovine hypothalamus, possesses potent neurotrophic and neuroprotective effects (Vaudry et al. 2009). It can mediate several physiological actions via the specific PACAP receptor type 1 (PAC1R) or VIP/PACAP receptors (Reichenstein et al. 2008; Kellogg et al. 2010; Miura et al. 2013). PACAP and its receptors have been observed broadly throughout the brain, and they are also identified in the retina (Dénes et al. 2014). PACAP administration has been demonstrated to exert protective effects in cases of retinal injury induced by optic nerve transection (Seki et al. 2008), chronic retinal hypoperfusion (Atlasz et al. 2010b), diabetic retinopathy (Szabadfi et al. 2012), oxidative stress (Fabian et al. 2012), and ischemia (Werling et al. 2017).
Our previous study showed that the expression of PACAP and its high-affinity receptor PAC1R were notably up-regulated after optic nerve crush (ONC), and were mainly expressed in the ganglion cell layer (GCL) of the retina (Ye et al. 2018). These results indicated that endogenous PACAP is implicated in retinal ganglion cell (RGC) death in the ONC model, which effectively mimics RGC apoptosis in glaucoma. In the current study, we conducted a series of experiments to investigate the specific effects and underlying mechanism of exogenous PACAP in RGC death based on the ONC-induced glaucoma model.
Materials and Methods
Reagents
Phosphate-buffered saline (PBS) solution and 4′,6-diamidino-2-phenylindole (DAPI) were obtained from Life Technologies (California, USA). Optimal cutting temperature (OCT) compound was provided by Sakura Finetek (California, USA). Bovine serum albumin (BSA) was obtained from Sigma Chemical (Missouri, USA). Monoclonal antibodies against cleaved caspase-3 (catalog no. 9664S), cyclic AMP response element-binding protein (CREB; catalog no. 9197S), and Phospho-CREB (p-CREB; catalog no. 9198S) were obtained from Cell Signaling Technology (Massachusetts, USA). Monoclonal antibody against neuronal nuclei (NeuN; catalog no. MAB378) was obtained from Millipore (Massachusetts, USA). Monoclonal antibody against B-cell lymphoma 2 (Bcl-2; catalog no. ab182858) was provided by Abcam (Cambridge, UK). PACAP38 was obtained from the Peptide Institute (Osaka, Japan).
Animals and ONC Model
Sprague-Dawley (SD) rats were purchased from the Animal Laboratory of Zhongshan Ophthalmic Center (Guangzhou, China). All animal studies were approved by the Institutional Animal Care and Use Committee of Zhongshan Ophthalmic Center. ONC was carried out in SD rats as previously described (Xu et al. 2014a; Cui et al. 2016). Briefly, the rats were anesthetized with 10% chloral hydrate (0.3 ml/100 g body weight), after which the optic nerve was exposed about 3–4 mm and clamped at 2 mm behind the eye for 10 s. After the procedure, the retina was assessed by funduscopy to eliminate the rats without grossly intact vascular integrity.
PACAP Administration
PACAP was injected into the vitreous humor immediately after building the ONC model. The rats were anesthetized with 10% chloral hydrate (0.3 ml/100 g body weight). PACAP (1 nM to 200 nM) in saline solution was injected into the vitreous cavity with a 33-gauge syringe. The concentration gradient was based on previous studies (Szabo et al. 2012) and pre-experimental results. In the PACAP-treated groups, PACAP38 at concentrations of 1, 10, 100, and 200 nM was administered in a final total volume of 2 μL when performing retrograde staining; the dosage we used in the remaining experiments was 100 nM. The animals underwent eye examinations before injection and at 1, 3, 5, and 7 days through slit-lamp biomicroscopy and indirect ophthalmoscopy. There were no cases of retinal detachment, vitreous hemorrhage, or intraocular inflammation. After 7 days, the retinas were harvested for further experiments. Six to ten eyes were used for each group of experiments. Both eye balls were enucleated and were prepared for the subsequent experiments.
Retrograde Retinal Ganglion Cell Labeling
Rats were anesthetized and fixed in a stereotaxic apparatus (Stoelting; Kiel, Germany), and the skin covering the skull was cut. The lambda and bregma sutures acted as marks of six boreholes. A solution of 4% Fluoro-Gold (FG; Fluorochrome LLC; Colorado, USA) in dimethyl sulfoxide was injected into the superior colliculus. The animals were allowed 7 days of recovery before further experimental intervention to ensure proper RGC labeling. The retinas were then harvested and fixed in 4% paraformaldehyde, and flat-mounted on glass slides. Labeled RGCs were observed with a fluorescence microscope (Leica Microsystems; Mannheim, Germany) and counted in five areas of 1 × 1 mm2 per retina. Each group contained five retinas.
