Abstract
CK2 shows disease-associated alteration in the scrapie experimental rodents and human prion diseases. In this study, mammalian expressing plasmids for human CK2 subunits, CK2α and CK2β, were generated. Immunoprecipitation assays revealed stronger signals of PrP–CK2α complexes in the HEK293 cells co-transfected with plasmids expressing CK2α and various PrP constructs, including PG5, CytoPrP, PG9, and PG12. Meanwhile, obviously weaker signals of PrP–CK2β complexes were also observed in the cells co-expressing CK2β and PrPs. Tubulin-specific Western blots and immunofluorescence assays revealed that similar as the observations in the presences of PrP-specific siRNA, the abnormal PrPs-induced reductions of tubulin and disruptions of microtubule structures were completely restored in the cells when co-expressing CK2α and CK2β. Moreover, co-expressions of CK2α and PrPs induced phosphorylation on p53 at the position of serine 6 (p53-Ser6), although much weaker than that in the cells expressing CK2α and CK2β, while expressions of either PrPs or CK2 subunits did not change the cellular p53 level or induce phosphorylation on p53 at Ser9. Our data here verify again the molecular interaction between CK2 and PrP. Co-presences of CK2 subunits restore the down-regulated tubulin and disrupted microtubule structures caused by expressions of the abnormal PrP proteins in HEK293 cells.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Prion diseases, also called transmissible spongiform encephalopathies (TSEs), are a group of fatal neurodegenerative diseases characterized by neuronal loss and spongiform degeneration in the central nervous system (CNS), which can affect a series of mammalian hosts, mainly Creutzfeldt–Jakob disease (CJD), fatal familial insomnia (FFI), Gerstmann–Straussler–Scheinker (GSS) syndrome, and kuru in human, scrapie in sheep and goat, bovine spongiform encephalopathy (BSE) in cattle, and chronic wasting disease (CWD) in deer and elk (Prusiner 1998; Johnson 2005). About 80–85 % human TSEs have no unambiguous cause, nominated as sporadic CJD. Approximately 10–15 % of human TSE cases refer to genetic or familial CJD, which is related with prion protein gene (PRNP) mutation on chromosome 20. Additionally, about 1 % human TSE cases are iatrogenically acquired4 and more than 220 vCJD cases have been reported worldwide following contamination by BSE agent. All prion diseases are characterized by the conversion of the constitutive cellular prion protein (PrPC) into the pathogenic form, which is also known as scrapie PrP (PrPSc) (Prusiner 1998). The accumulation of PrPSc has been thought to be linked to the pathogenesis of prion disease.
Protein kinase CK2 is a Ser/Thr kinase highly conserved in eukaryotic cells, involving in the control of various cellular processes, such as cell cycle, apoptosis, transcriptional regulation, and signal transduction (Guerra et al. 1999; Ahmed et al. 2002; Litchfield 2003). CK2 is much more abundant in the brains than in other tissues. In neural cells, there appears to be a myriad of CK2 substrates that have clear implications in neural development, neuritogenesis, synaptic transmission, and plasticity (Blanquet 1998). The CK2 holoenzyme generally composes of two catalytic subunits (CK2α or CK2α’) and two regulatory subunits (CK2β), which form a tetrameric structure through the dimerization of the two β subunits. The α- or α’-subunit shows catalytic activity, while the β-subunit modulates enzyme activity and substrate specificity through targeting the enzyme to its substrates (Ahmed et al. 2002; Litchfield 2003). In addition, several lines of evidences have indicated that the α- and β-subunits can exist individually in vivo to interact with other cellular proteins, implying that the CK2 subunits may have biological functions other than those assigned to the holoenzyme (Meggio and Pinna 2003).
CK2 levels in some neurodegenerative diseases, e.g., Alzheimer’s disease (AD), showed the disease-related alteration, in which the amount and activity of CK2 is decreased (Iimoto et al. 1990; Aksenova et al. 1991; Pigino et al. 2009). Previously, we have identified that in the brains of experimental scrapie-infected hamsters and mice, as well as in cerebella homogenates from one fCJD case and one FFI case, the amounts of CK2α and CK2β decreased, while that of CK2α’ or/and CK2α” increased at the terminal stages (Chen et al. 2008a, 2008b). Subsequently, remarkable molecular interaction between PrP and CK2α has been addressed, in which the interacting region within PrP for CK2α locates at its C-terminal segment (residues 91–231) (Chen et al. 2008a, 2008b). The changes of CK2 level and pattern in scrapie-infected animals and human TSE cases as well as the molecular interaction between PrP and CK2 highly indicate the linkage of CK2 and prion diseases. However, the biological significance of protein interaction between those two proteins remains unclear.
