Abstract
Recent studies have suggested antidepressant involvement in synaptic plasticity, possibly mediated by neurotrophins and neuropeptides. Pituitary adenylate cyclase activating polypeptide (PACAP) is a neuropeptide and neuromodulator. Since its discovery, PACAP has been extensively investigated with regard to its neurotrophic properties including regulation of brain-derived neurotrophic factor (BDNF) expression, a neurotrophin postulated to be involved in the mechanism of antidepressant action and etiology of affective disorders. Using real-time polymerase chain reaction (PCR) technique, we demonstrate in this paper a robust upregulation of BDNF messenger RNA (mRNA) expression in rat primary cortical neurons following a 6-hour incubation with PACAP, and subsequently elevated BDNF expression after prolonged treatment. Additional experiments were conducted to evaluate the effects of antidepressants on the expression of PACAP, its receptors and BDNF. In rat hippocampal neurons, prolonged (72-hour) treatment with selective serotonin reuptake inhibitors paroxetine and citalopram significantly up-regulated BDNF and PACAP expression and down-regulated PACAP receptor (PAC1 and VPAC2) expression; the tricyclic antidepressant imipramine had an opposite effect. These alterations in BDNF expression correlated negatively with PAC1 and VPAC2 expression, and positively with PACAP mRNA levels. Thus, our findings suggest the possible involvement of PACAP signaling in the neuronal plasticity induced by antidepressant treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pituitary adenylate cyclase activating polypeptide (PACAP) is widely expressed in the central and peripheral nervous systems, exerting an extensive profile of biological activities including its function as a neurohormone, neurotransmitter, and neuromodulator (Arimura and Shioda 1995; Sherwood et al. 2000; Vaudry et al. 2000; Waschek 2002). It is a member of the vasoactive intestinal peptide (VIP)/secretine/glucagone family of peptides (Miyata et al 1989) and exists in two amidated forms that result from alternative splicing: PACAP27 and PACAP38. The latter is the prevalent form in mammalian tissues (Miyata et al. 1989, 1990). The effects of PACAP are mediated by two types of G-protein coupled receptors. Type I receptor (PAC1) is the predominant PACAP receptor in the brain (Cauvin et al. 1991; Shioda 2000), exhibiting a high affinity for PACAP (Kd = 0.5 nM) and a much lower affinity for VIP (Kd > 500 nM) (Gottschall et al. 1990; Lam et al. 1990; Cauvin et al. 1991; Suda et al. 1991). Type II PACAP receptors, VPAC1 and VPAC2, have equal affinity for PACAP and VIP (Kd = 1 nM). Previous studies indicated that PACAP plays a role during neural development, and may be involved in pathological conditions as a part of developmental mechanisms reemployed following neuronal injury (Waschek 2002). PACAP exhibits trophic effects in various neuronal systems and is considered to interact with growth factors influencing neuronal survival (Somogyvari-Vigh and Reglodi 2004). In primary cultured neurons, PACAP was reported to stimulate the expression of brain-derived neurotrophic factor (BDNF) (Frechilla et al. 2001), one of the most studied neurotrophins that is critical for axonal growth, neuronal survival, and synaptic plasticity. It is differently regulated by stress and antidepressants, and is postulated to be involved in the etiology of depression and the clinical action of antidepressants (Altar 1999; Duman and Monteggia 2006; Pittenger and Duman 2008). Concomitantly, PACAP represents an essential element of the hypothalamic–pituitary–adrenal (HPA) axis, a mechanism activated in reaction to stress. HPA axis hyperactivity had been proposed to play a role in stress-related depressive conditions (Vinet et al. 2004; Malkesman et al. 2006) leading to increased plasma glucocorticoids, while antidepressants were demonstrated to reduce brain glucocorticoid levels (Weber et al. 2006).
It was reported that different classes of antidepressants, including the selective serotonin reuptake inhibitors (SSRI) and tricyclic antidepressants (TCA), significantly induce the expression of BDNF messenger RNA (mRNA) in the major subfields of the hippocampus in rodents (for review, see Duman and Monteggia 2006). Additionally, analyses of post-mortem hippocampal tissues obtained from antidepressant-treated subjects revealed significantly higher BDNF protein levels compared to tissues obtained from untreated subjects (Chen et al. 2001; Dwivedi et al. 2003), suggesting that antidepressants exert their effects at least partially through an up-regulation of BDNF expression. Moreover, several clinical trials demonstrate a reduction in serum BDNF levels in subjects diagnosed with severe depression (Karege et al. 2002; Shimizu et al. 2003), while antidepressants were shown to reverse this effect (Aydemir et al. 2005; Gervasoni et al. 2005; Gonul et al. 2005).
The goal of the current study was to explore PACAP involvement in the mechanism of antidepressants action. To address this question, we first evaluated the effect of PACAP38 on the expression of BDNF mRNA in rat primary cortical neurons. Subsequently, we examined the effects of three commonly prescribed antidepressants, citalopram, paroxetine, and imipramine, on the expression of PACAP, its receptors, and BDNF in rat primary hippocampal neurons using a quantitative real-time polymerase chain reaction (PCR) approach.
