Abstract
GTP-binding protein Gem, a member protein of the Ras superfamily, can regulate actin cytoskeleton reorganization mediated by Rho-associated coiled-coil-containing protein kinase (ROCK). One attractive activity of the ROCK is playing a potential role in physiological and pathological process in retinal ganglion cells (RGCs) apoptosis. However, the function of Gem in retina is still with limited understanding. To investigate whether Gem is involved in optic nerve injury, we performed an optic nerve crush (ONC) model in adult rats. Western blot analysis indicated that Gem was significantly increased in the retina at the 3rd day after ONC. Meanwhile, double-immunofluorescent staining showed that Gem expression was mainly up-regulated in ganglion cell layer and co-localized with NeuN (a marker of RGCs). Additionally, the co-localizations of Gem/active-caspase-3 and Gem/TUNEL-positive cells were detected in RGCs. Furthermore, the expression of active-caspase-3 and TUNEL-positive cells was parallel with that of Gem. Finally, expression pattern of ROCK family (only ROCK2 but not ROCK1) was increased in the differentiated process, which was collected with the expression of GEM and active-caspase-3. Based on the present results, it is suggested that Gem might play a crucial role in RGCs apoptosis after ONC, which might be involved in ROCK pathway.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Injury to the optic nerve (ON) caused by glaucoma and other ON diseases is characterized by rapid apoptosis of retinal ganglion cells (RGCs) (Quigley 1993). Unfortunately, treatment to restore visual function has not yet been established (Diekmann and Fischer 2013). The optic nerve crush (ONC) model was extensively used to analyze RCGs survival and apoptosis (Osborne et al. 1999; Quigley 1993). But the molecular mechanisms that initiate the RGCs apoptosis after ONC remains poorly understood.
GTP-binding protein Gem, a member protein of the Ras superfamily has been given the name RGK for Rad/Gem/Kir, Kir is the mouse orthologue of Gem (Cohen et al. 1994; Maguire et al. 1994; Reynet and Kahn 1993). The basic structure of RGK proteins consists of a Ras-related core, a non-CAAX containing both large N- and C-terminal extensions. Although the RGK proteins have many characteristics with Ras superfamily members in common, every family member may interact with relatively unique subsets of downstream signaling proteins and each may have different functions.
Gem had been found to be involved in the inhibition of actin cytoskeleton reorganization mediated by Rho-associated coiled-coil-containing protein kinase (ROCK) (Hatzoglou et al. 2007; Maguire et al. 1994), which was found to be with various nervous system disorders involvement, such as Parkinson’s disease (Tonges et al. 2012; Villar-Cheda et al. 2012), stroke (Vesterinen et al. 2013), Alzheimer’s disease (Herskowitz et al. 2013; Wen et al. 2014), epilepsy (Inan and Buyukafsar 2008), chronic pain (Yoshimi et al. 2010) and autoimmune neuritis (Pineda et al. 2011). Moreover, it has been demonstrated that active-ROCK may act as an upstream event that responsible for RGCs apoptosis after ONC (Lingor et al. 2007, 2008). As an inhibitor for ROCK, it is reasonable for us to hypothesize that Gem may be involved in physiological and pathological process in RGCs apoptosis.
Herein, for the first time, we studied temporal-spatial patterns of Gem at protein level using an ONC model in adult rat. Our work may provide deeper insight into the functions of Gem in the pathology of RGCs apoptosis and the molecular mechanisms underlying neurodegenerative diseases.
Materials and methods
Animals and surgery
Male Sprague–Dawley rats (10 weeks) with an average body weight of 250 g (220–275 g) were obtained from the Medical Laboratory Animal Center of Guangxi Medical University, China. In the ONC groups (Normal, 1, 2, 3, 5 and 7 days) and the SHAM groups (Normal and 3 days), all animals were killed at different survival times after injury. In the ONC groups, no animals were lost before these determined time points. The animals were used for Western blotting analysis (n: 8 group × 8 = 64), immunofluorescence studies (n: 2 group × 8 = 16) and TUNEL staining (n: 2 group × 8 = 16). All surgical interventions and postoperative animal care were carried out in accordance with the Guide for the Care and Use of Laboratory Animals (National Research Council, 1996, USA) and were approved by the Ethics Committee of the People’s Hospital of Guangxi Zhuang Autonomous Region.
