Abstract
In the subgranular zone (SGZ) of the hippocampus, neurogenesis persists throughout life and is upregulated following ischemia. Accumulating evidence suggests that enhanced neurogenesis stimulated by ischemic injury contributes to recovery after stroke. However, the mechanisms underlying the upregulation of neurogenesis are unclear. We have demonstrated that a neuropeptide, pituitary adenylate cyclase-activating polypeptide (PACAP), exerts a wide range of effects on neural stem cells (NSCs) during neural development. Here, we examined the effects of endogenous and exogenous PACAP in adult NSCs of the SGZ. Immunostaining showed expression of the PACAP receptor PAC1R in nestin-positive NSCs of adult naive mice. PACAP injection into the lateral ventricle increased bromodeoxyuridine (BrdU)-positive proliferative cells in the SGZ. These data suggest that PACAP promoted the proliferation of NSCs. In global ischemia model mice, the number of BrdU-positive cells was increased in wild-type mice but not in PACAP heterozygous knockout mice. The BrdU-positive cells that increased in number after ischemia were immunopositive for SOX2, a marker of NSCs, and differentiated into NeuN-positive mature neurons at 4 weeks after ischemia. These findings suggest that PACAP contributes to the proliferation of NSCs and may be associated with recovery after brain injury.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pituitary adenylate cyclase-activating polypeptide (PACAP) is a neuropeptide that was first isolated from the ovine hypothalamus based on its ability to stimulate adenylate cyclase activity in pituitary cells (Miyata et al. 1989). This neuropeptide belongs to the secretin/glucagon/vasoactive intestinal peptide (VIP) superfamily and exists as two biologically active forms, PACAP38 and PACAP27 (Kimura et al. 1990). Three receptors mediate the actions of PACAP: PAC1R, VPAC1R, and VPAC2R. Among these receptors, PACAP binds to PAC1R specifically with high affinity, while VIP and PACAP bind to VPAC1R or VPAC2R with similar affinities (Arimura 1998). These receptors are widely distributed in the body (Vaudry et al. 2009). PACAP exerts a wide range of effects on different cell types in the brain as early as fetal stage (Watanabe et al. 2007; Zhou et al. 1999). PACAP and its receptors are expressed in germinative neuroepithelia, suggesting that PACAP may be involved in neurogenesis (Sheward et al. 1998). PACAP has been shown to regulate cell fate in various developmental contexts, in a manner dependent on dose, region, signaling, and receptor subtype (Jaworski and Proctor 2000; Waschek et al. 1998). Recent studies suggest the contribution of PACAP to proliferation and differentiation of neural stem cells (NSCs) (Nakamachi et al. 2011).
It is well known that NSCs exist not only in the developing nervous system but also in the adult nervous system of mammals. The locations of NSCs are limited in the adult, including the subgranular zone (SGZ) of the hippocampus and the subventricular zone (SVZ) of the lateral ventricle. In particular, adult hippocampal neurogenesis has garnered great interest because of its potential to influence learning and memory (Deng et al. 2010). Multipotent NSCs are located within the SGZ, which are capable of repeated self-renewal and the generation of both neurons and astrocytes (Bonaguidi et al. 2011). Continuous adult hippocampal neurogenesis functions in cognition and mood regulation, and studies suggest that dysfunction of hippocampal neurogenesis cause learning and behavioral impairments (Christian et al. 2014). Recent studies also suggest that hippocampal neurogenesis is responsible for some aspects of recovery following brain injury (Yu et al. 2016). Increased hippocampal neurogenesis is known to occur following cerebral ischemia (Liu et al. 1998). Several genetic and environmental factors have shown to modulate cell fate of NSCs (Kempermann et al. 2015); however, the entire mechanisms that regulate neurogenesis after neural damage are still unclear.
Previous studies have evaluated the role of PACAP in NSCs during development of the nervous system. PACAP and PAC1R are known to be expressed in embryonic and postnatal brains of rodents (Watanabe et al. 2007). During the early stages of neural development, PAC1R messenger RNA (mRNA) expression is observed in the ventricular zone throughout the neuraxis. After birth, its expression in central nervous system is predominantly limited to the SVZ, olfactory bulb, hippocampus, and cerebellum. Many reports indicate that PACAP plays crucial roles in the proliferation and differentiation of neurons, astrocytes, and oligodendrocytes (Nakamachi et al. 2011; Watanabe et al. 2007). However, the involvement of PACAP in adult neurogenesis is not clearly understood.