Materials and Methods
Cell Cultures
Primary hippocampal and cortical neuron-enriched cell cultures were established from postnatal day 1 (P1) Sprague–Dawley rat pups (Harlan Laboratories, Israel, Institutional License number IL-13-10-06). Dissected tissues were incubated for 20 min at 37°C in serum-free Dulbecco’s Modified Eagle Medium (DMEM) (Gibco, 11960-028) containing 100 units/ml penicillin–streptomycin (P/S) (Gibco, 15140-122) and 0.25% trypsin solution (Gibco, 15090-046). The trypsin digestion was terminated by transferring the tissues into DMEM containing 10% fetal calf serum (FCS), followed by mechanical cell dissociation by trituration through a Pasteur pipette. The cell suspension was centrifuged at 1300×g for 3 min followed by resuspension in neurobasal medium (NB) (Gibco, 211030-49) supplemented with 1 × B-27 (Gibco, 17504-044), 0.5 mM l-glutamine and 100 units/ml P/S solution, which was used further for cell culturing. For the gene expression assay, cortical or hippocampal cells were plated onto six-well plates (Costar) coated with poly-d-lysine (PDL) (0.1 μg/ml, Sigma, P1024) at a density of 2 × 105 cells per well. For the cell viability assay, cells were plated onto 96-well plates, coated with PDL at the density of 3 × 104 cells per well. All cultures were maintained in NB medium at 37°C in an atmosphere containing 5% CO2 for 7 days prior to treatment.
Drug and Peptide Treatments
Cortical neuron-enriched cultures were treated with peptides PACAP38 (H-8430, Bachem) and PACAP(6-38) (H-2734, Bachem). Hippocampal neuron-enriched cultures were treated with the antidepressants imipramine hydrochloride (17379-5G, Sigma-Aldrich, Israel), citalopram hydrobromide (LU10-171-B, H. Lundbeck, Denmark) and paroxetine hydrochloride hemihydrate (P9623-10MG, Sigma-Aldrich, Israel). The medium was changed daily prior to drug/peptide treatment to avoid unwanted cumulative effects. All the compounds were initially dissolved in water followed by preparation of working concentrations in an appropriate medium. Control samples were treated with vehicle (water).
RNA Extraction, Reverse Transcription, and Quantitative PCR
Total RNA was isolated using a Versagene RNA purification kit, including DNAse treatment procedure (Gentra, 200561). A total of 0.5–1.0 μg RNA was reverse-transcribed according to the manufacturer’s instructions (Stratascript 5.0 multi-temp RT kit, Stratagene), using oligo-dT 18-bp primers (C1101, Promega). The relative expression of the investigated genes was measured by real-time quantitative PCR on an M×3000P detection system (Stratagene) using the Dynamo HS SYBR Green master kit (Finnzymes, FZ-F-410L). As an endogenous normalization factor, the transcript levels of the hypoxanthine-guanine phosphoribosyltransferase (HPRT) gene were used. All real-time PCR reactions were carried out in triplicate and the results were analyzed with the comparative CT method using MxPRO software (Stratagene). Primers were designed using Primer3 Software (http://frodo.wi.mit.edu/). Primer sequences and features are depicted in Table 1. Product specificity was confirmed in the initial experiments by agarose gel electrophoresis and routinely by melting curve analysis.
Quantitation of Cell Viability (MTS)
Metabolic activity of viable cells in culture was measured by a calorimetric method using a tetrazolium compound (3-(4,5-dimethylthiazol-2-y1-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H tetrazolium) and an electron-coupling reagent phenazine methasulfate using CellTiter96 Aqueous Non-Radioactive Cell Proliferation assay (Promega, G5421) applied directly onto 96-well plates. The tetrazolium compound is bio-reduced by the living cells to the formazan form that is detected at 490 nm by an enzyme-linked immunosorbent assay (ELISA) reader.
Statistical Analyses
Data are presented as the mean ± SEM from three independent experiments performed in triplicate. Statistical analyses were performed on PrismPad software using student t-test. A p value of < 0.05 was accepted as a statistically significant difference.