In the present study, we reconfirmed the molecular interaction of wild-type and other abnormal forms of human PrPs with CK2α expressed in a human cell line HEK293. Overexpression of CK2 subunits (CK2αand CK2β) in the cultured cells did not affect the expressions of PrP proteins, but almost completely reversed the abnormal PrP mutants induced reduction of cellular tubulin and destruction of cellular microtubule. Additionally, we illustrated that the presences of CK2α and PrP together induced weak but similar phosphorylation activity on the cellular agent p53 at the position of serine 6 as that of CK2α and CK2β.
Materials and Methods
Plasmid Construction
Human CK2α and CK2β specific cDNA sequences were obtained by reverse transcriptional PCR reaction (RT–PCR). Briefly, 1 μg SH-SY5Y RNA was mixed with 5 U avian myeloblastosis virus reverse transcriptase (Invitrogen), 10 U RNAsin, 20 mM deoxyribonucleotide triphosphate, and 20 pM oligo-dT in 20 μl volume at 37 °C for 30 min. The product (2 μl) was mixed with 5 U Taq polymerase, 20 mM deoxyribonucleotide triphosphate, and the specific primers for human CK2α (P1 = 5′-TAGGATCCATGAGCAGCTCAGAGGAGGT-3′, with a BamHI site underlined and P2 = 5′-CAAAGCTTCATTACTGCTGAGCGCCAGCGGC-3′, with a Hind III site underlined) or CK2β (P1 = 5′-TAGGATCCATGAGCAGCTCAGAGGAGGT-3′, with a BamHI site underlined and P2 = 5′-ACAAGCTTTCAGCGAATCGTCTTGACTGG-3′, with a Hind III site underlined). PCR reactions were conducted with following conditions: 94 °C for 30 s, 58 °C for 30 s, and 72 °C for 60 s, totally 25 cycles. The PCR products were individually inserted into T-vector (Promega) and subcloned into pcDNA3.1 after verified with sequencing assays, generating plasmid pcDNA3.1–CK2α and pcDNA3.1–CK2β.
The recombinant plasmids expressing human wild-type PrP (pcDNA3.1–PrP-PG5), mutated PrPs with nine- (pcDNA3.1–PrP-PG9) and 12- (pcDNA–PrP-PG12) octarepeats insertion, and cytosolic PrP (pcDNA3.1–CytoPrP) were generated previously (Wang et al. 2009; Wang et al. 2011a, 2011b). The expressions and distributions of various expressed PrPs in the cultured cells were also described elsewhere (Xu et al. 2011). The expressing plasmid for PrP-specific siRNA (pPrP-Ri3) and the plasmid containing the same compositions of nucleosides as Ri3 but randomly arrayed sequence (pPrP-Ri3null) were constructed previously (Wang et al. 2011a, 2011b).
Cell Culture and Transfection
Human embryonic kidney (HEK) 293 T cells without detectable endogenous PrP protein were maintained in Dulbecco’s modified Eagle’s medium (DMEM; Gibco BRL, USA) supplemented with 10 % (v/v) fetal cattle serum (FCS, USA). Cells at the logarithmic growth stage were plated into six-well plates (Falcon, USA) 24 h before transfection. Two micrograms of each plasmid DNA was transiently transfected per well with FugeneTM regent (Roche, Switzerland), according to the manufacturer’s instructions. Then, 24–48 h after transfection, cells were harvested and employed into further experiments.
Preparations of Cell Lysates
Cultured cells treated with different transfections or reagents were harvested and the whole cell lysates were prepared in cold lysis buffer (100 mM NaCl, 10 mM EDTA, 0.5 % Nonidet P-40, 0.5 % sodium deoxycholate, 10 mM Tris, pH 7.5) containing a mixture of protease inhibitors. After centrifugation at 10,000 × g at 4°C for 10 min, the supernatants were collected for further experiments.
Western Blots
The cellular lysates were separated by 12 % SDS–PAGE and electro-transferred onto nitrocellulose membranes. After blocking with 5 % non-fat dried milk in PBST (phosphate buffered saline, pH 7.6, containing 0.05 % Tween-20) for 1 h at room temperature, the membranes were incubated with 1:4,000 PrP specific monoclonal antibody (mAb) 3 F4 (Dako, Denmark), 1:2,000 diluted pAb anti-CK2α or -CK2β (Santa Cruz, USA), 1:4,000 diluted mAb anti-tubulin (Sigma, USA), 1:5,000 diluted mAb anti-p53 (R&D, USA), 1:2,000 diluted mAb anti-p53-Ser6 or anti-p53-Ser9 (CST, USA), 1:2,000 diluted mAb anti-human β-actin (Santa Cruz, USA) for 2 h at room temperature, and then incubated with 1:10,000 diluted horseradish peroxidase (HRP)-conjugated anti-mouse IgG (Santa Cruz, USA). The reactive signals were visualized by ECL kit (PE Applied Biosystems, Foster City, CA, USA).