PACAP is well known to be a promising neuroprotective peptide. The protective effects of PACAP have been shown in various models of brain injuries, including cerebral ischemia, Parkinson’s disease, trauma, and nerve transections (Lee and Seo 2014; Reglodi et al. 2011; Reglodi et al. 2015). The upregulation of PACAP following several types of nerve injuries indicates that endogenous PACAP plays a role in the post-traumatic recovery of the nervous system (Somogyvári-Vigh and Reglodi 2004). About stroke, a number of papers have reported that PACAP suppressed neuronal cell death or decreased infarct volume after global and focal ischemia in rodents (Dohi et al. 2002; Ohtaki et al. 2006; Ohtaki et al. 2008a; Reglodi et al. 2000; Uchida et al. 1996). Interestingly, recent study reported that the delivery of PACAP in the vicinity of the infarct zone 3 days poststroke improved functional recovery by inducing M2 microglia/macrophage polarization (Brifault et al. 2015). This study suggested that PACAP could also play an important role in the modulation of inflammation after stroke. As recent studies reported that inflammation after neural disease is necessary for neurogenesis (Belarbi and Rosi 2013), PACAP could also be involved in the proliferation and differentiation of NSCs after brain damage. Though the neuroprotective effect of PACAP is well established, its role in neurogenesis after stroke is still unclear.
In the present study, we hypothesized that PACAP is involved in hippocampal neurogenesis of adult mice. Therefore, we examined the neurogenetic effect of PACAP using intraventricular PACAP treatment and a global ischemia model in PACAP-deficient mice.
Materials and Methods
Animals
PACAP-deficient mice have been reported previously (Hashimoto et al. 2001). The experiment comparing the effects of endogenous PACAP in PACAP-deficient and wild-type mice was carried out using littermates (PACAP+/+ and +/− mice) from the breeding of PACAP+/− mice. Other experiments using wild-type mice were carried out using C57BL/6 wild-type mice obtained from Charles River Japan (Tokyo, Japan). Male mice were used in all experiments. Animal care and all experimental procedures involving animal use were approved by the Institutional Animal Care and Use Committee of Showa University. Mice were housed in a controlled environment (23 °C, 12/12-h light/dark cycle) and provided with free access to water and standard rodent chow.
Mouse NSC Culture
Primary cell culture of NSCs was essentially carried out as described previously (Watanabe et al. 2013). Briefly, telencephalons were prepared from embryos (embryonic day 14.5) of ICR mice and gently dissociated by pipetting. The dissociated cells, which included neural progenitor cells, were seeded onto a culture dish precoated with 15 μg/ml poly-l-ornithine and 1 μg/ml fibronectin (Sigma-Aldrich, St. Louis, MO). The cells were cultured in Dulbecco’s modified Eagle’s medium/F12 (Life Technologies, Carlsbad, CA) containing 25 μg/ml insulin, 100 μg/ml human apotransferrin, 20 nM progesterone, 100 μM putrescine, 30 nM sodium selenite, antibiotic antimycotic solution (Sigma-Aldrich), and 10 ng/ml recombinant human basic fibroblast growth factor (R&D Systems, Minneapolis, MN) for 4 days.
PACAP and Vehicle Treatments
Wild-type C57/BL6 male mice were anesthetized with sevoflurane. A stainless steel cannula was inserted into the right lateral ventricle at the position of 0.5 mm posterior, 1.0 mm lateral, 2.2 mm ventral from bregma and fixed with dental cement. At 1 week after cannulation, PACAP38 (480 pmol/kg/day, Peptide Institute, Osaka, Japan) dissolved in 0.1 % bovine serum albumin/saline or the vehicle only were continuously injected by an Alzet micro-osmotic pump (Model 1007D, 0.5 μl/h, Alzet, Cupertino, CA) until day 7.
Common Carotid Artery Occlusion and Reperfusion
Global ischemia was induced by common carotid artery occlusion and reperfusion as described previously (Nakamachi et al. 2013; Ohtaki et al. 2008b). Wild-type and PACAP+/− mice were anesthetized with sevoflurane. Anesthesia was maintained with 2 % sevoflurane in NO2/O2 (7:3) via a facemask. A midline incision was made, and then, both common carotid arteries were exposed. Both arteries were isolated and occluded with artery clips. After 12 min, the clips were removed for reperfusion and the neck incision was sutured. The animals were kept in an incubator room at 37 °C and then moved to room temperature.
BrdU Treatment
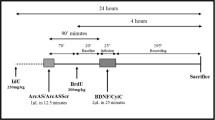
For bromodeoxyuridine (BrdU) staining and cell counts, BrdU (Sigma-Aldrich) was injected prior to sacrifice. Schematic experimental schedule is described in Supplemental Fig. 1. In the experiment assessing the effects of exogenous PACAP, BrdU was injected intraperitoneally starting from 14 h prior to sacrifice at a dose of 50 mg/kg every 2 h for a total of six times. Animals were sacrificed at 2 h after the last BrdU injection (Supplemental Fig. 1a). In the experiment assessing the effects of endogenous PACAP, BrdU was injected intraperitoneally at 3 h prior to sacrifice at a dose of 300 mg/kg. Animals were sacrificed on days 0, 1, 3, 7, 14, 28, and 56 in this experiment (Supplemental Fig. 1b).