The left ON was crushed as those described previously (Huang et al. 2014). Briefly, animals were anesthetized with an intraperitoneal injection of 7 % chloral hydrate solution (6 ml/kg body weight). ON was exposed under a surgical microscope and partially crushed for 10 s with a cross-action calibrated crush forceps placed 2 mm behind the ON head. Vascular integrity of the retina remained grossly intact, as judged by a fundoscopic examination while and after the procedure. For sham operations, the ON of right eye was exposed, but with no crush.
Western blotting
The rats were sacrificed with overdose anesthesia at different time points. Retina tissues were harvested and stored at −80 °C until use. Total protein was obtained by using a lysis buffer (50 mM/I Tris, pH 7.5, 1 % Triton X-100, 1 % NP-40, 10 % sodium dodecyl sulfate, 0.5 % sodium deoxycholate, 5 mM/l EDTA, 10 μg/ml leupeptin, and 10 μg/ml aprotinin) and clarified by centrifuging for 20 min in a microcentrifuge at 4 °C to collect the supernatant. Then, protein was separated with 10 % SDS-PAGE and transferred to polyvinylidine difluoride filter membranes (Millipore, Bedford, MA). The membranes were blocked with 5 % nonfat milk and incubated with primary antibody against Gem (anti-rabbit, 1:500; Santa Cruz), active-caspase-3 (anti-mouse, 1:1,000; Cell Signaling), ROCK1 (anti-rabbit, 1:500; BD Bioscience), ROCK2 (anti-rabbit, 1:500; BD Bioscience) and β-actin (anti-rabbit, 1:1,000, Santa Cruz) at 4 °C overnight. After incubating with a goat-anti-rabbit or goat-anti-mouse horseradish peroxidase-conjugated secondary antibody (1:2,000; Southern-Biotech) for 2 h, protein was visualized using an enhanced chemiluminescence system (ECL, Pierce Company, USA). The experiment was repeated at least three times.
Sections and immunofluorescence staining
After defined survival times, sham and injured rats were terminally anesthetized and perfused through the ascending aorta with saline, followed by 4 % paraformaldehyde. The eyes were enucleated and immediately fixed in 4 % neutral-buffered formalin for overnight at 4 °C, and then were dehydrated and made into frozen sections (7 μm). For double labeling, the cryosections were blocked with 1 % BSA to prevent nonspecific staining. Then, the sections were incubated with rabbit primary antibodies for anti-Gem (1:100; Santa Cruz) and mouse monoclonal primary antibodies anti-NeuN (a marker of RGCs, 1:600; Chemicon), and goat polyclonal antibody for anti-active-caspase-3 (a marker of apoptosis, 1:200; Santa Cruz) overnight at 4 °C. After washing in PBS three times, the secondary antibodies was added in the dark room and incubated for 2 h at 4 °C. The images were captured on a Leica fluorescence microscope (Leica Microsystems, Mannheim, Germany).
TUNEL staining
Terminal deoxynucleotidyl transferase-mediated biotinylated-dUTP nick-end labeling (TUNEL) staining was employed using the In Situ Cell Death Detection Kit, Fluorescence (Roche Applied Science, Mannheim, Germany). The cryosections were rinsed with PBS and treated with 1 % Triton-100 in PBS for 2 min on ice. The slides were rinsed in PBS and incubated for 60 min at 37 °C with 50 μl of TUNEL reaction mixture. After washing with PBS three times, the slides were analyzed on a Leica fluorescence microscope (Leica Microsystems, Mannheim, Germany).
Quantitative and statistical analysis
Cell double labeled for Gem with phenotype-specific markers (NeuN and active-caspase-3) used in the experiment were quantified and recorded. Two or three adjacent sections (50 μm apart) per animal sections for every animal were sampled. We also count RGCs density changes in dependence in the same distance from the optic disk. The number of TUNEL and Gem/TUNEL-positive cells in the ganglion cell layer (GCL) was counted. Three separate GCL regions were examined for each section. The cell counts in the three or four sections were then used to determine the total number of TUNEL-positive cells per square millimeter. The number of cell double-labeled for Gem and TUNEL-positive cells used in the experiment was quantified. To identify the proportion of TUNEL-positive cells expressing Gem, a minimum of 200 TUNEL-positive cells were counted in each section. Then, double-labeled cells for Gem and TUNEL-positive cells were recorded.