Immunoprecipitation
Immunoprecipitation (IP) was carried out using whole cell lysates (about 400 μg total protein), 2–4 μg of antibody, and 20 μl of Dynabeads® coated Protein G (Invitrogen). Cell lysates were mixed with different antibodies at 4 °C for 3–4 h and incubated with Protein G Sepharose for another 2 h. The immunocomplexes were collected by short spin and washed five times in washing buffer before being resolved by SDS–PAGE. The complexes were detected by further Western blots.
Microtubule in the Cultured Cells
After transfected for 48 h, cells receiving different plasmids were fixed in 4 % paraformaldehyde for 30 min at room temperature and then permeabilized with 0.5 % Triton X-100 in PBS for 30 min. After blocking with 5 % fetal bovine serum, cells were incubated with anti-α-tubulin (1:1,000) antibody overnight at 4 °C. After washing with PBS, cells were stained with Alexa Fluor 488 conjugated anti-mouse IgG (1:200, Invitrogen, USA) for 1 h at room temperature. In parallel, the cells treated with 10 μM colchicines for 48 h were used as positive control. Fluorescently stained cells were analyzed with confocal laser scanning microscope (Leica ST2, Germany).
Results
Wild-Type PrP Formed Complexes with CK-2 Subunits in the Cultured HEK293T Cells
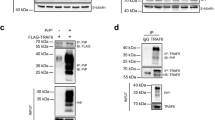
Our previous study identified the molecular interaction between the recombinant human PrP and CK2α subunit (Chen et al. 2008a, 2008b). To see the PrP–CK2 interaction in mammalian cells, human CK2α and/or CK2β subunits expressing plasmids co-transfected with wild-type human PrP expressing plasmid (PrP-PG5) into HEK293T cells, which was confirmed without detectable PrP and CK2 signals in Western blots. In the cells expressing CK2α and CK2β subunits, as well as expressing PrP, clear PrP-specific signal or CK2-specific signal was detected in the immunoprecipitation assays, after being captured with CK2 or PrP antibodies and blotted with opposite ones (Fig. 1a). To assess the binding activities of CK2α and CK2β subunits with PrP, cells were separately received with the plasmids expressing CK2α and PrP or CK2β and PrP. Immunoprecipitation assays, either using anti-PrP as the precipitating antibody and anti-CK2α as the blotting one or anti-CK2α as the precipitating antibody and anti-PrP as the blotting one, revealed clear PrP–CK2α complexes (Fig. 1b). Meanwhile, weak but repeated PrP–CK2β complexes were also observed in the cell lysates containing human PrP and CK2β (Fig. 1c). Those data indicate that PrP and CK2 subunits form the complex when transiently expressed in the cultured cells.
Molecular interactions between wild-type PrP (PG5) and CK2 subunits expressed in HEK293T cells. Cells receiving plasmids expressing human PrP, CK2α, and/or CK2β were harvested 48 h post-transfection. a PrP-PG5 with CK2α. The protein complexes in cell lysates were precipitated with PrP mAb 3 F4 (IP: 3 F4) and blotted with CK2α pAb (IB: CK2α) (the first panel from above), or precipitated with CK2α pAb (IP: CK2α) and blotted with PrP mAb 3 F4 (IB: 3 F4) (the second panel from above), respectively. b PrP-PG5 with CK2β. The protein complexes in cell lysates were precipitated with PrP mAb 3 F4 (IP: 3 F4) and blotted with CK2β pAb (IB: CK2β) (the first panel from above), or precipitated with CK2β pAb (IP: CK2β) and blotted with PrP mAb 3 F4 (IB: 3 F4) (the second panel from above), respectively. c PrP-PG5 with CK2α and CK2β. The protein complexes in cell lysates were precipitated with PrP mAb 3 F4 (IP: 3 F4) and blotted with CK2α pAb (IB: CK2α) (the first panel from above), or blotted with CK2β pAb (IB: CK2β) (the second panel from above). The expressed PrP, CK2α, or CK2β in the transfected cells were blotted directly with individual antibodies illustrated as inputs in the two (a, b) or three (c) panels from bottom, respectively
Octarepeat-Inserted PrP Mutants and Cytosolic PrP Formed Complexes with CK2 in the Cultured HEK293T Cells
To test the interaction between CK2 and some abnormal forms of PrP, two gCJD-associated PrP mutants (PrP-PG9 and PrP-PG12) and cytosolic PrP (CytoPrP) were transiently expressed in HEK293K cells, together with human CK2α and CK2β subunits, respectively. Immunofluorescent assays showed that unlike wild-type PrP-PG5 that mainly located on the cell surface, three abnormal forms of PrPs were largely distributed in cytoplasm, which were deposited as particles or granules. Immunoprecipitation assays with anti-PrP as the capturing antibody and anti-CK2α and CK2β as the blotting ones detected obvious CK2-specific signals in the tested cell lysates (Fig. 2). It suggests that octarepeat-inserted PrP mutants and cytosolic PrP interact with the expressed CK2 in the cultured cells.