Results
Effect of PACAP38 and the Antagonist PACAP (6-38) on BDNF and PAC1 Expression in Primary Cortical Neurons
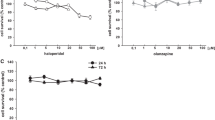
The effect of PACAP on BDNF gene expression was evaluated either after single (acute) or extended (3 days, daily) treatment. Cortical neurons were treated with PACAP38 (10 nM or 100 nM) or its antagonist PACAP (6-38) (10 nM) for 6, 24, 48, or 72 hours. The medium was changed daily prior to peptide treatment. After the incubation period total RNA was extracted and subjected to gene expression studies. Both acute (6 h) and prolonged (48 h and 72 h) incubation with PACAP38 significantly up-regulated BDNF mRNA expression (Fig. 1, ** at p < 0.001, and ***at p < 0.0001). The dose-dependent effect of PACAP38 on BDNF expression was observed only after 6-hour incubation. Daily treatment with the neuropeptide for 48 and 72 hours maintained elevated levels of BDNF mRNA compared to the control; however, the dose-dependent effect was absent. Additionally, we report a robust effect of PACAP38 treatment on BDNF expression observed after 6 hours, compared to the milder yet significant effect of the extended exposure to PACAP38 after both 48 and 72 hours. Interestingly, no effect of PACAP38 on BDNF expression was observed after 24 hours. PACAP (6-38) exhibited an opposite effect to PACAP38, causing BDNF down-regulation either after 48 or 72 hours of treatment. No effect of the antagonist on BDNF expression was observed after either 6- or 24-hour incubation. In parallel, the effect of exogenous PACAP38 on its specific receptor PAC1 was evaluated in this cell model. Both concentrations of PACAP38 (10 and 100 nM) markedly decreased the expression of PAC1 after 48 or 72 hours (Fig. 2). The results of this experiment confirm that acute PACAP38 administration up-regulates BDNF mRNA expression while the prolonged treatment maintains the elevated levels of the neurotrophin. In contrast, repeated exposure to antagonist down-regulates BDNF expression in cortical neurons. The extended exposure to PACAP38 resulted in gradual down-regulation of its specific receptor PAC1.
Effect of PACAP on BDNF expression in primary cortical neurons. Cortical neurons were incubated daily with PACAP38 or its antagonist PACAP(6-38), for 6 hours (A), 24 hours (B), 48 hours (C), or 72 hours (D) and subjected to gene expression studies. Control cells were treated with vehicle (water). Total RNA, 0.5–1 μg was reverse transcribed and 15–20 ng of cDNA was used for the real-time PCR analysis. All real-time PCR reactions were performed in triplicate, using HPRT gene as an endogenous normalization factor. Results are expressed as relative units (r.u.)—a number resulting from the normalization procedure; values are the mean ± SEM (n = 4). Statistical differences are shown as two asterisks at p < 0.01 and three asterisks at p < 0.001
Effects of PACAP on PAC1 expression in primary cortical neurons. Cortical neurons were incubated daily with PACAP38 for 6 hours (A), 24 hours (B), 48 hours (C), or 72 hours (D) and subjected to gene expression studies. Control cells were treated with vehicle. Total RNA (0.5–1 μg), was reverse transcribed and the obtained cDNA (15–20 ng) was used for the real-time PCR analyses. All reactions were performed in triplicate, using HPRT gene as an endogenous normalization factor. Results are expressed as relative units (r.u.)—a number resulting from the normalization procedure; values are the mean ± SEM (n = 4). Statistical differences are shown as two asterisks at p < 0.01 and three asterisks at p < 0.001
Effect of Antidepressants on BDNF and PACAP Receptors Expression in the Rat Hippocampal Neurons
Hippocampal neurons were incubated for 72 hours with 1- to 10-μM concentrations of antidepressants: citalopram, paroxetine (SSRIs), and imipramine (TCA). At the end of the incubation period, cell cultures were subjected to molecular studies.
Effect of Citalopram
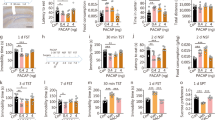
Daily treatments for 72 hours with citalopram dose-dependently down-regulated PACAP specific receptors PAC1 and VPAC2 (Fig. 3A,B; ** at p < 0.001) and simultaneously up-regulated BDNF mRNA expression (Fig. 3C; ** at p < 0.001, and *** at p < 0.0001). No changes in VPAC1 mRNA expression were observed (data not shown).
Effect of citalopram (72 hours) on BDNF and PACAP receptors expression in the rat hippocampal neurons. Hippocampal neurons were daily exposed to 1–10 μM of citalopram for 72 hours followed by molecular studies. Expression levels of PAC1 (A), VPAC2 (B), and BDNF (C) were measured. Real-time PCR reactions were performed in triplicate, using HPRT gene as an endogenous normalization factor. Results are expressed as relative units (r.u.)—a number resulting from the normalization procedure. Values are the mean ± SEM (n = 6). Statistical differences are shown as two asterisks at p < 0.01 and three asterisks at p < 0.001
Effect of Paroxetine
Similar to citalopram, daily administration of paroxetine for 72 hours caused a reduction in PAC1 and VPAC2 expression (Fig. 4A,B; ** at p < 0.001) and an increase in BDNF expression (Fig. 4C; * at p < 0.01 and ** at p < 0.001). Microscopic observations and later cell viability examination revealed that 10 μM of paroxetine had a cytotoxic effect on hippocampal neurons; therefore, this dose was excluded from further analyses. In agreement with the results of citalopram treatment, VPAC1 mRNA expression was not affected by the tested doses of paroxetine (data not shown).