Statistical analyses were carried out with SPSS software (SPSS, version 13.0, SPSS, Chicago). All values were expressed as mean ± standard error of mean (SEM). The statistical significance of difference between groups was determined by one-way ANOVA (ANOVA) followed by Tukey’s post hoc multiple comparison tests. A P value less than 0.05 was considered statistically significant. Each experiment consisted of at least three replicates per condition.
Results
The expression of Gem in retina after ONC by western blot analysis
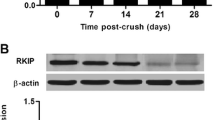
To explore the change of Gem in retina after ONC, Western blot was employed to analyze the temporal patterns of Gem expression at different time points. Standardizing densitometry against β-actin showed that Gem was low in normal retina and maintained that level on day 1 and 2 after ONC. Then, its expression was significantly increased and reached a peak on day 3. Finally, it returned to normal level on day 5 and 7 (*P < 0.05, Fig. 1a, b).
The expression of Gem in the adult rat retina after ONC. a Western blot analysis of the protein expression profiles Gem in the sham and injured retina at various survival times after ONC were homogenized and subjected to immunoblot analysis. b Quantification graphs (relative optical density) of the intensity of staining of Gem to β-actin at each time point. β-actin was used to confirm equal amount of protein run on gel. The data are mean ± SEM (n = 3; *P < 0.05, significantly different from the sham groups). ONC optic nerve crush, OD optical density
The staining and distribution changes of Gem positive cells after ONC
To assess the changes of Gem staining and distribution in retina after ONC, double-labeling immunocytochemistry was performed to analyze the cellular localization of Gem and NeuN which was known as a RGCs marker. In the normal retina, the expression of Gem was extremely weak in all retinal layers (Fig. 2a). At 3 days after ONC, the immunostaining of Gem was progressively increased in the GCL (Fig. 2b). Interestingly, Gem expression was significantly increased in NeuN-positive cells (Fig. 2c–f). These results suggested the temporal pattern of Gem after ONC was consistent with the western blot results.
Double immunofluorescence staining for Gem and cells markers in adult rat retina at different times after ONC. a The normal retina showed weak immunoreactivity for Gem antibody. b At 3 days after ONC, the number of positive cells largely increased in the GCL. c–f At 3 days after ONC, increased Gem costained with RGCs in the GCL. Gem (green, c); NeuN (red, d); Nuclear Hoechst staining (blue, e). The yellow color visualized in the merged images represented the co-localization of Gem with NeuN f. Scale bars are equivalent to 60 μm. ONC optic nerve crush, GCL ganglion cell layer, RGCs retinal ganglion cells. (Color figure online)
Detection of RGCs apoptosis and relative Gem alteration after ONC
RGCs apoptosis is a significant event after ON injury. Caspase-3 activation is a characteristic feature of apoptosis. To further learn the apoptosis of RGCs, Western blot was employed to analyze the expression of active-caspase-3. The level of active-caspase-3 was weakly present in normal retina, then gradually up-regulated after ONC and reached a peak at 3 days (*P < 0.05, Fig. 3a, b). Immunofluorescent staining showed that active-caspase-3 expression was significantly increased at 3 days after ONC. Also, the co-localization of active-caspase-3 and Gem was detected at 3 days after ONC (Fig. 3c–f).