TUNEL Staining
Terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) staining was performed for analysis of apoptosis in the experiments from a subset of samples using a commercially available kit (In Situ Cell Death Detection Kit; Roche Applied Science; Mannheim, Germany). After preparing the cryosections, TUNEL staining was performed following the manufacturer’s instructions. Cell nuclei were counterstained with DAPI. Stained cells were visualized under a fluorescence microscope (Leica Microsystems; Mannheim, Germany). Four fluorescent images per slide were quantitatively assessed using Image-Pro Plus software (Media Cybernetics; Maryland, USA).
Western Blot
Retinas were harvested and isolated from rats of each group, then stored at −80 °C for further use. The samples were homogenized in radioimmunoprecipitation assay (RIPA) buffer including protease inhibitors and centrifuged at 12,000 rpm at 4 °C for 20 min to obtain supernatant. Protein concentrations were measured using a bicinchoninic acid (BCA) protein assay kit (Sigma; Missouri, USA). Protein samples were heated at 99 °C for 5 min and subjected to sodium dodecyl sulfate (SDS)-PAGE electrophoresis, and then transferred to a polyvinylidene difluoride filter (PVDF) membrane (Millipore; Massachusetts, USA) at 250 mA for 1 h. The membranes were blocked with 5% nonfat milk for 2 h at 25 °C, and then incubated with diluted CREB (1:1000), p-CREB (1:1000), and Bcl-2 (1:2000) antibodies at 4 °C overnight. The following day, the PVDF membranes were washed three times and incubated with horseradish peroxidase-conjugated secondary antibody (HRP; 1:10000) for 2 h. Finally, HRP activity was visualized using an enhanced chemiluminescence system (Millipore; Massachusetts, USA).
Immunofluorescence
The cryosections were first blocked with 1% BSA at 25 °C for 2 h to avoid unspecific staining, and then incubated with antibodies against CREB (1:800), p-CREB (1:800), and Bcl-2 (1:800) at 4 °C overnight. For double immunofluorescence staining, the cryosections were co-incubated with monoclonal antibody for cleaved caspase-3 (1:200) and NeuN (1:200). On the next day, fluorescein isothiocyanate (FITC)- and/or CY3-conjugated secondary antibodies and DAPI were mixed and incubated for 2 h in a room protected from light. The stained cryosections were examined with a confocal fluorescence microscope (Leica; Frankfurt, Germany).
Statistical Analysis
All counting and measurements were executed in a blind fashion. Data were expressed as mean ± SEM (standard error of the mean). A p value less than 0.05 was considered to indicate a statistically significant difference. Student’s t test was used when comparing two groups, while one-way analysis of variance (ANOVA), followed by Tukey’s multiple comparison test, was applied when comparing three or more groups. Statistical analyses were performed using GraphPad Prism (v6.0, GraphPad Software; California, USA).
Results
PACAP Reduced the Loss of RGCs in the Retina after ONC
To confirm the indirect modulatory role performed by PACAP with regard to RGC survival, we used FG retrograde labeling based on quantification of retrogradely labeled RGC soma on the flat-mounted retina after ONC. The surviving RGCs with a round shape were visible by FG labeling (Fig. 1a), and the number of surviving RGCs decreased after ONC (Fig. 1b; ***P < 0.001). With PACAP treatment, RGC survival was up-regulated in a dose-dependent manner. The protective effect of PACAP was not evident at a dose of 1 nM or 10 nM, but it was considerable at a dose of 100 nM compared with the ONC group (Fig. 1b; ***P < 0.001). The neuroprotective effects of PACAP at 100 nM and 200 nM were statistically equal (Fig. 1b). Thus, a concentration of 100 nM was selected for subsequent experiments.