Effect of paroxetine (72 hours) on BDNF and PACAP receptors expression in the rat hippocampal neurons. Hippocampal neurons were daily exposed to 1–10 μM of paroxetine for 72 hours followed by molecular studies. 10 μM paroxetine-treated cells were excluded from the molecular studies due to observed cytotoxic effect of this dose. Expression levels of PAC1 (A), VPAC2 (B), and BDNF (C) were measured. All real-time PCR reactions were performed in triplicate using HPRT gene as an endogenous normalization factor. Results are expressed as relative units (r.u.)—a number resulting from the normalization procedure. Values are the mean ± SEM (n = 6). Statistical differences are shown as asterisk at p < 0.05 and two asterisks at p < 0.01
Effect of Imipramine
In contrast to the effect of citalopram and paroxetine on the expression levels of the investigated genes, 72-hour daily exposure of hippocampal cells to imipramine caused marked up-regulation of PAC1 and VPAC2 (Fig. 5A,B; * at p < 0.01, and ** at p < 0.001) and in parallel a reduction of BDNF mRNA expression (Fig. 5; ** at p < 0.001). The highest dose of imipramine tested in this study (10 μM) had an adverse effect on cell viability, resembling the outcome of exposure to paroxetine 10 μM; therefore, this dose was not included in further molecular studies. In agreement with the experimental data from paroxetine and citalopram testing, imipramine also had no effect on VPAC1 expression (data not shown), which may lead to the conclusion that VPAC1 receptor is not involved in the mechanism of antidepressant action in hippocampal neurons.
Effect of imipramine on (72 hours) BDNF and PACAP receptors expression in the rat hippocampal neurons. Hippocampal neurons were daily exposed to 1–10 μM of imipramine for 72 hours followed by molecular studies. Imipramine-treated (10 μM) cells were excluded from the molecular studies due to observed cytotoxic effect. Expression levels of PAC1 (A), VPAC2 (B), and BDNF (C) were measured. All real-time PCR reactions were performed in triplicate, using HPRT gene as an endogenous normalization factor. Results are expressed as relative units (r.u.)—a number resulting from the normalization procedure. Values are the mean ± SEM (n = 6). Statistical differences are shown as asterisk at p < 0.05 and two asterisks at p < 0.01
Effect of Antidepressants on PACAP Expression
The effect of 72-hour exposure of hippocampal neurons to antidepressants on PACAP expression greatly resembled their effect on BDNF mRNA levels shown previously in Figs. 3, 4, and 5C. While the SSRIs paroxetine and citalopram induced PACAP mRNA expression, the tricyclic compound imipramine caused a converse effect (Fig. 6; * at p < 0.01, and ** at p < 0.001).
Effect of antidepressants (72 hours) on PACAP expression. Hippocampal neurons were treated daily with 3 μM of citalopram (CIT), paroxetine (PAR) and imipramine (IMI), followed by molecular studies after 72 hours. The changes in PACAP mRNA levels were measured using HPRT gene as a normalizing factor. All real-time PCR reactions were performed in triplicate. Results are expressed as relative units (r.u.)—a number resulting from the normalization procedure. Values are the mean ± SEM (n = 6). Statistical differences are shown as asterisk at p < 0.05 and two asterisks at p < 0.01
Correlation Between BDNF, PACAP and its Receptor’s mRNA Expression
Strong correlations were found comparing BDNF and PACAP expression levels (r = 0.8979, p < 0.0025) as well as BDNF and PAC1 (r = −0.9137, p < 0.0013) or BDNF and VPAC2 (r = −0.8961, p < 0.0026). Up-regulation of BDNF expression resulted from SSRI treatment correlated with the up-regulation of PACAP expression and down-regulation of its receptors. At the same time, reduction in BDNF expression caused by imipramine accompanied the reduction of PACAP and an increase in PAC1 and VPAC2 expression.
Effect of Antidepressants on Neuronal Cell Viability
To assess the effect of antidepressants on cell viability, the neurons were seeded on 96-well plates and grown for 1 week in a culture medium; then cells were treated with various concentrations of drugs or vehicle for 24 or 72 hours. Following the incubation period cell viability was measured (Fig. 7). The results revealed that 24-hour incubation with antidepressants had no effect on cell viability relative to control cells (data not shown), whereas 72-hour treatment demonstrated that the highest of the tested doses of both paroxetine (10 μM) and imipramine (10 μM) caused a marked reduction of neuronal cell viability, which manifested as an increased rate of neuronal death. For this reason, the effect of this dose of paroxetine and imipramine on gene expression was not evaluated.
Effect of antidepressants (72 hours) on neuronal cell viability. Hippocampal neurons were seeded on 96-well plates coated with PDL at the density of 3 × 104 cells per well, and grown for one week prior to daily treatment with various concentrations of antidepressants or vehicle for 72 hours followed by cell viability analyses. The results are the mean ± SEM (n = 9) and expressed as a percentage of control values. Statistical differences between control and antidepressants (citalopram, paroxetine and imipramine; 0.3-10μM) are at p < 0.05
Discussion
This is the first study addressing the possibility of PACAP system involvement in the mechanisms of antidepressant action. Our results suggest that PACAP up-regulation by both the SSRI compounds tested, citalopram and paroxetine, may contribute to maintenance of up-regulated BDNF expression levels, which in turn may underlie some of the antidepressant beneficial effects, including increased cell survival. It is accepted that the increase in BDNF mRNA levels does not necessarily correspond with the changes in mature BDNF protein levels. Moreover, it may reflect an increased translation of pro-BDNF protein, that selectively binds p75 receptor, which participates in pro-apoptotic signaling with a known adverse effect on neuronal survival (Friedman 2000). Nevertheless, our cell viability measurements (Fig. 7) suggest an increase in cell viability rather than a toxic effect of the various doses of antidepressants tested, with the exception of high (10 μM) doses of paroxetine and imipramine.