Histology
Animals were deeply anesthetized by intraperitoneal injection of sodium pentobarbital (50 mg/kg) and then perfused transcardially with 0.9 % saline followed by 2 % paraformaldehyde (PFA) in 50 mM phosphate buffer. Brains were removed and postfixed in fixative solution overnight, followed by immersion in 0.1 M phosphate buffer containing 20 % sucrose for 2 days. Sections were prepared as frozen sections for immunohistochemistry and immunocytochemistry. Brains were cut from 2 mm anterior to 3 mm posterior to the bregma, embedded in O.C.T. compound (Sakura Finetech, Tokyo, Japan), and then frozen in liquid nitrogen-cooled isopentane. Sections of 14 μm were prepared on a cryostat. Primary cultures of NSCs (described above) were fixed with 2 % PFA for 30 min and subjected to immunostaining.
For PACAP and PAC1R staining, sections were pretreated with 10 mM citrate for 30 min at 80 °C. For BrdU staining, sections were pretreated with 2 N HCl for 30 min at 37 °C and then incubated in 5 % NHS/0.1 % Tween 20 in PBS (PBST). Tissue sections were washed several times with PBST and then incubated in 5 % NHS/PBST for 1 h. Subsequently, the sections were incubated overnight with primary antibodies. The sections were then rinsed with PBST and incubated with appropriate secondary antibodies for 2 h. Primary and secondary antibodies are listed in Table 1.
For single immunostaining, in addition to the pretreatment described above, sections were pretreated with 0.3 % H2O2. Sections were visualized using avidin-biotin complex/diaminobenzidine as the chromogen (Vector Laboratories, Burlingame, CA). Single immunostaining was detected using a microscope (AX70, Olympus, Tokyo, Japan).
For double immunostaining, fluorescently labeled antibodies were used and some sections were counterstained with 4,6-diamidine-2-phenylindole dihydrochloride (DAPI; 1:10,000; Roche, Mannheim, Germany). Fluorescence was detected using an Axio Imager optical sectioning microscope with ApoTome (Zeiss, Oberkochen, Germany) or a confocal microscope (A1, Nikon, Japan) for Fig. 1a.
PAC1R is expressed in NSCs. a Double-immunostained sections of the SGZ in the adult mouse hippocampus. PAC1R (red) was colocalized with nestin (green), a marker of NSCs. Sections were counterstained with DAPI (blue). b Double immunostaining showed PAC1R (red) colocalized with nestin (green) in cultured NSCs. Cell nuclei were stained with DAPI (blue)
BrdU-positive cells in mice were manually counted in both SGZs along the superior and inferior blades of the dentate gyrus. The experiment comparing wild-type and PACAP-deficient mice was performed by randomly choosing 22–45 sections from three to six mice for each day after ischemia. BrdU-positive cells/section were calculated by the average of the sum of the left and right BrdU-positive cells. For the experiment comparing BrdU-positive cells/SGZ in vehicle and PACAP treatments, serial sections including the hippocampus were prepared from eight to ten mice, and all BrdU-positive cells in the SGZ were counted in each animal.
Real-Time PCR
Real-time polymerase chain reaction (PCR) was performed according to our previous report (Nakamachi et al. 2012). In brief, brains were removed from intact wild-type mice (0 h) and wild-type mice that underwent global ischemia at 6 h, 12 h, 1, 3, 7, 14, 28, and 56 days. Hippocampi of both hemispheres were immediately separated on ice and then snap frozen in liquid nitrogen. The samples were stored at −80 °C until analysis. PACAP mRNA in tissues was quantified by real-time PCR using SYBR Premix Ex Taq II reagent (Takara Bio. Inc., Shiga, Japan) with an ABI PRISM 7900 sequence detection system (Applied Biosystems, Lincoln, CA). Total RNA was isolated from the tissue and reverse transcribed into cDNA using a PrimeScript RT reagent Kit (Takara Bio, Inc.). PCR amplification of cDNA with primers specific for PACAP and β-actin as a housekeeping gene was carried out. The primer sequences are shown in Table 2. Relative gene expression was calculated using the comparative ΔCt method in which gene expression levels were compared with Ct values of the housekeeping gene. Mouse PACAP mRNA levels were subsequently normalized to the percentage values obtained from intact control groups (considered as 100 %).
Statistical Analysis
Data are expressed as the mean ± standard error of the mean (SEM). Statistical comparisons were made by nonrepeated measures analysis of variance after the Tukey–Kramer post hoc test. Data for comparison of two groups were analyzed by two-tailed Student’s t test. Statistical significance was accepted at p < 0.05.