The expression and location of caspase-3 in the adult rat retina after ONC. a Western blotting showing active-caspase-3 in retina after ONC. The expression of active-caspase-3 was increased after ONC and peaked at 3 days. b The bar chart showed the ratio of active-caspase-3 to β-actin at each time point; these data were mean ± SEM (n = 3, *P < 0.05, significantly different from the normal group). c–f Double immunofluorescence staining for Gem immunoreactivity (green) and active-caspase-3 (red) in retina after ONC. c Active-caspase-3 (red, d) activity colocalized with Gem (green, c) in the merged image (yellow, f), and nuclear Hoechst staining (blue, e). Scale bars are equivalent to 30 μm. ONC optic nerve crush, OD optical density. (Color figure online)
To further confirm whether Gem expression was involved in RGCs apoptotic process, TUNEL staining was employed on retina sections at 3 days after ONC. In the normal retina, the number of TUNEL-positive cells was low (Fig. 4a). But the TUNEL-positive cells were apparent at 3 days after ONC (Fig. 4b). The co-localization of TUNEL-positive RGCs and Gem was detected at 3 days after ONC (Fig. 4b–e). Semi-quantitative analysis also indicated a significantly increase in the density of TUNEL and Gem/TUNEL-positive RGCs in the GCL at 3 days after ONC (*P < 0.05, Fig. 4f, g). These results indicated that Gem might play an important role in neuronal apoptosis after ONC.
The expression and location of TUNEL-positive cells and Gem in the adult rat retina after ONC. a TUNEL staining showing the expression of TUNEL-positive cells (red) was weak in normal retinal. (b–e) The number of TUNEL-positive cells was increased at 3 days after ONC and co-location with Gem in GCL. TUNEL-positive cells (red, b); Gem (green, c); Nuclear Hoechst staining (blue, d). e The yellow color visualized in the merged images represented the co-localization of Gem with TUNEL-positive cells. Scale bars are equivalent to 100 μm. f, g Semi-quantitative analysis indicated a significantly increase in the density of TUNEL and Gem/TUNEL-positive RGCs in the GCL at 3 days after ONC. The data are mean ± SEM (n = 3; *P < 0.05, significantly different from the normal groups). ONC optic nerve crush, GCL ganglion cell layer, RGCs retinal ganglion cells. (Color figure online)
Previous studies showed that Gem was a negative regulator of the ROCK pathway, which was involved in caspase-3-independent neuronal apoptosis. Furthermore, we performed Western blot to examine the expression of two main members of ROCK pathway: ROCK1 and ROCK2. After ONC, only ROCK2 but not ROCK1 were up-regulated, which kept increasing until 3 days and arrived at the peak then it decreased gradually (**P < 0.01; *P < 0.05, Fig. 5a–c). It is noteworthy that the ratio of ROCK2 is parallel with the expression profiles of Gem and activate-caspase-3 in retina after ONC (data not shown); the results were consistent with the previous studies, suggesting that ROCK2 is a major regulator of RGCs death (Koch et al. 2014). Based on these results, we further found that Gem might be related with RGCs apoptosis, which process might be relevant to ROCK family.
Association of GEM with ROCK pathway after ONC. a The time courses of ROCK1 and ROCK2 after ONC. The expressions of ROCK2 but not ROCK1 was increased after ONC and peaked at 3 days. b The bar chart showed the ratio of ROCK1 to β-actin at each time point. c The bar chart showed the ratio of ROCK2 to β-actin at each time point; these date were mean ± SEM (n = 3, **P < 0.01, *P < 0.05, significantly different from the normal group)
Discussion
The biochemical and physiological responses of RGCs death and axonal degeneration after ONC are still little known. After ONC, a fraction of RGCs immediate death results from mechanical axotomy (Bien et al. 1999), whereas others are subjected to delayed apoptotic death (Pettmann and Henderson 1998; Raff et al. 1993). After the short initial stage of immediate death, the chronic progressive loss of RGCs in the retina may attain characteristics similar to the loss observed in glaucoma. Thus, it is worthwhile to explore the exactly molecular and cellular events involved in RGCs death.
This study mimicked the expression profiles of Gem in adult rat retina after ONC. Western blot analysis indicated that Gem was significantly increased in the retina at 3 days after ONC. Meanwhile, double-immunofluorescent staining showed that Gem expression was mainly up-regulated in RGCs and co-localized with NeuN (a marker of RGCs). Additionally, the co-localizations of Gem/active-caspase-3 and Gem/TUNEL-positive cells were detected in RGCs. Finally, expression patterns of active-caspase-3, ROCK2 and TUNEL-positive cells were parallel with that of Gem. Taken together, Gem might play a crucial role in RGCs apoptosis after ONC, and this process might be relevant to ROCK family.