Effect of PACAP on RGC survival after ONC. Fluoro-Gold labeling was performed to analyze the number of surviving RGCs after ONC at different PACAP concentrations. a Representative image of Fluoro-Gold labeling in each group. b Graph of RGC survival rates showing significant differences. Data are presented as mean ± SEM. ***P < 0.001. PACAP, pituitary adenylate cyclase-activating polypeptide; RGCs, retinal ganglion cells; ONC, optic nerve crush
PACAP Prevented Apoptosis of RGCs in the Retina after ONC
To identify the anti-apoptotic effects of PACAP, TUNEL staining was performed to investigate whether PACAP could attenuate ONC-induced RGC apoptosis (Fig. 2a). Marked upregulation of TUNEL-positive cells was detected in the retina after ONC compared to the control group (Fig. 2b; ***P < 0.001), most of which were located in the GCL (Fig. 2a), while the number of TUNEL-positive cells after ONC declined significantly with the application of 100 nM PACAP treatment (Fig. 2b; **P < 0.01), indicating an anti-apoptotic effect of PACAP on RGCs after ONC.
Effect of PACAP (100 nM) on RGC apoptosis after ONC. TUNEL staining was performed to analyze the changes in the number of apoptotic cells in the retina after ONC with PACAP treatment. a Representative image of TUNEL staining in each group. b Graph of TUNEL-positive cells in the ganglion cell layer showing significant differences. Data are presented as mean ± SEM. ***P < 0.001, **P < 0.01. PACAP, pituitary adenylate cyclase-activating polypeptide; RGCs, retinal ganglion cells; ONC, optic nerve crush; TUNEL, terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling
PACAP Attenuated the Activation of Caspase-3 in the Retina after ONC
As shown previously (Yang et al. 2016; Xu et al. 2017), caspase-3 activity was found to be involved in regulating RGC apoptosis. To verify the effect of PACAP on RGC apoptosis, double immunofluorescence staining was performed to explore the cleaved caspase-3 expression in the retina after ONC (Fig. 3a). Co-localization of cleaved caspase-3 and NeuN was observed in RGCs, and increased cleaved caspase-3 was detected after ONC compared with the control group (Fig. 3b; ***P < 0.001). After intravitreal injection of PACAP, cleaved caspase-3 was notably decreased compared with the ONC group (Fig. 3b; **P < 0.01). These data suggest that PACAP inhibits RGC apoptosis after ONC in a caspase-3-dependent way.
Effect of PACAP (100 nM) on the activity of caspase-3 in the retina after ONC. Double immunofluorescence staining was performed to analyze the activation of caspase-3 with cleaved caspase-3 and NeuN from the control group, ONC group, and ONC-plus-PACAP group. a Representative image of double immunofluorescence staining with cleaved caspase-3 (red) and NeuN (green) in each group. b Graph of cleaved caspase-3 intensity showing significant differences. Data are presented as mean ± SEM. ***P < 0.001, **P < 0.01. Scale bar = 100 μm. PACAP, pituitary adenylate cyclase-activating polypeptide; ONC, optic nerve crush; NeuN, neuronal nuclei
PACAP Promoted the Activation of Bcl-2 in the Retina after ONC
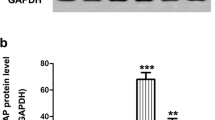
As a member of Bcl-2 gene family, Bcl-2 plays an important role in anti-apoptosis, which can be mediated by CREB (Almasieh et al. 2012). To better understand the association between PACAP and RGC protection, western blot analysis was initially performed to examine the expression of Bcl-2 at day 7 after ONC (Fig. 4a). The expression of Bcl-2 was slightly up-regulated in the ONC group, with no statistical difference compared to the control group (Fig. 4b), but was substantially increased in the ONC-plus-PACAP group (Fig. 4b; ****P < 0.000, ***P < 0.001). In addition, the results of immunofluorescence staining were consistent with those of western blot, showing that PACAP promoted the expression of Bcl-2 in the retina after ONC (Fig. 4c). These results suggest that PACAP may reduce RGC apoptosis after ONC by up-regulating the expression of Bcl-2.