Results
Double Immunostaining of PAC1R and Nestin in the Adult Mouse SGZ and Cultured NSCs
Double immunostaining of PAC1R and nestin was performed to confirm the presence of PAC1R in NSCs. PAC1R and nestin were colocalized in the adult mouse SGZ of the hippocampus (Fig. 1a). Similar results were also obtained by double immunostaining of PAC1R and nestin in cultured NSCs (Fig. 1b).
BrdU-Positive Proliferative Cells Are Increased in the Mouse SGZ After PACAP Administration
To evaluate the effect of exogenous PACAP, we examined proliferative cells in the adult mouse hippocampus after PACAP or vehicle treatment using BrdU labeling. PACAP38 (480 pmol/kg/day) or the vehicle only were injected into the lateral ventricle of C57/BL6 wild-type mice continuously for 7 days. BrdU was injected at a dose of 50 mg/kg six times before sacrifice on day 7. BrdU immunohistochemistry showed greater numbers of BrdU-positive cells in PACAP-treated mice than in vehicle-treated mice (Fig. 2a). During this time, BrdU-positive cells were mainly observed in the SGZ of the hippocampus. BrdU-positive cells/SGZ were significantly (p < 0.05) higher in PACAP-treated mice than in vehicle-treated mice (Fig. 2b).
PACAP treatment increases BrdU-positive proliferative cells in the hippocampal SGZ of adult mice. a BrdU-immunostained hippocampal SGZ after PACAP or vehicle administration. BrdU-immunopositive cells were increased after PACAP administration. b Quantitative data of BrdU-positive cells in the hippocampal SGZ after PACAP or vehicle treatment. BrdU-positive cells in the SGZ were significantly increased in the PACAP-treated group compared with the vehicle-treated group. Bars represent the means ± standard error of the mean (SEM). n = 8, vehicle group; n = 10, PACAP group.*P < 0.05
BrdU-Positive Cells Do Not Increase in the Hippocampus of PACAP-Deficient Mice After Global Ischemia
Cerebral ischemia is known to cause neuronal degeneration in the hippocampus. A recent study showed that NSCs proliferate after ischemia and are involved in functional recovery (Christian et al. 2014). To investigate the contribution of endogenous PACAP to increasing NSCs, we compared the numbers of BrdU-positive cells in wild-type and PACAP-deficient (PACAP+/−) mice after global ischemia. We used PACAP+/− mice because PACAP−/− pups had a high mortality rate. PACAP expression in PACAP+/− mice was reported to be greatly decreased (about 70 % reduction) compared with wild-type mice (Hashimoto et al. 2001). C57/BL6 wild-type and C57/BL6 PACAP-deficient mice underwent global ischemia as described in the “Materials and Methods.” Mice were sacrificed on days 0, 1, 3, 7, 14, 28, and 56. BrdU (300 mg/kg) was injected at 3 h prior to sacrifice. BrdU-positive cells/section were significantly decreased in PACAP-deficient mice compared with wild-type mice on days 3 and 7 (Fig. 3a, b). No significant increase was observed in PACAP-deficient mice after global ischemia throughout the experiment (Fig. 3b).
BrdU-positive cells do not increase after global ischemia in PACAP knockout (KO) mice. a BrdU-immunostained hippocampus before or at 7 days after global ischemia in wild-type and PACAP KO mice. BrdU staining increased after ischemia in wild-type mice but not in PACAP KO mice. b Quantitative data of BrdU-positive cells in the hippocampal SGZ after global ischemia in wild-type and PACAP KO mice over a time course. BrdU-positive cells/section were significantly increased on days 3 and 7 in wild-type mice, while no such increase was observed in PACAP KO mice throughout the experiment. Bars represent the means ± SEM. n = 22–45 per group.*P < 0.05
Identification of BrdU-Positive Cells After Global Ischemia
We confirmed an increase in BrdU-positive cells in the SGZ after global ischemia in wild-type mice, the highest of which was at day 7. To examine which cells were proliferating, we stained for BrdU and several neural markers at day 7 after global ischemia in wild-type mice. BrdU was colocalized with the NSC marker SOX2 (Fig. 4a). No coexpression was observed with the microglial marker IBA1 or astrocyte marker S100β (Fig. 4a). Next, we investigated the fate of BrdU-positive cells after a longer period of time. BrdU was injected into wild-type mice at day 7 after global ischemia, and then, samples were collected at day 28. Double immunostaining was performed for BrdU and NeuN. BrdU was colocalized with NeuN at day 28 (Fig. 4b). These data suggest that BrdU-labeled cells at day 7 after ischemia were NSCs and had subsequently differentiated into mature neurons.
