Abstract
Pituitary adenylate cyclase-activating polypeptide (PACAP) is a neuropeptide acting as a neuroprotectant. We previously showed that PACAP receptor (PAC1R) immunoreactivity was elevated in reactive astrocytes after stab wound injury. However, the pattern of PAC1R expression in astrocytes after brain injury is still unknown. In this study, PAC1R expression was evaluated in mouse hippocampal astrocytes after bilateral common carotid artery occlusion. PAC1R mRNA levels in the hippocampus peaked on day 7, and glial fibrillary acidic protein (GFAP) mRNA levels increased from day 3 to day 7 after ischemia. We then observed co-localization of PAC1R and GFAP by double immunostaining. GFAP-immunopositive cells showed signs of hypertrophy 3 days after the ischemia, and by day 7 had fine processes, were hypertrophied, and are known as reactive astrocytes. A low number of PAC1R-immunopositive astrocytes were detectable in the hippocampal area until 3 days after ischemia. PAC1R-positive astrocytes were widely distributed in the hippocampus between day 7 and day 14 after ischemia, and they were converging around the damaged CA1 pyramidal cell layer by day 28. These results suggest that PAC1R might be expressed in the middle to late stage of reactive astrocytes and PACAP plays an important role in the reactive astrocytes after brain injury.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
- PACAP receptor
- Brain ischemia
- Reactive astrocyte
- Hippocampus
- Mouse
- Bilateral common carotid artery occlusion
- Real-time PCR
- Immunohistochemistry
Introduction
Pituitary adenylate cyclase-activating polypeptide (PACAP) was isolated from ovine hypothalamus, based on its ability to stimulate the accumulation of cAMP in rat pituitary cell cultures [2]. PACAP is a member of the vasoactive intestinal polypeptide (VIP) secretin glucagon family, and its closest analog is VIP. PACAP and VIP share three types of receptors: the PAC1 receptor (PAC1R), and the VPAC1 and VPAC2 receptors (VPAC1R, VPAC2R). The affinity of PAC1R for PACAP is more than 1,000 times higher than its affinity for VIP, indicating that PAC1R is a relatively selective receptor for PACAP [7, 30]. PAC1R is widely distributed in the brain, and is expressed in neurons and astrocytes [1 21]. PACAP has been shown to have pleiotropic functions, such as neurotransmission, neuroprotection, vasodilatation, and immunomodulation, and it also regulates neural development [10, 20, 25, 31]. PACAP has a great neuroprotective effect on the brain, retina, spinal cord, heart, etc. [4, 12, 18, 24, 28]. The neuroprotective effects of PACAP after infarction appear at a very low concentration after intracerebroventricular or intravenous infusion in the case of global or focal ischemia [21, 29]. We previously showed that PACAP receptor (PAC1R) immunoreactivity was elevated in reactive astrocytes after stab wound injury [26]. However, the expression pattern of PAC1R in ischemic brain has not yet been clarified. Moreover, the astrocytic PAC1R distribution in resting and activated astrocytes after ischemia is unknown. In this study, we examined the expression and distribution of PAC1R in hippocampal regions after global ischemia.
Materials and Methods
Animals
All experimental procedures involving animals were approved by the Institutional Animal Care and Use Committee of the Showa University (51010, 51031). All experiments were performed on male C57BL/6 mice. The animals were housed at 23 °C on a 12-h light/dark cycle with ad libitum access to food and water.
Global Cerebral Ischemia Model
The bilateral common carotid artery occlusion (BCCAO) procedure was carried out as described in our previous report [13]. In brief, mice were anesthetized with 2.0 % sevoflurane in N2O/O2. The body temperature was maintained at 37.0–38.0 °C by a heat blanket. The common carotid arteries on both sides were exposed and occluded with Zen temporary clips (Oowa-tusho, Tokyo, Japan). After 15 min the clips were removed for reperfusion.