Effect of PACAP (100 nM) on the activity of Bcl-2 in the retina after ONC. Western blot and immunofluorescence staining were performed to analyze the activation of Bcl-2 in the control group, ONC group, and ONC-plus-PACAP group. a The protein level of Bcl-2 was evaluated by western blot analysis. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used to ensure equal loading. b Densitometric analysis of the effects of PACAP on Bcl-2 expression. Data are presented as mean ± SEM. ****P < 0.000, ***P < 0.001. c Representative image of immunofluorescence staining with Bcl-2 (red) and DAPI (blue) in each group. Scale bar = 100 μm. PACAP, pituitary adenylate cyclase-activating polypeptide; ONC, optic nerve crush; Bcl-2, B-cell lymphoma 2; GAPDH, glyceraldehyde-3-phosphate dehydrogenase
PACAP Up-Regulated the Activation of CREB in the Retina after ONC
A wide variety of reports show that CREB is involved in the survival mechanism of neurons. The activity of the transcription factor CREB is enhanced when Ser133 is phosphorylated (Sakamoto et al. 2011). We conducted western blot analysis to examine whether PACAP affects the phosphorylation status of CREB. The results showed that expression of CREB was nearly equal among the three groups (Fig. 5a, c); however, p-CREB was increased after ONC compared with the control group (Fig. 5a, b; *P < 0.05), and PACAP treatment enhanced the phosphorylation of CREB (Fig. 5a, b; ***P < 0.001, **P < 0.01). Immunofluorescence staining results mirrored those of western blot, showing that PACAP promoted p-CREB expression in the retina after ONC (Fig. 5d). These results suggest that PACAP may reduce RGC apoptosis after ONC by enhancing the phosphorylation of CREB.
Effect of PACAP (100 nM) on the activity of CREB in the retina after ONC. Western blot and immunofluorescence staining were performed to analyze the activation of CREB in the control group, ONC group, and ONC-plus-PACAP group. a The protein levels of CREB and p-CREB were evaluated by western blot analysis. b Densitometric analysis of p-CREB expression. The phosphorylation status of CREB was determined by the ratio of phosphorylated proteins to un-phosphorylated proteins. Data are presented as mean ± SEM. ***P < 0.001, **P < 0.01, *P < 0.05. c Densitometric analysis of CREB expression. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used to ensure equal loading. Data are presented as mean ± SEM. d Representative image of immunofluorescence staining with p-CREB (red) and DAPI (blue) in each group. Scale bar = 100 μm. PACAP, pituitary adenylate cyclase-activating polypeptide; CREB, cyclic AMP response element-binding protein; ONC, optic nerve crush; p-CREB, Phospho-CREB; GAPDH, glyceraldehyde-3-phosphate dehydrogenase
Discussion
Glaucoma, a progressive optic neuropathy caused by pathological high IOP, is characterized by RGC apoptosis and progressive loss of visual function (Quigley 2011; Xu et al. 2018). Treatment strategies include lowering of IOP and administration of neuroprotective agents. In addition, intravitreal injection of brain-derived neurotrophic factor has been shown to prevent ganglion cell death (Kido et al. 2000). Further research is thus needed on neuroprotective treatment in glaucoma to reduce the rate of blindness.
The ONC model is an important experimental disease model that mimics typical changes in the optic nerve head, progressive optic nerve degeneration, and gradual RGC apoptosis. It is widely used to investigate the overall course and mechanisms of neuronal death and survival, which is fundamental for the development of therapeutic measures (Tang et al. 2011). In addition, this model is reproducible and quantifiable, and has been well tested. For these reasons, we chose the ONC model to explore the underlying mechanisms of RGC survival and to investigate the potential neuroprotective efficacy of PACAP in experimental glaucoma.
In this study, we aimed to determine whether topical application of PACAP could elicit neuroprotective effects, and to elucidate the molecular mechanisms involved. Our results revealed that PACAP had direct protective effects on RGC death via inhibition of the caspase-3-dependent apoptotic pathway. In addition, we found that PACAP could reduce RGC loss through activation of CREB-Bcl-2 signaling pathways.
PACAP, a pleiotropic peptide, is found to exert a wide variety of biological effects, including the regulation of neurotransmitter release, vasodilation, bronchodilation, activation of intestinal motility, increased insulin and histamine secretion, immunomodulation, and stimulation of cell proliferation and/or differentiation (Vaudry et al. 2009). Dozens of studies have reported that PACAP exhibits neuroprotective effects after brain ischemic injury (Uchida et al. 1996; Gillardon et al. 1998), and dramatically reduces neuronal death by inhibiting the activation of c-Jun N-terminal kinase (JNK), which contributes to the induction of apoptosis. Additionally, the ipsilateral hemispheres of hetero- and homozygous PACAP knockout mice were observed to have accumulated cytochrome C released from the mitochondria to the cytoplasm (Shioda et al. 2006), which is an initial stage of apoptosis. Accordingly, there is a strong possibility that PACAP acts to protect neurons against cell death via anti-apoptotic pathways. Furthermore, studies showed that PACAP was effective in protecting retinal pigment epithelial cells from oxidative stress-induced apoptosis (Mester et al. 2011), and PACAP significantly ameliorated diabetic retinal injury by promoting increased levels of anti-apoptotic factors (p-Akt, p-ERK1, p-ERK2, PKC, Bcl-2) and reduced levels of pro-apoptotic factors (p-p38MAPK; activated caspases 8, 3, 12) (Szabadfi et al. 2014). These results demonstrate the potential anti-apoptotic effect of PACAP in retinal diseases.