Characterization of BrdU-positive cells in the hippocampus of wild-type mice at days 7 and 28 after global ischemia. a Double-immunostained brain sections at day 7 after global ischemia. BrdU colocalized with the NSC marker SOX2. No coexpression was observed with the microglial marker IBA1 or astrocyte marker S100β. b Double immunostaining at day 28 after global ischemia. BrdU was colocalized with the mature neuronal marker NeuN
Localization of PACAP After Global Ischemia
PACAP immunostaining at day 7 showed an increase of PACAP in the SGZ and hilus of the hippocampus after global ischemia (Fig. 5a). We also assessed the mRNA expression of PACAP by real-time PCR. Consistent with our other results, the mRNA expression of PACAP was significantly increased and the highest at day 7 after global ischemia (Fig. 5b). Next, we performed double immunostaining of PACAP and neuronal markers and determined the localization of PACAP in the SGZ and hilus. PACAP was coexpressed with nestin in the SGZ but not with NeuN or Tuj1. In the hilus, PACAP was coexpressed with NeuN, but not with Tuj1 or glial fibrillary acidic protein (GFAP) (Fig. 5c). These data suggest that PACAP was secreted from NSCs in the SGZ and mature neurons in the hilus.
PACAP expression and localization at day 7 after global ischemia. a PACAP-immunopositive cells in the hippocampus at day 7 after global ischemia. PACAP immunoreactivity was increased after global ischemia in the SGZ and hilus. b PACAP mRNA expression in the hippocampus. Real-time RT-PCR showed a significant increase in the mRNA expression of PACAP at day 7 after ischemia. c PACAP immunoreactivity was colocalized with nestin but not NeuN or Tuj1 in the SGZ. In the hilus, PACAP immunoreactivity was colocalized with NeuN but not Tuj1 or GFAP
Discussion
In the present study, we demonstrated the effects of exogenous and endogenous PACAP in NSCs of the SGZ in the hippocampus. Among the three receptors that mediate PACAP actions, PACAP binds to PAC1R specifically with high affinity (Arimura 1998). This receptor is distributed highly in neurogenic areas such as the SVZ and hippocampus (Vaudry et al. 2009), and studies suggest the contribution of PACAP to proliferation and differentiation of NSCs (Nakamachi et al. 2011; Watanabe et al. 2007). PACAP affects NSCs via PAC1R in these areas. Therefore, we first examined the localization of PAC1R in the SGZ and cultured NSCs by performing double immunostaining of PAC1R and the NSC marker nestin. Immunoreactivities of PAC1R and nestin were colocalized in both cultured NSCs and NSCs in the SGZ (Fig. 1a, b). These results suggest that NSCs in the SGZ of adult mice express PAC1R, indicating that PACAP has a role in NSCs. Our previous study reported strong expression of PAC1R in nestin- and GFAP-positive adult NSCs in the SVZ of the lateral ventricle (Matsuno et al. 2008). Another group also showed expression of PAC1R in the adult neural germinal area such as the SVZ and SGZ (Ohta et al. 2006). Here, we first identified that cells expressing PAC1R in the adult SGZ of the hippocampus as nestin-positive NSCs.
We then examined the effect of PACAP in the SGZ. First, to evaluate the effect of exogenous PACAP, we examined proliferative cells in the adult mouse hippocampus after PACAP or vehicle injection into the lateral ventricle. BrdU immunohistochemistry showed greater numbers of BrdU-positive cells in PACAP-treated mice than in vehicle-treated mice, which were mainly in the SGZ (Fig. 2a, b). This result suggests that exogenous PACAP increases the proliferation of NSCs in the SGZ. A previous study reported that administration of a high dose of PACAP (approximately 61 nmol/kg/day) increases BrdU-positive cells in the SGZ (Ohta et al. 2006). A high concentration of PACAP can activate VPAC2R and result in vasodilation, tachycardia, and water retention (Farnham et al. 2011; Lamine et al. 2015; Tsutsumi et al. 2002; Warren et al. 1992). These effects would limit the use of PACAP as a neuroprotective drug. Here, we found that a relatively low dose of PACAP (480 pmol/kg/day) increased BrdU-positive cells in the SGZ. These data indicate not only that PACAP is biologically important for NSCs but also potentially useful for treatment of neurodegenerative diseases.