Real Time PCR
Mice were sacrificed by decapitation and brains were immediately removed on day 0, 3, 7 or 14 after BCCAO. The hippocampi were then collected and immediately frozen by liquid nitrogen, and kept in a deep freezer at −80 °C. Total RNA was extracted using Trisol (Invitrogen, Carlsbad, CA, USA). The total RNA was reverse-transcribed into cDNA and then amplified using the reagents and the protocol of the PrimeScript RT reagent kit (TaKaRa BIO, Kyoto, Japan). RT reaction and the PCR amplification were performed with GeneAmp PCR System 2700 (Perkin-Elmer, Boston, MA, USA). The primers specific for the mouse GFAP, PAC1R, and β-actin primers were purchased from TaKaRa BIO. For quantification of GFAP and PAC1R mRNA levels, real-time PCR was performed in a SYBR Premix Ex Taq II reagent (TaKaRa BIO INC) setting with heating to 95 °C for 30 s followed by 45 amplification cycles of 95 °C for 5 s and 60 °C for 31 s using an ABI PRISM 7900 (Applied Biosystems, Lincoln, CA, USA). Standard curves were generated using a serial dilution of a reference sample and included in each real-time run to correct for possible variations in product amplification. Relative copy numbers were obtained from standard curve values, and were normalized to the values obtained for the house keeping gene, β-actin. The levels of GFAP and PAC1R mRNA were normalized as percentages of the intact controls.
Histology
Mice were anesthetized with sodium pentobarbital (50 mg/kg, i.p.) 0, 1, 3, 7, 14 or 28 days after BCCAO, and brains were fixed by perfusion with saline followed by 2 % paraformaldehyde in 50 mM phosphate buffer. Post-fixation, the brain tissues containing bregma −1.6 to −2.2 mm were embedded in OCT compound after immersion in 20 % sucrose for cytoprotection as a frozen section. The frozen sections (thickness 8 μm) were used for immunostaining.
Double Immunofluorescent Staining
The frozen sections were washed by PBS, incubated, and blocked with 5 % normal horse serum. The sections were then incubated overnight at 4 °C with rabbit anti-PAC1R antibody raised by using the N-terminal residue as an antigen [26] and mouse anti-glial fibrillary acidic protein (GFAP) antibody (1:500; Sigma, St. Louis, MO, USA) as a marker of astrocytes. The immunoreactivity of PAC1R was detected using Alexa 546-labeled goat anti-rabbit IgG, while that of GFAP were detected using Alexa 488-labeled goat anti-mouse or anti-rat IgG antibodies following 90 min incubation at room temperature. Double immunolabeling was detected using a fluorescence microscope.
Results
Hippocampal GFAP and PAC1R mRNA levels after BCCAO were evaluated using real-time PCR. The GFAP mRNA level was significantly elevated and peaked 3 days after ischemia (456 ± 84 %; Fig. 1). It then decreased gradually by day 7 (280 ± 73 %), and reached the basal level on day 14 (110 ± 18 %; Fig. 1). The PAC1R mRNA level did not change on day 3 (102 ± 13 %), but increased significantly on day 7, where it peaked (155 ± 21 %; Fig. 1).
Glial fibrillary acidic protein (GFAP) mRNA (a) and pituitary adenylate cyclase-activating polypeptide receptor (PAC1R) mRNA (b) levels in mouse hippocampus after global ischemia. Data on the mRNA levels are shown as the mean ± SE (n = 6). **P < 0.01, *P < 0.05 vs 0 day (one-way ANOVA followed by Dunnett’s test)
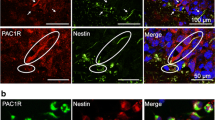
Immunoreactivity of GFAP and PAC1R was observed in the ischemic mouse hippocampus. GFAP-positive PAC1R-negative cells were observed in the hippocampus, and weak PAC1R immunoreactivity was observed in CA1 pyramidal neurons on 0 (intact) and 1 day after ischemia (Fig. 2a, b). GFAP-immunopositive cells became hypertrophied on day 3 without PAC1R immunoreactivity (Fig. 2c). On day 7, PAC1R immunoreactivity was well co-localized with GFAP-positive cells, which had fine processes and were hypertrophied, and are known as reactive astrocytes (Fig. 2d). GFAP-positive with PAC1R-positive cells were observed around the hippocampal CA1 area, where delayed neuronal death was induced by BCCAO. The double-immunopositive cells were widely distributed from there on day 14 (Fig. 2e), and were only found in the CA1 region on day 28 (Fig. 2f). Although PAC1R and GFAP double-immunopositive cells were observed in the cerebral cortex, in the amygdala, and in the fimbria hippocampi, the distribution did not change after BCCAO (data not shown). The distribution of GFAP and PAC1R immunoreactivity after BCCAO was summarized in Fig. 2g.