Molecular interactions between mutated PrPs and CK2 subunits expressed in HEK293T cells. Cells co-transfected with the plasmids expressing various human PrP mutants, as well as human CK2α and CK2β, were harvested 48 h post-transfection. The protein complexes in cell lysates were precipitated with PrP mAb 3 F4 (IP: 3 F4) and blotted with CK2α pAb (IB: CK2α) (the first panel from above), or blotted with CK2β pAb (IB: CK2β) (the second panel from above). The expressed PrP, CK2α, or CK2β in the transfected cells were blotted directly with individual antibodies illustrated as inputs in the three panels from bottom, respectively. The preparations of various PrP mutants are shown on the left (CytoPrP), middle (PG9), and right (PG12)
Expressions of CK2 Subunits Rescued the Down-Regulated Effects of the PrP Mutants on the Levels of Cellular Tubulin
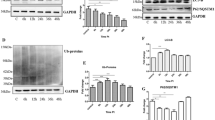
To see the potential effect of expression of CK2 on the abolishment of cellular tubulin due to the presences of abnormal PrPs, the tubulin levels in the cells expressing wild-type and three forms of the mutated PrPs were comparatively evaluated by Western blots under the conditions of mock-transfected with vector pcDNA3.1 and transfected with CK2α and CK2β expressing plasmids. In parallel, PrP siRNA transcribing plasmids pPrP-Ri3 and pPrP-Ri3null were subjected into the experiments as controls. Forty-eight hours post-transfection, cells were harvested. It showed that expressions of abnormal PrPs, including CytoPrP, PrP-PG9, and PrP-PG12, resulted in remarkable reductions of tubulin levels, while expression of PrP-PG5 did not change the tubulin level (Fig. 3a). Co-expression of PrP-specific siRNA Ri3 efficiently recovered the tubulin levels in the preparations of PrP mutants (Fig. 3b), whereas co-expression of Ri3null did not reverse the down-regulations of tubulin levels resulted from the expressions of different PrP mutants (Fig. 3c). Interestingly, the tubulin levels in the cells challenged with three abnormal PrPs were almost totally improved when CK2α and CK2β were co-expressed, making them comparable as that of the mock and PrP-PG5 (Fig. 3d).
Influences of the expressions of various PrP constructs on the levels of cellular tubulin. a Expressions of various PrP constructs alone. b Co-expressions of various PrP constructs with PrP siRNA Ri3. c Co-expressions of various PrP constructs with PrP siRNA Ri3null. d Co-expressions of various PrP constructs with CK2α and CK2β. Cells transfected with various plasmids were harvested 48 post-transfection and the tubulin levels were evaluated by tubulin-specific Western blots. Mock represents the cells receiving blank vector pcDNA3.1. The different plasmid compositions are indicated above the graphs. The specific immunoblots of tubulin and β-actin are indicated on the left
Expressions of CK2 Subunits Antagonized the Destructive Actions of the Abnormal PrPs on the Cellular Microtubule Structures
Furthermore, microtubule structures in various cell preparations were analyzed with tubulin-specific immunofluorescent staining. As shown in Fig. 4, expression of PrP-PG5 maintained the cellular microtubule structures similar as that of mock, with clear fibrillating networks in cytoplasm, whereas expressions of PrP-PG9, PrP-PG12, and CytoPrP caused severe disruption of microtubule, showing different sizes of vacuolation in cytoplasm. Co-expression of PrP siRNA Ri3 in the cells receiving abnormal PrPs markedly improved the cellular microtubule networks that were almost comparable with those of mock and PrP-PG5, but expression of Ri3null failed, highlighting that PrP-specific siRNA Ri3 can remove the disruption effects of the abnormal PrPs on cellular microtubule structures via the down-regulation of the expressing levels of the PrP mutants. Similar to the improvement of cellular tubulin levels observed above, the microtubule structures in the cells co-expressed with two CK2 subunits remained almost intact, implying that introduction of CK2 in the cells will successfully antagonize the abnormal PrPs-induced destructive actions on cellular microtubule structures.