Next, to evaluate the effect of PACAP in NSCs after neuronal damage, we compared wild-type and PACAP+/− mice in a global ischemia model. Increased hippocampal neurogenesis is known to occur following cerebral ischemia (Liu et al. 1998). We hypothesized that PACAP plays a role in this process by increasing cell numbers after cerebral ischemia in the hippocampus. To test this hypothesis, we performed BrdU immunostaining after global ischemia in the SGZ of the two groups. PACAP+/− mice showed significantly less BrdU-positive cells in the SGZ after global ischemia compared with wild-type mice (Fig. 3a, b). This result suggests that endogenous PACAP plays an important role in increasing cell numbers in the SGZ after global ischemia. While growth factors and inflammation modulators, such as brain-derived neurotrophic factor and matrix metalloproteinases, have been reported to play important roles in neurogenesis after stoke (Barkho and Zhao 2011), PACAP is also considered to be crucial for stoke-induced neurogenesis. Interestingly, BrdU-positive cells did not differ between the two groups before global ischemia (Fig. 3b, day 0). Endogenous PACAP did not appear to be involved in the proliferation of cells in the SGZ under normal conditions. However, PACAP may be needed during conditions that require stimulation of proliferation. A study comparing wild-type and PACAP-deficient mice under standard and enriched environment conditions obtained similar findings indicating that the survival of newly divided cells under enriched conditions increases in wild-type mice but does not differ under standard conditions (Ago et al. 2011).
We next investigated the fate of BrdU-positive cells that increased after global ischemia in the SGZ. Although hippocampal neurogenesis is known to occur in the SGZ, and NSCs are the prominent proliferative cells in the SGZ, astrocytes and microglia can also be activated and proliferate after stroke. To verify that the cells proliferating after global ischemia were NSCs, we performed double immunohistochemistry. At day 7 after global ischemia, BrdU-positive cells colocalized with the NSC marker SOX2 (Fig. 4a). At day 28 after global ischemia, the cells labeled with BrdU at day 7 were immunopositive for the mature neuronal marker NeuN (Fig. 4b). These results suggest that NSCs increased in the SGZ after global ischemia and then differentiated into mature neurons. These results are consistent with past studies suggesting that global ischemia stimulates cell proliferation in the SGZ by performing BrdU injection after global ischemia (Kawai et al. 2004; Yagita et al. 2001). On the other hand, although NSCs proliferate after global ischemia, many of these new cells were reported to not survive (Yagita et al. 2001). In our study, cells that survived at day 28 had differentiated into neurons (Fig. 4b). Another study also suggested that newly generated cells can mature into functional neurons after adult hippocampal neurogenesis (van Praag et al. 2002). PACAP promoted the proliferation of NSCs in the SGZ, which differentiated into neurons. This finding indicates the potential of PACAP as a factor that could be useful for neural repair and functional improvement after neuronal damage. Promoting the survival of proliferating NSCs should be a challenge to consider in future studies.
Finally, we performed immunohistostaining and real-time PCR to determine the source of PACAP after global ischemia. Real-time PCR showed that PACAP mRNA levels were highest at day 7 after global ischemia (Fig. 5b). Unexpectedly, PACAP mRNA levels 3 days after ischemia was not upregulated while BrdU-positive cells were significantly increased at this time. We consider that not only the expression of PACAP but that of PAC1R in NSCs could be upregulated after stroke. This hypothesis could be supported by our previous study that showed the expression of PAC1R was upregulated after traumatic brain injury (Morikawa et al. 2009). PACAP immunostaining revealed that PACAP-positive cells increased in the SGZ and hilus of the hippocampus after global ischemia (Fig. 5a). Double immunostaining showed that PACAP in the SGZ colocalized with nestin, but not with the mature neural marker NeuN or immature neural marker Tuj1 (Fig. 5c). During this time, PACAP in the hilus was colocalized with NeuN but not Tuj1 or the astrocyte marker GFAP. These results suggest that NSCs in the SGZ and neurons in the hilus could be the cells producing PACAP after global ischemia, which are supposedly responsible for increasing NSCs in the SGZ in an autocrine/paracrine manner.
In conclusion, PACAP is an important neuropeptide that contributes to increasing NSCs in the SGZ of the hippocampus. This increase can lead to hippocampal neurogenesis and may regenerate neurons that are damaged after neurodegenerative diseases such as cerebral ischemia. Future studies addressing the molecular basis and pathophysiological implications of PACAP-associated responses of NSCs may contribute to the development of treatments for neural disorders.