The inducible effect of PACAP on BDNF mRNA expression in rat primary cortical neurons was measured using a real-time quantitative PCR approach. In our experimental setting, treatment with 10 nM and 100 nM PACAP38 produced a marked and dose-dependent upregulation of BDNF mRNA expression after a 6-hour incubation, although this effect was abolished after 24 hours. Moreover, daily incubation for 48 and 72 hours with both concentrations of PACAP38 (10 and 100 nM) maintained the elevated levels of BDNF mRNA compared to control, although they were significantly lower in comparison to the effect observed after 6 hours. Georg and Fahrenkrug 2000 demonstrated a similar effect of PACAP38 on endogenous VIP gene transcription in human neuroblastoma cell line, with maximal effect on VIP expression achieved subsequent to 6-hour incubation, followed by a steep decline 24 hours after the initial treatment. The rapid and robust upregulation of BDNF transcript levels measured 6 hours after the exposure to PACAP38 may reflect an inhibition of degradation of the existing BDNF transcripts, while the delayed long-term up-regulated BDNF mRNA levels may represent the activation of transcriptional mechanisms, involving cyclic adenosine monophosphate (cAMP)-responsive element-binding protein (CREB) phosphorylation and a subsequent production of new BDNF transcripts. Consequently, we propose that repetitive prolonged treatment of cultured cells with efficacious concentrations of PACAP38 (10 nM and 100 nM) (Onoue et al. 2002) may lead to adaptation changes in BDNF expression following 48- and 72-hour treatment (Fig. 1). These results are in agreement with previous studies demonstrating a significant upregulation of BDNF expression after exposure to nanomolar concentrations of PACAP38 in rat cortical neurons (Frechilla et al. 2001) as well as in rat hippocampal neurons (Yaka et al. 2003). In addition, the PAC1 specific antagonist PACAP (6-38) caused a significant down-regulation of BDNF mRNA expression following extended, but not acute, daily exposure, in comparison to both control and PACAP38 treatments. Previous studies have indicated that PACAP stimulates its own mRNA expression in rat cortical neurons (Shintani et al. 2005), thus we found interesting to investigate the possible effect of exogenous PACAP on the specific PACAP receptor (PAC1) expression. A marked down-regulation of PAC1 mRNA was observed after 24 hours incubation with both concentrations of PACAP38 (10 and 100 nM) compared to control (Fig. 2). This effect sustained further following 48- and 72-hour daily treatment with PACAP38. In contrast, there was no significant effect of PACAP on the expression level of PAC1 6 hours after the treatment. This may represent the time interval necessary for the adaptation changes in the receptor expression levels to take place, which may be a result of constant addition of PACAP to the culture medium compared to control. It is likely that system adaptation to repeated administration of PACAP/synaptic plasticity underlie both the elevated BDNF and the down-regulated PAC1 mRNA levels seen after 48 and 72 hours (Fig. 2). Numerous reports suggest the involvement of BDNF in the mechanism of antidepressant action and the etiology of affective disorders as well as its possible regulatory role in the process of neurogenesis in the hippocampus (Duman et al. 1997, 2000; Nestler et al. 2002; Duman 2004; Castren 2005).
In this study, we attempt to combine the current understanding of the relationship between PACAP and BDNF with the available data suggesting the role of BDNF in antidepressant treatment and the etiology of affective disorders.
Thus, our second battery of experiments was designed to assess the effect of antidepressants on PACAP, its receptors and BDNF mRNA expression in primary rat hippocampal neurons.
We choose two distinct classes of antidepressants—SSRIs, represented by citalopram and paroxetine, and TCA, represented by imipramine. Several studies indicate that SSRIs up-regulate BDNF expression in the brain and also in the serum of depressed patients (Martinez-Turrillas et al. 2005; Yoshimura et al. 2007).