NeuN is a reliable nuclear protein marker used to verify and quantitate RGCs in the retina (Gusel’nikova and Korzhevskiy 2015); cleaved caspase-3, which is known as a marker of apoptosis, is an active form of caspase-3 (Almasieh et al. 2012). In the current study, the number of cells co-expressed with cleaved caspase-3 and NeuN was clearly greater in the ONC group than in the control group, and likewise was decreased with PACAP administration at a dose of 100 nM. To further verify the presence of apoptosis in RGCs, TUNEL staining was conducted to identify whether PACAP could attenuate ONC-induced RGC apoptosis. Similarly, a greater number of TUNEL-positive cells were detected in the retina after ONC than in controls. After PACAP treatment, fewer TUNEL-positive cells were observed in the GCL of the retina compared with the untreated ONC group. Therefore, our results confirm the anti-apoptotic effect of PACAP on RGCs in the retina after ONC.
The molecular mechanisms of RGC apoptosis involve diverse stimuli including intrinsic and extrinsic pathways (Almasieh et al. 2012), which can both be induced after ONC. These molecular pathways are considered to be critical signals in regulating RGC death in glaucoma. The extrinsic pathway includes a series of complex processes beginning with the activation of the death receptor pathway, which results in the recruitment of the intracellular adaptor Fas-associated death domain (FADD), leading to the recruitment of the initiator procaspase-8 triggering caspase-8 activation, and ultimately activates executioner caspase-3 and cell death. In the intrinsic pathway, cytochrome C released from the mitochondria forms the apoptosome together with the apoptotic protease-activating factor-1 (Apaf-1) and procaspase-9, which enhances the activation of caspase-9 and downstream cleavage of caspase-3. The above mechanisms indicate that the activation of executioner caspase-3 is downstream of both the extrinsic and intrinsic pathways (Almasieh et al. 2012). A wide array of research has demonstrated that the upregulation of active, cleaved caspase-3 has been detected in RGCs after optic nerve injury (Weishaupt et al. 2003; Grosskreutz et al. 2005), and the inhibition of caspases increases RGC survival. Consistent with this, we found that expression of cleaved caspase-3 in NeuN-positive RGCs was significantly increased after ONC, which indicates that activation of the caspase-3-dependent pathway was involved. However, vitreous injection of PACAP significantly reduced the expression of cleaved caspase-3. These results suggest that PACAP can prevent RGC apoptosis in the retina after ONC via inhibition of caspase-3-dependent pro-apoptotic pathways.
CREB is a crucial transcription factor for various physiological processes involving neuronal protection and cell survival (Lonze and Ginty 2002; Fukuchi et al. 2005; Benito and Barco 2010; Sakamoto et al. 2011). Mechanistically, production of cyclic adenylyl cyclase monophosphate (cAMP) following activation of PAC1R induces phosphorylation of CREB. Specifically, PACAP binds to PAC1R, which is coupled to adenylyl cyclase (AC), and activates the cAMP-PKA pathway; PKA then activates CREB phosphorylation in the nucleus (Silveira et al. 2002; Atlasz et al. 2010a). PAC1R can also be coupled to phospholipase C (PLC), and mediates Ca2+ release through PLC, resulting in phosphorylation of CREB, which blocks the apoptotic machinery. However, some studies have reported that inhibition of PLC did not inhibit the protective effects of PACAP. Therefore, the cAMP/PKA pathway is responsible for neuroprotection modulated by CREB after PACAP administration (Silveira et al. 2002; Lakk et al. 2015).