References
Ago Y, Yoneyama M, Ishihama T, et al. (2011) Role of endogenous pituitary adenylate cyclase-activating polypeptide in adult hippocampal neurogenesis. Neuroscience 172:554–561
Arimura A (1998) Perspectives on pituitary adenylate cyclase activating polypeptide (PACAP) in the neuroendocrine, endocrine, and nervous systems. Jpn J Physiol 48:301–331
Barkho BZ, Zhao X (2011) Adult neural stem cells: response to stroke injury and potential for therapeutic applications. Curr Stem Cell Res Ther 6:327–338
Belarbi K, Rosi S (2013) Modulation of adult-born neurons in the inflamed hippocampus. Front Cell Neurosci 7:145
Bonaguidi MA, Wheeler MA, Shapiro JS, et al. (2011) In vivo clonal analysis reveals self-renewing and multipotent adult neural stem cell characteristics. Cell 145:1142–1155
Brifault C, Gras M, Liot D, et al. (2015) Delayed pituitary adenylate cyclase-activating polypeptide delivery after brain stroke improves functional recovery by inducing m2 microglia/macrophage polarization. Stroke 46:520–528
Christian KM, Song H, Ming GL (2014) Functions and dysfunctions of adult hippocampal neurogenesis. Annu Rev Neurosci 37:243–262
Deng W, Aimone JB, Gage FH (2010) New neurons and new memories: how does adult hippocampal neurogenesis affect learning and memory? Nat Rev Neurosci 11:339–350
Dohi K, Mizushima H, Nakajo S, et al. (2002) Pituitary adenylate cyclase-activating polypeptide (PACAP) prevents hippocampal neurons from apoptosis by inhibiting JNK/SAPK and p38 signal transduction pathways. Regul Pept 109:83–88
Farnham MM, Inglott MA, Pilowsky PM (2011) Intrathecal PACAP-38 causes increases in sympathetic nerve activity and heart rate but not blood pressure in the spontaneously hypertensive rat. Am J Physiol Heart Circ Physiol 300:H214–H222
Hashimoto H, Shintani N, Tanaka K, et al. (2001) Altered psychomotor behaviors in mice lacking pituitary adenylate cyclase-activating polypeptide (PACAP). Proc Natl Acad Sci U S A 98:13355–13360
Jaworski DM, Proctor MD (2000) Developmental regulation of pituitary adenylate cyclase-activating polypeptide and PAC(1) receptor mRNA expression in the rat central nervous system. Brain Res Dev Brain Res 120:27–39
Kawai T, Takagi N, Miyake-Takagi K, et al. (2004) Characterization of BrdU-positive neurons induced by transient global ischemia in adult hippocampus. J Cereb Blood Flow Metab 24:548–555
Kempermann G, Song H, Gage FH (2015) Neurogenesis in the adult hippocampus. Cold Spring Harb Perspect Biol 7:a018812
Kimura C, Ohkubo S, Ogi K, et al. (1990) A novel peptide which stimulates adenylate cyclase: molecular cloning and characterization of the ovine and human cDNAs. Biochem Biophys Res Commun 166:81–89
Lamine A, Létourneau M, Doan ND, et al. (2015) Characterizations of a synthetic pituitary adenylate cyclase-activating polypeptide analog displaying potent neuroprotective activity and reduced in vivo cardiovascular side effects in a Parkinson’s disease model. Neuropharmacology. doi:10.1016/j.neuropharm.2015.05.014
Lee EH, Seo SR (2014) Neuroprotective roles of pituitary adenylate cyclase-activating polypeptide in neurodegenerative diseases. BMB Rep 47:369–375
Liu J, Solway K, Messing RO, et al. (1998) Increased neurogenesis in the dentate gyrus after transient global ischemia in gerbils. J Neurosci 18:7768–7778
Matsuno R, Ohtaki H, Nakamachi T, et al. (2008) Distribution and localization of pituitary adenylate cyclase-activating polypeptide-specific receptor (PAC1R) in the rostral migratory stream of the infant mouse brain. Regul Pept 145:80–87
Miyata A, Arimura A, Dahl RR, et al. (1989) Isolation of a novel 38 residue-hypothalamic polypeptide which stimulates adenylate cyclase in pituitary cells. Biochem Biophys Res Commun 164:567–574
Morikawa K, Dohi K, Yofu S, et al. (2009) Expression and localization of pituitary adenylate cyclase-activating polypeptide (PACAP) specific receptor (PAC1R) after traumatic brain injury in mice. In: Shioda S, Homma I, Kato N (eds) Transmitters and modulators in health and disease. Springer Japan, Tokyo, Japan, pp. 207–210
Nakamachi T, Farkas J, Kagami N, et al. (2013) Expression and distribution of pituitary adenylate cyclase-activating polypeptide receptor in reactive astrocytes induced by global brain ischemia in mice. Acta Neurochir Suppl 118:55–59
Nakamachi T, Farkas J, Watanabe J, et al. (2011) Role of PACAP in neural stem/progenitor cell and astrocyte–from neural development to neural repair. Curr Pharm Des 17:973–984
Nakamachi T, Tsuchida M, Kagami N, et al. (2012) IL-6 and PACAP receptor expression and localization after global brain ischemia in mice. J Mol Neurosci 48:518–525