Our data have shown that 72-hour daily incubation of primary rat hippocampal neurons with these antidepressants affected the expression of BDNF, PACAP and its receptors, even though in animal models the effect of antidepressants on gene expression is usually reported only after chronic treatment (Martinez-Turrillas et al. 2005). Both paroxetine and citalopram up-regulated the levels of BDNF and PACAP mRNA, and at the same time reduced the levels of PACAP receptors PAC1 and VPAC2 transcripts (Figs. 3 and 4). Interestingly, the effect of imipramine was opposite to the effect exhibited by the tested SSRIs (Fig. 5). Imipramine is a non-selective antidepressant drug with known anticholinergic side effects (Bohman et al. 1982; Rana et al. 1993; Izaguirre et al. 1997), while blockade or disruption of the cholinergic system was reported to down-regulate BDNF (da Penha Berzaghi et al. 1993; Kotani et al. 2006). This may be one of the explanations for the suppressive effect of imipramine on BDNF and PACAP mRNA expression observed in our experiments. An alternative possibility rises from a study where the effect of acute and repeated treatment with antidepressants on BDNF expression in rat brain was investigated. A bi-phasic and time-dependent influence of some antidepressants on BDNF expression was found (Coppell et al. 2003). It has also been suggested that stimulation of BDNF mRNA expression is dependent on the pharmacological profile and on the time course of drug treatment (Larsen et al. 2008). Another study suggests that the negative effect on BDNF expression correlates with simultaneous neurogenic and apoptotic processes, both inflicted by imipramine administration, resulting in increased cellular turnover in mouse hippocampus (Sairanen et al. 2005). This study also supports our results regarding the cell viability measurements (Fig. 7), which show a negative effect of imipramine (10 μM) and paroxetine (10 μM) on cultured neurons. Furthermore, along with the down-regulation of BDNF and PACAP after daily extended (72-hour) treatment with imipramine, we observed a corresponding up-regulation of PACAP receptors (PAC1 and VPAC2), further reinforcing our speculations that PAC1 and VPAC2 receptor down-regulation is due to the increased levels of PACAP. These suggestions were further supported by correlative analyses that revealed a strong positive correlation between BDNF and PACAP expression on one hand and a negative correlation with PACAP receptors expression on the other. Finally, our experiments suggest that antidepressants may affect neuronal plasticity via mechanisms that involve changes in the activity of the PACAP signaling system. Neuropeptides, which are also neurotransmitters and neuromodulators, represent the major concern in the discovery of novel antidepressants. This work may open new avenues for exploration of PACAP’s role in the etiology of affective disorders and the action of antidepressants, and possibly provide new prospectives for novel therapeutic targets.
References
Altar, C. A. (1999). Neurotrophins and depression. Trends in Pharmacological Sciences, 20, 59–61. doi:10.1016/S0165-6147(99)01309-7.
Arimura, A., & Shioda, S. (1995). Pituitary adenylate cyclase activating polypeptide (PACAP) and its receptors: Neuroendocrine and endocrine interaction. Frontiers in Neuroendocrinology, 16, 53–88. doi:10.1006/frne.1995.1003.
Aydemir, O., Deveci, A., & Taneli, F. (2005). The effect of chronic antidepressant treatment on serum brain-derived neurotrophic factor levels in depressed patients: A preliminary study. Progress in Neuro-Psychopharmacology & Biological Psychiatry, 29, 261–265. doi:10.1016/j.pnpbp.2004.11.009.
Bohman, B. D., Karbowski, M. J., & Halaris, A. E. (1982). Central cholinergic effects of tricyclic antidepressants in mouse. Archives Internationales de Pharmacodynamie et de Therapie, 255, 68–80.
Castren, E. (2005). Is mood chemistry? Nature Reviews. Neuroscience, 6, 241–246. doi:10.1038/nrn1629.
Cauvin, A., Robberecht, P., De Neef, P., Gourlet, P., Vandermeers, A., Vandermeers-Piret, M. C., et al. (1991). Properties and distribution of receptors for pituitary adenylate cyclase activating peptide (PACAP) in rat brain and spinal cord. Regulatory Peptides, 35, 161–173. doi:10.1016/0167-0115(91)90478-Y.
Chen, B., Dowlatshahi, D., MacQueen, G. M., Wang, J. F., & Young, L. T. (2001). Increased hippocampal BDNF immunoreactivity in subjects treated with antidepressant medication. Biological Psychiatry, 50, 260–265. doi:10.1016/S0006-3223(01)01083-6.
Coppell, A. L., Pei, Q., & Zetterstrom, T. S. (2003). Bi-phasic change in BDNF gene expression following antidepressant drug treatment. Neuropharmacology, 44, 903–910. doi:10.1016/S0028-3908(03)00077-7.
da Penha Berzaghi, M., Cooper, J., Castren, E., Zafra, F., Sofroniew, M., Thoenen, H., et al. (1993). Cholinergic regulation of brain-derived neurotrophic factor (BDNF) and nerve growth factor (NGF) but not neurotrophin-3 (NT-3) mRNA levels in the developing rat hippocampus. The Journal of Neuroscience, 13, 3818–3826.
Duman, R. S. (2004). Role of neurotrophic factors in the etiology and treatment of mood disorders. Neuromolecular Medicine, 5, 11–25. doi:10.1385/NMM:5:1:011.
Duman, R. S., Heninger, G. R., & Nestler, E. J. (1997). A molecular and cellular theory of depression. Archives of General Psychiatry, 54, 597–606.
Duman, R. S., Malberg, J., Nakagawa, S., & D’Sa, C. (2000). Neuronal plasticity and survival in mood disorders. Biological Psychiatry, 48, 732–739. doi:10.1016/S0006-3223(00)00935-5.
Duman, R. S., & Monteggia, L. M. (2006). A neurotrophic model for stress-related mood disorders. Biological Psychiatry, 59, 1116–1127. doi:10.1016/j.biopsych.2006.02.013.
Dwivedi, Y., Rao, J. S., Rizavi, H. S., Kotowski, J., Conley, R. R., Roberts, R. C., et al. (2003). Abnormal expression and functional characteristics of cyclic adenosine monophosphate response element binding protein in postmortem brain of suicide subjects. Archives of General Psychiatry, 60, 273–282. doi:10.1001/archpsyc.60.3.273.