Distribution of GFAP and PAC1R immunoreactivities in the mouse hippocampal region after global ischemia. (a–f) Typical hippocampal pictures with GFAP (green) and PAC1R (red) immunoreactivities in intact mice (a) or mice at 1 (b), 3 (c), 7 (d), 14 (e), and 28 days (f) after bilateral common carotid artery occlusion (BCCAO). Double-immunopositive cells were indicated by arrows (d–f). (g) Schematic brain maps indicate the distribution of GFAP-positive and PAC1R-negative cells (black circles) and GFAP-positive and PAC1R-positive cells (red squares)
Conclusion
Astrocytes can be activated by central nervous system injury. The activated astrocytes, called reactive astrocytes, form dense scar tissue, known as the glial scar, around the lesion site, which serves to compact inflammatory cells and re-seal the blood–brain barrier after it has been breached by injury [5 11]. We previously reported that PAC1R-immunopositive astrocytes were observed close to the injury site on day 4 after stab wounding. However, the behavior of PAC1R-immunopositive astrocytes after nervous tissue injury is not well understood. In this study, we used the BCCAO model which resulted in delayed neuronal death in the hippocampal CA1 region on day 4 after injury [13]. As a result, the number of PAC1R and GFAP double-immunopositive cells were elevated on day 7, diffused at 14 days, and converging on the area around the injury site 28 days after BCCAO (Fig. 2). To our knowledge, this is the first report to reveal the distribution and transition of PAC1R-immunopositive astrocytes after brain injury. Interestingly, peaks of the mRNA levels show a time lag: GFAP on day 3 and PAC1R on day 7 (Fig. 1). GFAP is well known as a normal astrocytic marker, but it is also up-regulated when these astrocytes become reactive [9]. Indeed, GFAP-immunopositive cells start hypertrophying on day 3, almost at the same time as neuronal death occurs, but the astrocytes were PAC1R-immunonegative. Between days 7 and 14, matured reactive astrocytes were PAC1R-immunopositive. These data suggest that PAC1R is expressed at the middle or late stage of reactive astrocytosis, but not in the earlier stage.
The effect of PACAP on cultured astrocytes has been reported. A number of growth factors and cytokines are released from reactive astrocytes [23], and it has been reported that PACAP administration induces the expression of neuroprotective proteins, such as ADNF and ADNP [3 15 17], and cytokines [6, 14, 27]. Recently, We reported that PAC1R immunoreactivity was observed in IL-6 reactive astrocyte with IL-6 immunoreactivity which is known as an neuroprotective factor associated with PACAP [19]. Neuroprotective effects of PACAP may mediate the release of such PACAP-inducible factors from astrocytes. Moreover, PACAP at a concentration of 10−11 to 10−13 M potentiates and stimulates the proliferation of resting and reactive astrocytes [8, 16].
We conclude that PAC1R expressed in mature reactive astrocytes may play an important role in glial scar formation. Further studies are required to fully elucidate the role of PACAP in the functional behavior of astrocytes, and to characterize the underlying mechanisms.
References
Arimura A (1998) Perspectives on pituitary adenylate cyclase activating polypeptide (PACAP) in the neuroendocrine, endocrine, and nervous systems. Jpn J Physiol 48:301–331
Arimura A, Shioda S (1995) Pituitary adenylate cyclase activating polypeptide (PACAP) and its receptors: neuroendocrine and endocrine interaction. Front Neuroendocrinol 16:53–88
Dejda A, Sokolowska P, Nowak JZ (2005) Neuroprotective potential of three neuropeptides PACAP, VIP and PHI. Pharmacol Rep 57:307–320