Previous studies have shown that activation of CREB, which can function as a constitutive transcriptional activator via phosphorylation, is also involved in RGC protection (Xu et al. 2014b). A substantial amount of work unraveling the molecular mechanisms by which CREB regulates neuronal survival has focused on two streams (Riccio et al. 1999; Lambert et al. 2001; Tabuchi et al. 2002; Fukuchi et al. 2005). First, CREB mediates the transcription of neurotrophic factors, such as PACAP, which confers direct neuroprotection (Fukuchi et al. 2005). Second, neurotrophins regulate neuronal survival by CREB-mediated expression of the anti-apoptotic gene Bcl-2 transcription (Riccio et al. 1999). Bcl-2 belongs to the Bcl-2 gene family regulating RGC apoptosis (Bonfanti et al. 1996), and seems to be a pivotal anti-apoptotic protein in the rat retina among anti-apoptotic family members for transgenic mice over-expressing Bcl-2, resulting in increased numbers of RGCs during developmental cell death and after optic nerve axotomy (Bonfanti et al. 1996; Cenni et al., 1996). Apart from the above-described molecular mechanisms, CREB also binds to the cAMP-responsive element (CRE), through which the promoters of PACAP can be specifically activated (Sakamoto et al. 2011). This is in line with our data showing that p-CREB was massively up-regulated with PACAP treatment in the retina after ONC. Additionally, upregulation of Bcl-2 expression was found in the ONC-plus-PACAP group, which implies that PACAP elicited RGC neuroprotection via the CREB-Bcl-2 signaling pathway after ONC. Taken together, these results lead us to infer that PACAP can play a neuroprotective role in the retina after ONC by blocking RGC apoptosis, as reflected by the increase in p-CREB, and subsequent induction of Bcl-2 transcription.
Collectively, the results of this study provide evidence that PACAP reduced apoptosis of RGCs after ONC by inhibiting the caspase-3-dependent pathway in the retina. The neuroprotective and anti-apoptotic function of PACAP was exerted by provoking the activation of the CREB-Bcl-2 signaling pathway. These observations imply that PACAP played a neuroprotective role via anti-apoptosis in experimental glaucoma. Therefore, the current study suggests that application of PACAP represents a promising strategy for the treatment of glaucoma or other optic nerve diseases.
References
Almasieh M, Wilson AM, Morquette B et al (2012) The molecular basis of retinal ganglion cell death in glaucoma. Prog Retin Eye Res 31:152–181. https://doi.org/10.1016/j.preteyeres.2011.11.002
Atlasz T, Szabadfi K, Kiss P et al (2010a) Pituitary adenylate cyclase activating polypeptide in the retina: focus on the retinoprotective effects. Ann N Y Acad Sci 1200:128–139. https://doi.org/10.1111/j.1749-6632.2010.05512.x
Atlasz T, Szabadfi K, Kiss P et al (2010b) Evaluation of the protective effects of PACAP with cell-specific markers in ischemia-induced retinal degeneration. Brain Res Bull 81:497–504. https://doi.org/10.1016/j.brainresbull.2009.09.004
Benito E, Barco A (2010) CREB’s control of intrinsic and synaptic plasticity: implications for CREB-dependent memory models. Trends Neurosci 33:230–240. https://doi.org/10.1016/j.tins.2010.02.001
Bonfanti L, Strettoi E, Chierzi S et al (1996) Protection of retinal ganglion cells from natural and axotomy-induced cell death in neonatal transgenic mice overexpressing bcl-2. J Neurosci 16:4186–4194. https://doi.org/10.4028/www.scientific.net/AMM.823.23
Cui L, He WJ, Xu F et al (2016) Alterations in the expression of Hs1-associated protein X-1 in the rat retina after optic nerve crush. Mol Med Rep 14:4761–4766. https://doi.org/10.3892/mmr.2016.5824
Dénes V, Czotter N, Lakk M et al (2014) PAC1-expressing structures of neural retina alter their PAC1 isoform splicing during postnatal development. Cell Tissue Res 355:279–288. https://doi.org/10.1007/s00441-013-1761-0
Donegan RK, Lieberman RL (2016) Discovery of molecular therapeutics for Glaucoma: challenges, successes, and promising directions. J Med Chem 59:788–809. https://doi.org/10.1021/acs.jmedchem.5b00828
Doozandeh A, Yazdani S (2016) Neuroprotection in glaucoma. J Ophthalmic Vis Res 11:209–220. https://doi.org/10.4103/2008-322X.183923