In the Ras superfamily of small GTPases, Gem, together with Rad, Rem and Rem2 comprise a subfamily, whose expressions is regulated by mitogenic stimulations in different cell types including primary endothelial cells, human embryonic fibroblasts, lymphocytes and monocytes (Maguire et al. 1994). One of distinct functions of Gem is the inhibition of actin cytoskeleton reorganization mediated by ROCK. ROCK, a member of the AGC (PKA/PKG/PKC) family, belonging to serine/threonine kinases family, is one of the best-characterized effectors of RhoA. ROCK, which is widely involved in regulation of neuronal development, survival, axon guidance, apoptosis, and regeneration (Bermel et al. 2009; Frisca et al. 2013; Herskowitz et al. 2013; Pernet et al. 2013), while its abnormal activation in a number of nervous system disorders has been shown (Herskowitz et al. 2013; Inan and Buyukafsar 2008; Pineda et al. 2011; Tonges et al. 2012; Vesterinen et al. 2013; Villar-Cheda et al. 2012; Wen et al. 2014; Yoshimi et al. 2010). Reports have pointed out that pharmacological inhibition of ROCK protects RGCs from apoptosis (Lingor et al. 2008) and increases the regeneration of RGCs after ONC in vitro and in vivo (Lingor et al. 2007). Moreover, it has been demonstrated that ROCK inhibitor significantly protected neuron against the ischemia-induced delayed neuronal death in association with suppression of abundant active-caspase-3 and TUNEL-positive cells (Satoh et al. 2007; Wu et al. 2012), suggesting that ROCK activity may be involved in neuronal apoptosis. Together, ROCK activation may act as an upstream event that responsible for caspase-3-independent neuronal apoptosis.
In a number of studies, the activation of caspases-3 has been shown to play a key role in regulating neuronal apoptosis (Lossi et al. 2004; Sang et al. 2013; Sun et al. 2013). Besides, inhibition of caspases-3 might be the most effective strategy in the treatment of nervous system injury, such as cerebral trauma and neurodegenerative diseases (Kerman et al. 2012). In retina, previous studies have shown that enhanced cleavage and activation of caspases-3 were detected in retinal tissue after injury, while caspases-3 was significantly increased in TUNEL-positive RGCs (Sang et al. 2013). Furthermore, it has been demonstrated that inhibition of caspase-3 was a very promising treatment strategy to protect adult rat RGCs from injury-induced secondary RGCs apoptosis (Kermer et al. 1998). These data suggested that caspases-3 was one of the main executors of apoptosis in RGCs after ON injury in the adult rat.
Interesting, as our results shown, Gem was observed mainly in the RGCs and significant increased as early as 3 days after ONC. Furthermore, the expression of Gem was increased in parallel with active-caspase-3 and TUNEL-positive cells. Recently, many researches have showed that Gem may be involved in the pathophysiology of nervous system injury (Wang et al. 2013; Wen et al. 2013). As mentioned above, Gem was a negative regulator of the ROCK pathway, which was involved in caspase-3-independent neuronal apoptosis. Accordingly, we speculated that Gem might be associated with neuronal apoptosis in RGCs after ONC via the ROCK pathway. The precise mechanism of Gem in the regulation of RGCs apoptosis needs further investigation.
On the whole, this study provided novel evidences that Gem was significantly up-regulated after ONC. It suggested that Gem might involve in regulating biochemical and physiological responses of RGCs apoptosis after ONC. Our results may provide a vital clue towards detecting the endogenous responses of RGCs after ONC. Further studies should aim to be done to confirm the intrinsic mechanisms of Gem in RGCs apoptosis after ONC.