Morphological analyses of the influences of the expressions of various PrP constructs on the structures of microtubule in HEK293T cells by a confocal microscopy. The structures of microtubules of the cells treated with various agents were monitored 48 h post-transfection. Mock represents the cells receiving blank vector pcDNA3.1. Colchicine is the cells treated with 10 μM colchicine for 6 h. Various preparations are indicated at the bottom of the graphs
Co-expression of CK2α and PrPs Induced Phosphorylation on p53 at the Position of Serine 6
CK2 is believed as a phosphorkinase for p53 at the position of serine 6 (p53-Ser6) (Finlan et al. 2006). To assess the potential function of transient expression of CK2 subunits, the plasmids expressing CK2α and CK2β were transfected into HEK293T cells together or separately. Western blots revealed p53-Ser6 signal in the preparation co-transfected with plasmids expressing CK2α and CK2β, but not in that of CK2α or CK2β alone (Fig. 5a), indicating that co-expression recombinant expressing CK2α and CK2β subunits in the cells works as phosphorkinase for p53 at the position of serine 6. Meanwhile, expressions of CK2α and CK2β, either together or separately, did not influence the cellular p53 level or induce phosphorylation on p53 at the position of serine 9 (Fig. 5a). To see the possible effects of expressing PrPs on the CK2 phosphorkinase activity, HEK293T cells were transfected with various PrP constructs, in the conditions of co-transfection with the plasmids expressing CK2α and CK2β together or co-transfection with the plasmid expressing CK2α or CK2β alone. No p53-Ser6 specific signal was observed in the lysates of the cells expressing PrP constructs or that expressing PrP constructs and CK2β subunit, whereas relatively weak but repeatedly identified p53-Ser6 bands were detected in the cells co-expressing PrP constructs and CK2α subunit together (Fig. 5b–e). In addition, the p53-Ser6 signals in the preparations co-expressing PrP and CK2α and CK2β subunits seemed to be relatively stronger than that expressing CK2α and CK2β (Fig. 5b–e). In line with the above observations, expressions of various PrPs in the cells, together with CK2 subunits or not, did not induce phosphorylation on p53 at serine 9 or affect the cellular p53 level. Those results highlight that PrP might act as a binding domain for CK2α to phosphorylate cellular p53 at the position of serine 6.
Influences of expressions of PrP constructs alone or together with CK2α and/or CK2β on the levels of p53-Ser6, p53-Ser9, and p53. Cells transfected with various plasmids were harvested 48 post-transfection and the signals of p53-Ser6, p53-Ser9, and p53 were analyzed by Western blots with individual antibodies. a Cell expression of CK2α, CK2β or CK2α and CK2β. b Cell co-expressions of wild-type PrP-PG5 with different CK2 subunits. c Cell co-expressions of CytoPrP with different CK2 subunits. d Cell co-expressions of PrP-PG9 with different CK2 subunits. e Cell co-expressions of PrP-PG12 with different CK2 subunits. The different plasmid compositions are indicated above the graphs. The specific immunoblots of p53-Ser6, p53-Ser9, p53, and β-actin are indicated on the left
Discussion
Using immunoprecipitation assays, we have confirmed the molecular interactions of wild-type and abnormal PrPs with CK2 subunits, especially CK2α, expressed in cultured cells. The specific binding between human PrP and CK2α has already been addressed based on the Escherichia coli expressed recombinant proteins; meanwhile, PrPC–CK2 and PrPSc–CK2 complexes have been repeatedly observed in the brain tissues of normal and scrapie-infected animals (Chen et al. 2008a, 2008b). Those results provide solid evidence that PrP forms complex with CK2, which may play roles either in the physiological activity of PrP protein or the pathogenesis of prion diseases. Like the observation in brain tissues, besides the PrP–CK2α, significantly weak PrP–CK2β complexes are seen in all cell preparations. These phenomena are distinct from what has been observed in the tests with recombinant proteins purified from E. coli, in which recombinant CK2β is not able to form complex with recombinant PrP (Chen et al. 2008a, 2008b). The difference between prokaryotic- and mammalian-derived recombinant CK2β proteins in interacting with PrP might be due to the difference in tetrameric structure of the proteins expressed in two systems. Nevertheless, it is implicit that the CK2 proteins expressed in mammalian cells may mimic their analog in brain tissues more precisely.