Fabian E, Reglodi D, Mester L et al (2012) Effects of PACAP on intracellular signaling pathways in human retinal pigment epithelial cells exposed to oxidative stress. J Mol Neurosci 48:493–500. https://doi.org/10.1007/s12031-012-9812-7
Fukuchi M, Tabuchi A, Tsuda M (2005) Transcriptional regulation of neuronal genes and its effect on neural functions: cumulative mRNA expression of PACAP and BDNF genes controlled by calcium and cAMP signals in neurons. J Pharmacol Sci 98:212–218
Gillardon F, Hata R, Hossmann KA (1998) Delayed up-regulation of Zac1 and PACAP type I receptor after transient focal cerebral ischemia in mice. Mol Brain Res 61:207–210. https://doi.org/10.1016/S0169-328X(98)00202-2
Grosskreutz CL, Hänninen VA, Pantcheva MB et al (2005) FK506 blocks activation of the intrinsic caspase cascade after optic nerve crush. Exp Eye Res. https://doi.org/10.1016/j.exer.2004.11.017
Gusel’nikova VV, Korzhevskiy DE (2015) NeuN as a neuronal nuclear antigen and neuron differentiation marker. Acta Nat 7:42–47. https://doi.org/10.1038/bjc.2017.28
Kellogg DL, Zhao JL, Wu Y, Johnson JM (2010) VIP/PACAP receptor mediation of cutaneous active vasodilation during heat stress in humans. J Appl Physiol 109:95–100. https://doi.org/10.1152/japplphysiol.01187.2009
Kido N, Tanihara H, Honjo M et al (2000) Neuroprotective effects of brain-derived neurotrophic factor in eyes with NMDA-induced neuronal death. Brain Res. https://doi.org/10.1016/S0006-8993(00)02887-0
Lakk M, Denes V, Gabriel R (2015) Pituitary adenylate cyclase-activating polypeptide receptors signal via phospholipase C pathway to block apoptosis in newborn rat retina. Neurochem Res 40:1402–1409. https://doi.org/10.1007/s11064-015-1607-0
Lambert HW, Weiss ER, Lauder JM (2001) Activation of 5-HT receptors that stimulate the adenylyl cyclase pathway positively regulates IGF-I in cultured craniofacial mesenchymal cells. Dev Neurosci. https://doi.org/10.1159/000048697
Lonze BE, Ginty DD (2002) Function and regulation of CREB family transcription factors in the nervous system. Neuron 35(4):605–623
Mester L, Kovacs K, Racz B et al (2011) Pituitary adenylate cyclase-activating polypeptide is protective against oxidative stress in human retinal pigment epithelial cells. J Mol Neurosci 43:35–43. https://doi.org/10.1007/s12031-010-9427-9
Miura A, Kambe Y, Inoue K et al (2013) Pituitary adenylate cyclase-activating polypeptide type 1 receptor ( PAC1 ) gene is suppressed by transglutaminase 2 activation. J Biol Chem 288:32720–32730. https://doi.org/10.1074/jbc.M113.452706
Prum BE (2014) Normal-tension glaucoma. In: Clinical glaucoma care: the essentials
Quigley HA (1996) Number of people with glaucoma worldwide. Br J Ophthalmol. https://doi.org/10.1136/bjo.80.5.389
Quigley HA (2011) Glaucoma. Lancet 377:1367–1377. https://doi.org/10.1016/S0140-6736(10)61423-7
Reichenstein M, Rehavi M, Pinhasov A (2008) Involvement of pituitary adenylate cyclase activating polypeptide (PACAP) and its receptors in the mechanism of antidepressant action. J Mol Neurosci 36:330–338. https://doi.org/10.1007/s12031-008-9116-0
Riccio A, Ahn S, Davenport CM, et al (1999) Mediation by a CREB family transcription factor of NGF-dependent survival of sympathetic neurons. Science (80- ). https://doi.org/10.1126/science.286.5448.2358
Sakamoto K, Karelina K, Obrietan K (2011) CREB: a multifaceted regulator of neuronal plasticity and protection. J Neurochem 116:1–9. https://doi.org/10.1111/j.1471-4159.2010.07080.x
Seki T, Itoh H, Nakamachi T, Shioda S (2008) Suppression of ganglion cell death by PACAP following optic nerve transection in the rat. J Mol Neurosci 36:57–60. https://doi.org/10.1007/s12031-008-9091-5
Shioda S, Ohtaki H, Nakamachi T et al (2006) Pleiotropic functions of PACAP in the CNS: neuroprotection and neurodevelopment. Ann N Y Acad Sci 1070:550–560. https://doi.org/10.1196/annals.1317.080
Silveira MS, Costa MR, Bozza M, Linden R (2002) Pituitary adenylyl cyclase-activating polypeptide prevents induced cell death in retinal tissue through activation of cyclic AMP-dependent protein kinase. J Biol Chem 277:16075–16080. https://doi.org/10.1074/jbc.M110106200