References
Bermel C, Tonges L, Planchamp V, Gillardon F, Weishaupt JH, Dietz GP, Bahr M, Lingor P (2009) Combined inhibition of Cdk5 and ROCK additively increase cell survival, but not the regenerative response in regenerating retinal ganglion cells. Mol Cell Neurosci 42:427–437
Bien A, Seidenbecher CI, Bockers TM, Sabel BA, Kreutz MR (1999) Apoptotic versus necrotic characteristics of retinal ganglion cell death after partial optic nerve injury. J Neurotrauma 16:153–163
Cohen L, Mohr R, Chen YY et al (1994) Transcriptional activation of a ras-like gene (kir) by oncogenic tyrosine kinases. Proc Natl Acad Sci USA 91:12448–12452
Diekmann H, Fischer D (2013) Glaucoma and optic nerve repair. Cell Tissue Res 353:327–337
Frisca F, Crombie DE, Dottori M, Goldshmit Y, Pebay A (2013) Rho/ROCK pathway is essential to the expansion, differentiation, and morphological rearrangements of human neural stem/progenitor cells induced by lysophosphatidic acid. J Lipid Res 54:1192–1206
Hatzoglou A, Ader I, Splingard A, Flanders J, Saade E, Leroy I, Traver S, Aresta S, de Gunzburg J (2007) Gem associates with Ezrin and acts via the Rho-GAP protein Gmip to down-regulate the Rho pathway. Mol Biol Cell 18:1242–1252
Herskowitz JH, Feng Y, Mattheyses AL et al (2013) Pharmacologic inhibition of ROCK2 suppresses amyloid-beta production in an Alzheimer’s disease mouse model. J Neurosci 33:19086–19098
Huang TL, Huang SP, Chang CH, Lin KH, Sheu MM, Tsai RK (2014) Factors influencing the retrograde labeling of retinal ganglion cells with fluorogold in an animal optic nerve crush model. Ophthalmic Res 51:173–178
Inan S, Buyukafsar K (2008) Antiepileptic effects of two Rho-kinase inhibitors, Y-27632 and fasudil, in mice. Br J Pharmacol 155:44–51
Kerman M, Kanter M, Coskun KK, Erboga M, Gurel A (2012) Neuroprotective effects of caffeic acid phenethyl ester on experimental traumatic brain injury in rats. J Mol Histol 43:49–57
Kermer P, Klocker N, Labes M, Bahr M (1998) Inhibition of CPP32-like proteases rescues axotomized retinal ganglion cells from secondary cell death in vivo. J Neurosci 18:4656–4662
Koch JC, Tonges L, Barski E, Michel U, Bahr M, Lingor P (2014) ROCK2 is a major regulator of axonal degeneration, neuronal death and axonal regeneration in the CNS. Cell Death Dis 5:e1225
Lingor P, Teusch N, Schwarz K, Mueller R, Mack H, Bahr M, Mueller BK (2007) Inhibition of Rho kinase (ROCK) increases neurite outgrowth on chondroitin sulphate proteoglycan in vitro and axonal regeneration in the adult optic nerve in vivo. J Neurochem 103:181–189
Lingor P, Tonges L, Pieper N, Bermel C, Barski E, Planchamp V, Bahr M (2008) ROCK inhibition and CNTF interact on intrinsic signalling pathways and differentially regulate survival and regeneration in retinal ganglion cells. Brain 131:250–263
Lossi L, Tamagno I, Merighi A (2004) Molecular morphology of neuronal apoptosis: analysis of caspase 3 activation during postnatal development of mouse cerebellar cortex. J Mol Histol 35:621–629
Maguire J, Santoro T, Jensen P, Siebenlist U, Yewdell J, Kelly K (1994) Gem: an induced, immediate early protein belonging to the Ras family. Science 265:241–244
Osborne NN, Wood JP, Chidlow G, Bae JH, Melena J, Nash MS (1999) Ganglion cell death in glaucoma: what do we really know? Br J Ophthalmol 83:980–986
Pernet V, Joly S, Jordi N, Dalkara D, Guzik-Kornacka A, Flannery JG, Schwab ME (2013) Misguidance and modulation of axonal regeneration by Stat3 and Rho/ROCK signaling in the transparent optic nerve. Cell Death Dis 4:e734