Endo K, Nakamachi T, Seki T, Kagami N, Wada Y, Nakamura K, Kishimoto K, Hori M, Tsuchikawa D, Shinntani N, Hashimoto H, Baba A, Koide R, Shioda S (2011) Neuroprotective effect of PACAP against NMDA-induced retinal damage in the mouse. J Mol Neurosci 43:22–29
Fawcett JW, Asher RA (1999) The glial scar and central nervous system repair. Brain Res Bull 49:377
Gottschall PE, Tatsuno I, Arimura A (1994) Regulation of interleukin-6 (IL-6) secretion in primary cultured rat astrocytes: synergism of interleukin-1 (IL-1) and pituitary adenylate cyclase activating polypeptide (PACAP). Brain Res 637:197–203
Harmar AJ, Arimura A, Gozes I, Journot L, Laburthe M, Pisegna JR, Rawlings SR, Robberecht P, Said SI, Sreedharan SP, Wank SA, Waschek JA (1998) International Union of Pharmacology. XVIII. Nomenclature of receptors for vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide. Pharmacol Rev 50:265–270
Hashimoto H, Kunugi A, Arakawa N, Shintani N, Fujita T, Kasai A, Kawaguchi C, Morita Y, Hirose M, Sakai Y, Baba A (2003) Possible involvement of a cyclic AMP-dependent mechanism in PACAP-induced proliferation and ERK activation in astrocytes. Biochem Biophys Res Commun 311:337–343
Lin RCS, Matesic DF, Marvin M, McKay RDG, Brüstle O (1995) Re-expression of the intermediate filament nestin in reactive astrocytes. Neurobiol Dis 2:79
Matsuno R, Ohtaki H, Nakamachi T, Watanabe J, Yofu S, Hayashi D, Takeda T, Nonaka N, Seki M, Nakamura M, Itabashi K, Shioda S (2008) Distribution and localization of pituitary adenylate cyclase-activating polypeptide-specific receptor (PAC1R) in the rostral migratory stream of the infant mouse brain. Regul Pept 145:80–87
Milos P, Michael N (2005) Astrocyte activation and reactive gliosis. Glia 50:427–434
Mori H, Nakamachi T, Ohtaki H, Yofu S, Sato A, Endo K, Iso Y, Suzuki H, Takeyama Y, Shintani N, Hashimoto H, Baba A, Shioda S (2010) Cardioprotective effect of endogenous pituitary adenylate cyclase-activating polypeptide on doxorubicin-induced cardiomyopathy in mice. Circ J 74:1183–1190
Nakamachi T, Endo S, Ohtaki H, Yin L, Kenji D, Kudo Y, Funahashi H, Matsuda K, Shioda S (2005) Orexin-1 receptor expression after global ischemia in mice. Regul Pept 126:49–54
Nakamachi T, Farkas J, Watanabe J, Ohtaki H, Dohi K, Arata S, Shioda S (2011) Role of PACAP in neural stem/progenitor cell and astrocyte – from neural development to neural repair. Curr Pharm Des 17:973–984
Nakamachi T, Li M, Shioda S, Arimura A (2006) Signaling involved in pituitary adenylate cyclase-activating polypeptide-stimulated ADNP expression. Peptides 27:1859–1864
Nakamachi T, Nakamura K, Oshida K, Kagami N, Mori H, Watanabe J, Arata S, Yofu S, Endo K, Wada Y, Hori M, Tsuchikawa D, Kato M, Shioda S (2011) Pituitary adenylate cyclase-activating polypeptide (PACAP) stimulates proliferation of reactive astrocytes in vitro. J Mol Neurosci 43:16–21
Nakamachi T, Ohtaki H, Yofu S, Dohi K, Watanabe J, Hayashi D, Matsuno R, Nonaka N, Itabashi K, Shioda S (2008) Pituitary adenylate cyclase-activating polypeptide (PACAP) type 1 receptor (PAC1R) co-localizes with activity-dependent neuroprotective protein (ADNP) in the mouse brains. Regul Pept 145:88–95
Nakamachi T, Ohtaki H, Yofu S, Dohi K, Watanabe J, Mori H, Sato A, Hashimoto H, Shintani N, Baba A, Shioda S (2010) Endogenous pituitary adenylate cyclase activating polypeptide is involved in suppression of edema in the ischemic brain. Acta Neurochir Suppl 106:43–46
Nakamachi T, Tsuchida M, Kagami N, Yofu S, Wada Y, Hori M, Tsuchikawa D, Yoshikawa A, Imai N, Nakamura K, Arata S, Shioda S (2012) IL-6 and PACAP receptor expression and localization after global brain ischemia in mice. J Mol Neurosci 48(3):518–525
Ohtaki H, Dohi K, Yofu S, Nakamachi T, Kudo Y, Endo S, Aruga T, Goto N, Watanabe J, Kikuyama S, Shioda S (2004) Effect of pituitary adenylate cyclase-activating polypeptide 38 (PACAP38) on tissue oxygen content – treatment in central nervous system of mice. Regul Pept 123:61–67