Normal PrPC seems to be as a protective factor for neuron (Sponne et al. 2004). Overexpression of mutated PrP proteins, such as octarepeats insertions and many CJD-associated point mutations (Xu et al. 2011) and orientation of PrP in cytoplasm, such as CytoPrP and ER-PrP (Wang et al. 2010; Wang et al. 2011a, 2011b; Shi and Dong 2011), even abolishment of glycosylation sites of PrP, such as aglycosyl PrP (Chen et al. 2007), will cause significant cytotoxicity. One of the obvious alterations in cellular sub-organelle is disruption of microtubule and/or reduction of tubulin level when overexpressed above PrP mutants. The exact mechanism seems to be complicated, minimally including down-regulations of a series of tubulin-associated proteins, e.g., TPPP (Zhou et al. 2011) and MAP2 (Guo et al. 2012), alterations of tau phosphorylating profiles, and changes of some kinases, e.g., CCK5 and GSK3β (Wang et al. 2010). Markedly decreased tubulin in the brains of scrapie experimental rodents at the late stage described previously (Li et al. 2011) and clearly reduced the cellular tubulin levels in the cultured cells overexpressed PrP mutants in this study highly indicate the possibility of direct effectiveness of PrPSc and PrP mutants. The interaction between PrP and tubulin (Dong et al. 2008; Li et al. 2009) supplies molecular basis for this direct effectiveness.
Our study has confirmed that the down-regulation of cellular tubulin levels and disruption of microtubule structures due to expressions of abnormal PrP proteins are effectively restored by co-expression of two CK2 subunits. These protective effects of CK2 are quite similar as that of a PrP-specific siRNA, which has been verified to be able to antagonize the cytotoxicity of some CJD-associated PrP mutants previously (Wang et al. 2011a, 2011b). However, unlike the knockdown of the expression levels of PrPs by PrP siRNA, expression of CK2 subunits does not influence the expression levels of PrPs in the cells. The CK2α binds directly to both microtubules and tubulin heterodimers, and CK2 holoenzyme exhibits a potent effect of inducing microtubule assembly and bundling in a phosphorylation-independent manner (Lim et al. 2004). CK2 activity is consistently enhanced in many human cancers as well as in experimental tumors (Seldin et al. 2005; Kim et al. 2007; Trembley et al. 2009) and down-regulation of CK2 leads to cell apoptosis, which is being used as a potential methodology for cancer therapy (Hamacher et al. 2007; Yde et al. 2007; Lee et al. 2011). Those data strongly indicate the direct effect of CK2 on microtubule stability. On the other hand, CK2 may contribute to the microtubule stability and cell protection via its activity of protein kinase, such as promoting the formation of kinetochore–microtubule attachments by phosphorylation of CLIP-170 (Li et al. 2010), regulating the formation of clearance of aggresomes in response to the stress of misfolded protein aggregates by phosphorylation of cytoplasmic deacetylase HDAC6 (Watabe and Nakaki 2011). Additionally, either octarepeat-inserted PrP or cytosolic PrP possesses active molecular interaction with both cellular tubulin and CK2 like the wild-type PrP (Dong et al. 2008; Chen et al. 2008a, 2008b). Possibly, interaction between mutated PrPs and CK2 will directly interfere in their interactions with cellular tubulin, therefore resulting in antagonizing their down-regulation of cellular tubulin levels and disruption of microtubule structure.
CK2 as a multifunctional protein kinase has been shown to impact cell growth and proliferation. More than 300 growth-related proteins are the substrates of CK2 (Kawaguchi et al. 2003). One of the well-characterized in vitro substrates for CK2 is the tumor suppressor protein, p53, whose accumulation and activation are believed to be regulated through protein phosphorylations and/or acetylations (Appella and Anderson 2001). At least 20 sites in human p53, located at the N-terminal transactivation domains, the C-terminal regulatory domain proximal or distal to the tetramerization domain, are modified in response to the activation of different stress signaling pathways (Saito et al. 2003). The phosphorylation p53 at Ser392 by CK2, which is described as the solo site modified by CK2, has been widely documented in literatures, either in vitro studies or in malignant carcinomas (Cox and Meek 2010; Meek and Cox 2011; Ruzzene et al. 2011). Using specific antibodies, we have observed that expression of CK2 subunits in the cells induces evidential phosphorylated p53-Ser6, but does not either alter the cellular p53 levels or induce phosphorylated p53 at Ser9. In the responses to genotoxic stress, such as ionizing radiation (IR) and ultraviolet (UV), or non-genotoxic stress, such as ALLN that is an inhibitor of ubiquitin-mediated degradation by the 26 S proteasome, taxol, and nocodazole that disrupt microtubules, and N-phosphonacetyl-l-aspartate that causes depletion of ribonucleotides without detectable DNA damage, some sites of p53 are phosphorylated following activations of several kinase-mediated signaling pathways (Saito et al. 2003), in which Ser9 is specially related with kinase CK1 and Ser6 is related with CK2 and CK1 (Knippschild et al. 1997). Interestingly, in our experimental condition, co-presences of CK2α and PrP in HEK293T cells cause relatively weak but clear phosphorylation of p53-Ser6, while presence of either CK2α or PrP alone fails to induce such phenomenon. It might be hypothesized that PrP substitutes CK2 subunit in some extents for its binding activity to target substrates. Further analysis of the interaction between PrP and p53 will disclose the mechanism of the phosphorylating activity of CK2α–PrP complex on p53-Ser6.