Pettmann B, Henderson CE (1998) Neuronal cell death. Neuron 20:633–647
Pineda AA, Minohara M, Kawamura N, Matsushita T, Yamasaki R, Sun X, Piao H, Shimokawa H, Kira J (2011) Preventive and therapeutic effects of the selective Rho-kinase inhibitor fasudil on experimental autoimmune neuritis. J Neurol Sci 306:115–120
Quigley HA (1993) Open-angle glaucoma. N Engl J Med 328:1097–1106
Raff MC, Barres BA, Burne JF, Coles HS, Ishizaki Y, Jacobson MD (1993) Programmed cell death and the control of cell survival: lessons from the nervous system. Science 262:695–700
Reynet C, Kahn CR (1993) Rad: a member of the Ras family overexpressed in muscle of type II diabetic humans. Science 262:1441–1444
Sang A, Xu Y, Jin N et al (2013) Involvement of transcription initiation factor IIB in the light-induced death of rat retinal ganglion cells in vivo. J Mol Histol 44:11–18
Satoh S, Toshima Y, Ikegaki I, Iwasaki M, Asano T (2007) Wide therapeutic time window for fasudil neuroprotection against ischemia-induced delayed neuronal death in gerbils. Brain Res 1128:175–180
Sun H, Li L, Zhou F et al (2013) The member of high temperature requirement family HtrA2 participates in neuronal apoptosis after intracerebral hemorrhage in adult rats. J Mol Histol 44:369–379
Tonges L, Frank T, Tatenhorst L, Saal KA, Koch JC, Szego EM, Bahr M, Weishaupt JH, Lingor P (2012) Inhibition of rho kinase enhances survival of dopaminergic neurons and attenuates axonal loss in a mouse model of Parkinson’s disease. Brain 135:3355–3370
Vesterinen HM, Currie GL, Carter S, Mee S, Watzlawick R, Egan KJ, Macleod MR, Sena ES (2013) Systematic review and stratified meta-analysis of the efficacy of RhoA and Rho kinase inhibitors in animal models of ischaemic stroke. Syst Rev 2:33
Villar-Cheda B, Dominguez-Meijide A, Joglar B, Rodriguez-Perez AI, Guerra MJ, Labandeira-Garcia JL (2012) Involvement of microglial RhoA/Rho-kinase pathway activation in the dopaminergic neuron death. Role of angiotensin via angiotensin type 1 receptors. Neurobiol Dis 47:268–279
Wang Y, Cheng X, Zhou Z, Wu H, Long L, Gu X, Xu G (2013) Increased expression of Gem after rat sciatic nerve injury. J Mol Histol 44:27–36
Wen H, Cao J, Yu X et al (2013) Spatiotemporal patterns of Gem expression after rat spinal cord injury. Brain Res 1516:11–19
Wen X, Wang L, Liu Z, Liu Y, Hu J (2014) Intracranial injection of PEG-PEI/ROCK II-siRNA improves cognitive impairment in a mouse model of Alzheimer’s disease. Int J Neurosci. doi:10.3109/00207454.2013.877014
Wu J, Li J, Hu H, Liu P, Fang Y, Wu D (2012) Rho-kinase inhibitor, fasudil, prevents neuronal apoptosis via the Akt activation and PTEN inactivation in the ischemic penumbra of rat brain. Cell Mol Neurobiol 32:1187–1197
Yoshimi E, Yamamoto H, Furuichi Y, Shimizu Y, Takeshita N (2010) Sustained analgesic effect of the Rho kinase inhibitor AS1892802 in rat models of chronic pain. J Pharmacol Sci 114:119–122
Acknowledgments
This study was supported by Natural Science Foundation of Guangxi Zhuang Autonomous Region (No. 2012GXNSFAA276039) and Science Fund Project of People’s Hospital of Guangxi Zhuang Autonomous Region (No. qn2014-1).
Author information
Authors and Affiliations
Corresponding author
Additional information
Fan Xu, Hui Huang and Yu Wu have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Xu, F., Huang, H., Wu, Y. et al. Upregulation of Gem relates to retinal ganglion cells apoptosis after optic nerve crush in adult rats. J Mol Hist 45, 565–571 (2014). https://doi.org/10.1007/s10735-014-9579-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10735-014-9579-y