Frechilla, D., Garcia-Osta, A., Palacios, S., Cenarruzabeitia, E., & Del Rio, J. (2001). BDNF mediates the neuroprotective effect of PACAP-38 on rat cortical neurons. Neuroreport, 12, 919–923. doi:10.1097/00001756-200104170-00011.
Friedman, W. J. (2000). Neurotrophins induce death of hippocampal neurons via the p75 receptor. The Journal of Neuroscience, 20, 6340–6346.
Georg, B., & Fahrenkrug, J. (2000). Pituitary adelylate cyclase-activating peptide is an activator of vasoactive intestinal polypeptide gene transcription in human neuroblastoma cells. Brain Research. Molecular Brain Research, 79, 67–76. doi:10.1016/S0169-328X(00)00101-7.
Gervasoni, N., Aubry, J. M., Bondolfi, G., Osiek, C., Schwald, M., Bertschy, G., et al. (2005). Partial normalization of serum brain-derived neurotrophic factor in remitted patients after a major depressive episode. Neuropsychobiology, 51, 234–238. doi:10.1159/000085725.
Gonul, A. S., Akdeniz, F., Taneli, F., Donat, O., Eker, C., & Vahip, S. (2005). Effect of treatment on serum brain-derived neurotrophic factor levels in depressed patients. European Archives of Psychiatry and Clinical Neuroscience, 255, 381–386. doi:10.1007/s00406-005-0578-6.
Gottschall, P. E., Tatsuno, I., Miyata, A., & Arimura, A. (1990). Characterization and distribution of binding sites for the hypothalamic peptide, pituitary adenylate cyclase-activating polypeptide. Endocrinology, 127, 272–277.
Izaguirre, V., Fernandez-Fernandez, J. M., Cena, V., & Gonzalez-Garcia, C. (1997). Tricyclic antidepressants block cholinergic nicotinic receptors and ATP secretion in bovine chromaffin cells. FEBS Letters, 418, 39–42. doi:10.1016/S0014-5793(97)01343-4.
Karege, F., Perret, G., Bondolfi, G., Schwald, M., Bertschy, G., & Aubry, J. M. (2002). Decreased serum brain-derived neurotrophic factor levels in major depressed patients. Psychiatry Research, 109, 143–148. doi:10.1016/S0165-1781(02)00005-7.
Kotani, S., Yamauchi, T., Teramoto, T., & Ogura, H. (2006). Pharmacological evidence of cholinergic involvement in adult hippocampal neurogenesis in rats. Neuroscience, 142, 505–514. doi:10.1016/j.neuroscience.2006.06.035.
Lam, H. C., Takahashi, K., Ghatei, M. A., Kanse, S. M., Polak, J. M., & Bloom, S. R. (1990). Binding sites of a novel neuropeptide pituitary-adenylate-cyclase-activating polypeptide in the rat brain and lung. European Journal of Biochemistry, 193, 725–729. doi:10.1111/j.1432-1033.1990.tb19392.x.
Larsen, M. H., Hay-Schmidt, A., Ronn, L. C., & Mikkelsen, J. D. (2008). Temporal expression of brain-derived neurotrophic factor (BDNF) mRNA in the rat hippocampus after treatment with selective and mixed monoaminergic antidepressants. European Journal of Pharmacology, 578, 114–122. doi:10.1016/j.ejphar.2007.08.050.
Malkesman, O., Maayan, R., Weizman, A., & Weller, A. (2006). Aggressive behavior and HPA axis hormones after social isolation in adult rats of two different genetic animal models for depression. Behavioural Brain Research, 175, 408–414. doi:10.1016/j.bbr.2006.09.017.
Martinez-Turrillas, R., Del Rio, J., & Frechilla, D. (2005). Sequential changes in BDNF mRNA expression and synaptic levels of AMPA receptor subunits in rat hippocampus after chronic antidepressant treatment. Neuropharmacology, 49, 1178–1188. doi:10.1016/j.neuropharm.2005.07.006.
Miyata, A., Arimura, A., Dahl, R. R., Minamino, N., Uehara, A., Jiang, L., et al. (1989). Isolation of a novel 38 residue-hypothalamic polypeptide which stimulates adenylate cyclase in pituitary cells. Biochemical and Biophysical Research Communications, 164, 567–574. doi:10.1016/0006-291X(89)91757-9.
Miyata, A., Jiang, L., Dahl, R. D., Kitada, C., Kubo, K., Fujino, M., et al. (1990). Isolation of a neuropeptide corresponding to the N-terminal 27 residues of the pituitary adenylate cyclase activating polypeptide with 38 residues (PACAP38). Biochemical and Biophysical Research Communications, 170, 643–648. doi:10.1016/0006-291X(90)92140-U.
Nestler, E. J., Barrot, M., DiLeone, R. J., Eisch, A. J., Gold, S. J., & Monteggia, L. M. (2002). Neurobiology of depression. Neuron, 34, 13–25. doi:10.1016/S0896-6273(02)00653-0.