Ohtaki H, Nakamachi T, Dohi K, Aizawa Y, Takaki A, Hodoyama K, Yofu S, Hashimoto H, Shintani N, Baba A, Kopf M, Iwakura Y, Matsuda K, Arimura A, Shioda S (2006) Pituitary adenylate cyclase-activating polypeptide (PACAP) decreases ischemic neuronal cell death in association with IL-6. Proc Natl Acad Sci U S A 103:7488–7493
Reglodi D, Somogyvari-Vigh A, Vigh S, Kozicz T, Arimura A (2000) Delayed systemic administration of PACAP38 is neuroprotective in transient middle cerebral artery occlusion in the rat. Stroke 31:1411–1417
Ridet JL, Malhotra SK, Privat A, Gage FH (1997) Reactive astrocytes: cellular and molecular cues to biological function. Trends Neurosci 20:570–577
Seki T, Itoh H, Nakamachi T, Endo K, Wada Y, Nakamura K, Shioda S (2011) Suppression of rat retinal ganglion cell death by PACAP following transient ischemia induced by high intraocular pressure. J Mol Neurosci 43:30–34
Shioda S, Ohtaki H, Nakamachi T, Dohi K, Watanabe J, Nakajo S, Arata S, Kitamura S, Okuda H, Takenoya F, Kitamura Y (2006) Pleiotropic functions of PACAP in the CNS: neuroprotection and neurodevelopment. Ann N Y Acad Sci 1070:550–560
Suzuki R, Arata S, Nakajo S, Ikenaka K, Kikuyama S, Shioda S (2003) Expression of the receptor for pituitary adenylate cyclase-activating polypeptide (PAC1-R) in reactive astrocytes. Brain Res Mol Brain Res 115:10–20
Tatsuno I, Morio H, Tanaka T, Uchida D, Hirai A, Tamura Y, Saito Y (1996) Pituitary adenylate cyclase-activating polypeptide (PACAP) is a regulator of astrocytes: PACAP stimulates proliferation and production of interleukin 6 (IL-6), but not nerve growth factor (NGF), in cultured rat astrocyte. Ann N Y Acad Sci 805:482–488
Tsuchikawa D, Nakamachi T, Tsuchida M, Wada Y, Hori M, Farkas J, Yoshikawa A, Kagami N, Imai N, Shintani N, Hashimoto H, Atsumi T, Shioda S (2012) Neuroprotective effect of endogenous pituitary adenylate cyclase-activating polypeptide on spinal cord injury. J Mol Neurosci 48(3):508–517
Uchida D, Arimura A, Somogyvari-Vigh A, Shioda S, Banks WA (1996) Prevention of ischemia-induced death of hippocampal neurons by pituitary adenylate cyclase activating polypeptide. Brain Res 736:280–286
Vaudry D, Gonzalez BJ, Basille M, Yon L, Fournier A, Vaudry H (2000) Pituitary adenylate cyclase-activating polypeptide and its receptors: from structure to functions. Pharmacol Rev 52:269–324
Watanabe J, Nakamachi T, Matsuno R, Hayashi D, Nakamura M, Kikuyama S, Nakajo S, Shioda S (2007) Localization, characterization and function of pituitary adenylate cyclase-activating polypeptide during brain development. Peptides 28:1713–1719
Acknowledgments
This work was supported in part by a Special Research Grant-in-Aid for Development of Characteristic Education and the High-Technology Research Center Project from the Ministry of Education, Culture, Sports, Science, and Technology (T.N. and S.S.) and Research on Health Sciences focusing on Drug Innovation from The Japan Health Sciences Foundation (S.S).
Conflict of InterestWe declare that we have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer-Verlag Wien
About this paper
Cite this paper
Nakamachi, T. et al. (2013). Expression and Distribution of Pituitary Adenylate Cyclase-Activating Polypeptide Receptor in Reactive Astrocytes Induced by Global Brain Ischemia in Mice. In: Katayama, Y., Maeda, T., Kuroiwa, T. (eds) Brain Edema XV. Acta Neurochirurgica Supplement, vol 118. Springer, Vienna. https://doi.org/10.1007/978-3-7091-1434-6_9
Download citation
DOI: https://doi.org/10.1007/978-3-7091-1434-6_9
Published:
Publisher Name: Springer, Vienna
Print ISBN: 978-3-7091-1433-9
Online ISBN: 978-3-7091-1434-6
eBook Packages: MedicineMedicine (R0)