References
Ahmed K, Gerber DA, Cochet C (2002) Joining the cell survival squad: an emerging role for protein kinase CK2. Trends Cell Biol 12:226–230
Aksenova MV, Burbaeva GS, Kandror KV, Kapkov DV, Stepanov AS (1991) The decreased level of casein kinase 2 in brain cortex of schizophrenic and Alzheimer’s disease patients. FEBS Lett 279:55–57
Appella E, Anderson CW (2001) Eur J Biochem 268:2764–2772
Blanquet PR (1998) Neurotrophin-induced activation of casein kinase 2 in rat hippocampal slices. Neuroscience 86:739–749
Chen JM, Gao G, Shi Q et al (2008a) Different expression levels of CK2 subunits in the brains of experimental animals and patients with transmissible spongiform encephalopathies. Arch Virol 153:1013–1020
Chen JM, Gao C, Shi Q et al (2008b) CK2 can interact with PrP in vitro and forms complex with native PrP. Acta Biochim Biophys Sin 40:1039–1047
Chen L, Yang Y, Han J et al (2007) Removal of the glycosylation of prion protein provoke apoptosis in SF126. J Biochem Mol Biol 30:662–629
Cox ML, Meek DW (2010) Phosphorylation of serine 392 in p53 is a common and integral event during p53 induction by diverse stimuli. Cell Signal 22:564–571
Dong CF, Shi S, Wang XF et al (2008) The N-terminus of PrP is responsible for interacting with tubulin and fCJD related PrP mutants possess stronger inhibitive effect on microtubule assembly in vitro. Arch Biochem Biophys 480:83–92
Finlan LE, Nenutil R, Ibbotson SH, Vojtesek B, Hupp TR (2006) CK2-site phosphorylation of p53 is induced in DeltaNp63 expressing basal stem cells in UVB irradiated human skin. Cell Cycle 5:2489–2494
Guerra B, Boldyreff B, Sarno S, Cesaro L, Issinger OG, Pinna LA (1999) CK2: a protein kinase in need of control. Pharmacol Ther 82:303–313
Guo Y, Gong HS, Zhang J et al (2012) Remarkable reduction of MAP2 in the brains of scrapie-infected rodents and human prion disease possibly correlated with the increase of calpain. PLoS One 7:e30163
Hamacher R, Saur D, Fritsch R, Reichert M, Schmid RM, Schneider G (2007) Casein kinase II inhibition induces apoptosis in pancreatic cancer cells. Oncol Rep 18:695–701
Iimoto DS, Masliah E, DeTeresa R, Terry RD, Saitoh T (1990) Aberrant casein kinase II in Alzheimer’s disease. Brain Res 507:273–280
Johnson RT (2005) Prion diseases. Lancet Neurol 4:635–642
Kawaguchi Y, Kovacs JJ, McLaurin A, Vance JM, Ito A, Yao TP (2003) The deacetylase HDAC6 regulates aggresome formation and cell viability in response to misfolded protein stress. Cell 115:727–738
Kim JS, Eom JI, Cheong JW et al (2007) Protein kinase CK2alpha as an unfavorable prognostic marker and novel therapeutic target in acute myeloid leukemia. Clin Cancer Res 13:1019–1028
Knippschild U, Milne DM, Campbell LE et al (1997) p53 is phosphorylated in vitro and in vivo by the delta and epsilon isoforms of casein kinase 1 and enhances the level of casein kinase 1 delta in response to topoisomerase-directed drugs. Oncogene 15:1727–1736
Lee SW, Song YS, Lee SY et al (2011) Downregulation of protein kinase CK2 activity facilitates tumor necrosis factor-α-mediated chondrocyte death through apoptosis and autophagy. PLoS One 6:e19163
Li H, Liu XS, Yang X et al (2010) Phosphorylation of CLIP-170 by Plk1 and CK2 promotes timely formation of kinetochore–microtubule attachments. EMBO J 29:2953–2965
Li XL, Dong CF, Shi S et al (2009) The octarepeats of hamster PrP (PrP51-91) enhance the formation of microtubule and antagonize Cu2+-induced microtubule-disrupting activity. Acta Biochim Biophys Sin 41:929–937
Li XL, Wang GR, Jing YY et al (2011) Cytosolic PrP induces apoptosis of cell by disrupting microtubule assembly. J Mol Neuroscience 43:316–325
Lim AC, Tiu SY, Li Q, Qi RZ (2004) Direct regulation of microtubule dynamics by protein kinase CK2. J Biol Chem 279:4433–4439
Litchfield DW (2003) Protein kinase CK2: structure, regulation and role in cellular decisions of life and death. Biochem J 369:1–15