Onoue, S., Ohshima, K., Endo, K., Yajima, T., & Kashimoto, K. (2002). PACAP protects neuronal PC12 cells from the cytotoxicity of human prion protein fragment 106–126. FEBS Letters, 522, 65–70. doi:10.1016/S0014-5793(02)02886-7.
Pittenger, C., & Duman, R. S. (2008). Stress, depression, and neuroplasticity: A convergence of mechanisms. Neuropsychopharmacology, 33, 88–109.
Rana, B., McMorn, S. O., Reeve, H. L., Wyatt, C. N., Vaughan, P. F., & Peers, C. (1993). Inhibition of neuronal nicotinic acetylcholine receptors by imipramine and desipramine. European Journal of Pharmacology, 250, 247–251. doi:10.1016/0014-2999(93)90388-X.
Sairanen, M., Lucas, G., Ernfors, P., Castren, M., & Castren, E. (2005). Brain-derived neurotrophic factor and antidepressant drugs have different but coordinated effects on neuronal turnover, proliferation, and survival in the adult dentate gyrus. The Journal of Neuroscience, 25, 1089–1094. doi:10.1523/JNEUROSCI.3741-04.2005.
Sherwood, N. M., Krueckl, S. L., & McRory, J. E. (2000). The origin and function of the pituitary adenylate cyclase-activating polypeptide (PACAP)/glucagon superfamily. Endocrine Reviews, 21, 619–670. doi:10.1210/er.21.6.619.
Shimizu, E., Hashimoto, K., Okamura, N., Koike, K., Komatsu, N., Kumakiri, C., et al. (2003). Alterations of serum levels of brain-derived neurotrophic factor (BDNF) in depressed patients with or without antidepressants. Biological Psychiatry, 54, 70–75. doi:10.1016/S0006-3223(03)00181-1.
Shintani, N., Suetake, S., Hashimoto, H., Koga, K., Kasai, A., Kawaguchi, C., et al. (2005). Neuroprotective action of endogenous PACAP in cultured rat cortical neurons. Regulatory Peptides, 126, 123–128. doi:10.1016/j.regpep.2004.08.014.
Shioda, S. (2000). Pituitary adenylate cyclase-activating polypeptide (PACAP) and its receptors in the brain. Kaibogaku Zasshi, 75, 487–507.
Somogyvari-Vigh, A., & Reglodi, D. (2004). Pituitary adenylate cyclase activating polypeptide: A potential neuroprotective peptide. Current Pharmaceutical Design, 10, 2861–2889. doi:10.2174/1381612043383548.
Suda, K., Smith, D. M., Ghatei, M. A., Murphy, J. K., & Bloom, S. R. (1991). Investigation and characterization of receptors for pituitary adenylate cyclase-activating polypeptide in human brain by radioligand binding and chemical cross-linking. The Journal of Clinical Endocrinology and Metabolism, 72, 958–964.
Vaudry, D., Gonzalez, B. J., Basille, M., Yon, L., Fournier, A., & Vaudry, H. (2000). Pituitary adenylate cyclase-activating polypeptide and its receptors: From structure to functions. Pharmacological Reviews, 52, 269–324.
Vinet, J., Carra, S., Blom, J. M., Brunello, N., Barden, N., & Tascedda, F. (2004). Chronic treatment with desipramine and fluoxetine modulate BDNF, CaMKKalpha and CaMKKbeta mRNA levels in the hippocampus of transgenic mice expressing antisense RNA against the glucocorticoid receptor. Neuropharmacology, 47, 1062–1069.
Waschek, J. A. (2002). Multiple actions of pituitary adenylyl cyclase activating peptide in nervous system development and regeneration. Developmental Neuroscience, 24, 14–23. doi:10.1159/000064942.
Weber, C. C., Eckert, G. P., & Muller, W. E. (2006). Effects of antidepressants on the brain/plasma distribution of corticosterone. Neuropsychopharmacology, 31, 2443–2448. doi:10.1038/sj.npp.1301076.
Yaka, R., He, D. Y., Phamluong, K., & Ron, D. (2003). Pituitary adenylate cyclase-activating polypeptide (PACAP(1-38)) enhances N-methyl-D-aspartate receptor function and brain-derived neurotrophic factor expression via RACK1. The Journal of Biological Chemistry, 278, 9630–9638. doi:10.1074/jbc.M209141200.
Yoshimura, R., Mitoma, M., Sugita, A., Hori, H., Okamoto, T., Umene, W., et al. (2007). Effects of paroxetine or milnacipran on serum brain-derived neurotrophic factor in depressed patients. Progress in Neuro-Psychopharmacology & Biological Psychiatry, 31, 1034–1037. doi:10.1016/j.pnpbp.2007.03.001.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Reichenstein, M., Rehavi, M. & Pinhasov, A. Involvement of Pituitary Adenylate Cyclase Activating Polypeptide (PACAP) and its Receptors in the Mechanism of Antidepressant Action. J Mol Neurosci 36, 330–338 (2008). https://doi.org/10.1007/s12031-008-9116-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-008-9116-0