Szabadfi K, Atlasz T, Kiss P et al (2012) Protective effects of the neuropeptide PACAP in diabetic retinopathy. Cell Tissue Res 348:37–46. https://doi.org/10.1007/s00441-012-1349-0
Szabadfi K, Szabo A, Kiss P et al (2014) PACAP promotes neuron survival in early experimental diabetic retinopathy. Neurochem Int 64:84–91. https://doi.org/10.1016/j.neuint.2013.11.005
Szabo A, Danyadi B, Bognar E et al (2012) Effect of PACAP on MAP kinases, Akt and cytokine expressions in rat retinal hypoperfusion. Neurosci Lett 523:93–98. https://doi.org/10.1016/j.neulet.2012.06.044
Tabuchi A, Sakaya H, Kisukeda T et al (2002) Involvement of an upstream stimulatory factor as well as cAMP-responsive element-binding protein in the activation of brain-derived neurotrophic factor gene promoter I. J Biol Chem. https://doi.org/10.1074/jbc.M204784200
Tang Z, Zhang S, Lee C et al (2011) An optic nerve crush injury murine model to study retinal ganglion cell survival. J Vis Exp. https://doi.org/10.3791/2685
Tham YC, Li X, Wong TY et al (2014) Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology
Uchida D, Arimura A, Somogyvári-Vigh A et al (1996) Prevention of ischemia-induced death of hippocampal neurons by pituitary adenylate cyclase activating polypeptide. Brain Res 736:280–286. https://doi.org/10.1016/0006-8993(96)00716-0
Vaudry D, Falluel-morel A, Bourgault S et al (2009) Pituitary adenylate cyclase-activating polypeptide and its receptors : 20 years after the discovery. Pept Res 61:283–357. https://doi.org/10.1124/pr.109.001370.283
Weishaupt JH, Diem R, Kermer P et al (2003) Contribution of caspase-8 to apoptosis of axotomized rat retinal ganglion cells in vivo. Neurobiol Dis. https://doi.org/10.1016/S0969-9961(03)00032-9
Werling D, Banks WA, Salameh TS et al (2017) Passage through the ocular barriers and beneficial effects in retinal ischemia of topical application of PACAP1-38 in rodents. Int J Mol Sci 18:1–12. https://doi.org/10.3390/ijms18030675
Xu F, Huang H, Wu Y et al (2014a) Upregulation of gem relates to retinal ganglion cells apoptosis after optic nerve crush in adult rats. J Mol Histol 45:565–571. https://doi.org/10.1007/s10735-014-9579-y
Xu Y, Yu S, Shu Q et al (2014b) Upregulation of CREM-1 relates to retinal ganglion cells apoptosis after light-induced damage in vivo. J Mol Neurosci 52:331–338. https://doi.org/10.1007/s12031-013-0153-y
Xu Y, Yang B, Hu Y et al (2017) Secretion of down syndrome critical region 1 isoform 4 in ischemic retinal ganglion cells displays anti-Angiogenic properties via NFATc1-dependent pathway. Mol Neurobiol 54:6556–6571. https://doi.org/10.1007/s12035-016-0092-z
Xu J, Lu P, Dai M et al (2019) The relationship between binocular visual field loss and various stages of monocular visual field damage in glaucoma patients. J Glaucoma 28:42–50. https://doi.org/10.1097/IJG.000000000000110
Yang C, Xu Y, Zhou H et al (2016) Tetramethylpyrazine protects CoCl2-induced apoptosis in human umbilical vein endothelial cells by regulating the PHD2/HIF/1α-VEGF pathway. Mol Med Rep 13:1287–1296. https://doi.org/10.3892/mmr.2015.4679
Ye D, Yang Y, Lu X et al (2018) Spatiotemporal expression changes of PACAP and its receptors in retinal ganglion cells after optic nerve crush. J Mol Neurosci 1. https://doi.org/10.1007/s12031-018-1203-2
Acknowledgements
This work is supported by the National Natural Science Foundation of China (81670850) and the Natural Science Foundation of Guangdong Province in China (2018A030310144).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical Approval
All procedures performed in studies involving animals were carried out according to the US National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals put forth by the US National Academy of Sciences, with the approval of the Administration Committee of Experimental Animals, Guangdong Province, China.
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ye, D., Shi, Y., Xu, Y. et al. PACAP Attenuates Optic Nerve Crush-Induced Retinal Ganglion Cell Apoptosis Via Activation of the CREB-Bcl-2 Pathway. J Mol Neurosci 68, 475–484 (2019). https://doi.org/10.1007/s12031-019-01309-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-019-01309-9