Meek DW, Cox M (2011) Induction and activation of the p53 pathway: a role for the protein kinase CK2? Mol Cell Biochem 356:133–138
Meggio F, Pinna LA (2003) One-thousand-and-one substrates of protein kinase CK2? FASEB J 17:349–368
Pigino G, Morfini G, Atagi Y et al (2009) Disruption of fast axonal transport is a pathogenic mechanism for intraneuronal amyloid beta. Proc Natl Acad Sci USA 106:5907–5912
Prusiner SB (1998) Prions. Proc Natl Acad Sci USA 95:13363–13383
Ruzzene M, Tosoni K, Zanin S, Cesaro L, Pinna LA (2011) Protein kinase CK2 accumulation in “oncophilic” cells: causes and effects. Mol Cell Biochem 356:5–10
Saito S, Yamaguchi H, Higashimoto Y et al (2003) Phosphorylation site interdependence of human p53 post-translational modifications in response to stress. J Biol Chem 278:37536–37544
Seldin DC, Landesman-Bollag E, Farago M, Currier N, Lou D, Dominguez I (2005) CK2 as a positive regulator of Wnt signaling and tumourigenesis. Mol Cell Biochem 274:63–67
Shi Q, Dong XP (2011) CtmPrP and ER stress: a neurotoxic mechanism of some special PrP mutants. Prion 5:123–125
Sponne I, Fifre A, Koziel V, Kriem B, Oster T, Pillot T (2004) Humanin rescues cortical neurons from prion-peptide-induced apoptosis. Mol Cell Neurosci 25:95–102
Trembley JH, Wang G, Unger G, Slaton J, Ahmed K (2009) Protein kinase CK2 in health and disease: CK2: a key player in cancer biology. Cell Mol Life Sci 66:1858–1867
Wang GR, Shi S, Gao C et al (2010) Changes of tau profiles in brains of the hamsters infected with scrapie strains 263 K or 139A possibly associated with the alteration of phosphate kinases. BMC Infect Dis 10:86–95
Wang X, Dong CF, Shi Q et al (2009) Cytosolic prion protein induces apoptosis in human neuronal cell SH-SY5Y via mitochondrial disruption pathway. BMB Rep 42:444–449
Wang X, Shi Q, Xu K et al (2011a) Familial CJD associated PrP mutants within transmembrane region induced Ctm-PrP retention in ER and triggered apoptosis by ER stress in SH-SY5Y cells. PLoS One 6:e14602
Wang ZY, Tian C, Jing YY et al (2011b) Knocking-down of the levels of prion protein by RNA interference (RNAi) weakened the protective activity of wild-type PrP against Cu2+ and antagonized the cytotoxicities of fCJD-associated PrP mutants in the cultured cells. Int J Mol Med 28:413–421
Watabe M, Nakaki T (2011) Protein kinase CK2 regulates the formation and clearance of aggresomes in response to stress. J Cell Sci 124:1519–1532
Xu K, Wang X, Shi Q et al (2011) Human prion protein mutants with deleted and inserted octarepeats undergo in different pathways to trigger cell apoptosis. J Mol Neuroscience 43:225–234
Yde CW, Frogne T, Lykkesfeldt AE, Fichtner I, Issinger OG, Stenvang J (2007) Induction of cell death in antiestrogen resistant human breast cancer cells by the protein kinase CK2 inhibitor DMAT. Cancer Lett 256:229–237
Zhou RM, Jing YY, Guo Y et al (2011) Molecular interaction of TPPP with PrP antagonized the CytoPrP-induced disruption of microtubule structures and cytotoxicity. PLoS One 6:e23079
Acknowledgments
This work was supported by Chinese National Natural Science Foundation Grants (81100980, 81101302, and 31100117), National Basic Research Program of China (973 Program) (2007CB310505), China Mega-Project for Infectious Disease (2009ZX10004-101), and SKLID Development Grant (2008SKLID102, 2011SKLID211).
Author information
Authors and Affiliations
Corresponding author
Additional information
Zhao-Yun Wang and Qi Shi contributed equally to this paper.
Rights and permissions
About this article
Cite this article
Wang, ZY., Shi, Q., Wang, SB. et al. Co-expressions of Casein Kinase 2 (CK2) Subunits Restore the Down-Regulation of Tubulin Levels and Disruption of Microtubule Structures Caused by PrP Mutants. J Mol Neurosci 50, 14–22 (2013). https://doi.org/10.1007/s12031-012-9845-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-012-9845-y