Ohta S, Gregg C, Weiss S (2006) Pituitary adenylate cyclase-activating polypeptide regulates forebrain neural stem cells and neurogenesis in vitro and in vivo. J Neurosci Res 84:1177–1186
Ohtaki H, Nakamachi T, Dohi K, et al. (2006) Pituitary adenylate cyclase-activating polypeptide (PACAP) decreases ischemic neuronal cell death in association with IL-6. Proc Natl Acad Sci U S A 103:7488–7493
Ohtaki H, Nakamachi T, Dohi K, et al. (2008a) Role of PACAP in ischemic neural death. J Mol Neurosci 36:16–25
Ohtaki H, Ylostalo JH, Foraker JE, et al. (2008b) Stem/progenitor cells from bone marrow decrease neuronal death in global ischemia by modulation of inflammatory/immune responses. Proc Natl Acad Sci U S A 105:14638–14643
Reglodi D, Kiss P, Lubics A, et al. (2011) Review on the protective effects of PACAP in models of neurodegenerative diseases in vitro and in vivo. Curr Pharm Des 17:962–972
Reglodi D, Renaud J, Tamas A, et al (2015) Novel tactics for neuroprotection in Parkinson’s disease: role of antibiotics, polyphenols and neuropeptides. Prog Neurobiol
Reglodi D, Somogyvari-Vigh A, Vigh S, et al. (2000) Delayed systemic administration of PACAP38 is neuroprotective in transient middle cerebral artery occlusion in the rat. Stroke 31:1411–1417
Sheward WJ, Lutz EM, Copp AJ, et al. (1998) Expression of PACAP, and PACAP type 1 (PAC1) receptor mRNA during development of the mouse embryo. Brain Res Dev Brain Res 109:245–253
Somogyvári-Vigh A, Reglodi D (2004) Pituitary adenylate cyclase activating polypeptide: a potential neuroprotective peptide. Curr Pharm Des 10:2861–2889
Tsutsumi M, Claus TH, Liang Y, et al. (2002) A potent and highly selective VPAC2 agonist enhances glucose-induced insulin release and glucose disposal: a potential therapy for type 2 diabetes. Diabetes 51:1453–1460
Uchida D, Arimura A, Somogyvári-Vigh A, et al. (1996) Prevention of ischemia-induced death of hippocampal neurons by pituitary adenylate cyclase activating polypeptide. Brain Res 736:280–286
van Praag H, Schinder AF, Christie BR, et al. (2002) Functional neurogenesis in the adult hippocampus. Nature 415:1030–1034
Vaudry D, Falluel-Morel A, Bourgault S, et al. (2009) Pituitary adenylate cyclase-activating polypeptide and its receptors: 20 years after the discovery. Pharmacol Rev 61:283–357
Warren JB, Cockcroft JR, Larkin SW, et al. (1992) Pituitary adenylate cyclase activating polypeptide is a potent vasodilator in humans. J Cardiovasc Pharmacol 20:83–87
Waschek JA, Casillas RA, Nguyen TB, et al. (1998) Neural tube expression of pituitary adenylate cyclase-activating peptide (PACAP) and receptor: potential role in patterning and neurogenesis. Proc Natl Acad Sci U S A 95:9602–9607
Watanabe J, Nakamachi T, Matsuno R, et al. (2007) Localization, characterization and function of pituitary adenylate cyclase-activating polypeptide during brain development. Peptides 28:1713–1719
Watanabe J, Nakamachi T, Ohtaki H, et al. (2013) Low dose of methylmercury (MeHg) exposure induces caspase mediated-apoptosis in cultured neural progenitor cells. J Toxicol Sci 38:931–935
Yagita Y, Kitagawa K, Ohtsuki T, et al. (2001) Neurogenesis by progenitor cells in the ischemic adult rat hippocampus. Stroke 32:1890–1896
Yu TS, Washington PM, Kernie SG (2016) Injury-induced neurogenesis: mechanisms and relevance. Neuroscientist 22:61–71
Zhou CJ, Shioda S, Shibanuma M, et al. (1999) Pituitary adenylate cyclase-activating polypeptide receptors during development: expression in the rat embryo at primitive streak stage. Neuroscience 93:375–391
Acknowledgments
This study was supported in part by JSPS KAKENHI Grants (23249079, 24592681, 25861289, 26293020, 26670122, 15K15670, and 15H01288) and the JSPS Program for Advancing Strategic International Networks to Accelerate the Circulation of Talented Researchers, Grant No. S2603 (HH).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Animal care and all experimental procedures involving animal use were approved by the Institutional Animal Care and Use Committee of Showa University.
Conflict of Interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Supplemental Figure 1
Schematic experimental schedule for BrdU labeling. (GIF 14 kb)
Rights and permissions
About this article
Cite this article
Matsumoto, M., Nakamachi, T., Watanabe, J. et al. Pituitary Adenylate Cyclase-Activating Polypeptide (PACAP) Is Involved in Adult Mouse Hippocampal Neurogenesis After Stroke. J Mol Neurosci 59, 270–279 (2016). https://doi.org/10.1007/s12031-016-0731-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-016-0731-x