Abstract
To address the possible alteration of casein kinase 2 (CK2) in transmissible spongiform encephalopathies (TSEs), the levels and patterns of CK2 in the brain tissues of hamsters or C57BL mice inoculated intracerebrally with scrapie agents 263K or 139A were evaluated by Western blots, followed by quantitative analysis. Specific semi-quantitative RT-PCR for evaluating the mRNA transcripts of CK2 subunits was performed in parallel. Compared with normal animals, the levels of CK2α and CK2β in the brains of infected hamsters and mice were significantly decreased, regardless of which scrapie agent was. However, the expression of CK2α′ or CK2α′/CK2α′′ in the animals infected with agents 263K or 139A was considerably increased. Furthermore, decreases of CK2α and CK2β and increases of CK2α′/CK2α′′ were observed in cerebella homogenates from one familial Creutzfeldt-Jakob disease (fCJD) case and one fatal familial insomnia (FFI) case. These results suggest that alterations of CK2 subunits in brains are illness-correlative phenomena in TSEs and indicate a potential linkage of CK2 changes with the pathogenesis of prion diseases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Prion diseases, or TSEs, are fatal neurodegenerative disorders of humans and animals [16, 25]. All forms of prion diseases are characterized by common histopathological features, such as spongiform degeneration, reactive gliosis, neuronal loss and formation of amyloid plaques in the central nervous system (CNS) [9, 16]. The conversion of normal prion protein (PrPC) to a protease-resistant isoform, PrPSc, is a key event in the pathogenesis of all transmissible prion diseases. This transition appears to involve only a conformational change and renders PrPSc partially resistant to proteolytic degradation. The biological role(s) of PrPC still remains unclear, although its conservation among species strongly suggests a relevance in physiological processes [8, 27, 32].
CK2 is a highly conserved serine/threonine protein kinase which is ubiquitous in eukaryotic organisms. CK2 from most sources consists of two catalytic subunits (α and/or α′) complexed with two β subunits, existing as an α2β2, αα′β2, or α′2β2 heterotetramer [24]. Meanwhile, CK2 is an extremely conserved pleiotropic protein kinase with a growing list of more than 300 substrates, the majority of which are proteins implicated in signal transduction, gene transcription, proliferation, apoptosis and various steps of development [21].
CK2 has some possible neurophysiological functions in normal tissues [4, 5, 7, 22]. It enables neural cells to control their developmental programs, adapt to environmental changes by modulating the strength of synaptic connections, and participate in key decision-making events that lead to survival or death. A reduced CK2 activity and a decreased amount of total CK2 are observed in Alzheimer’s disease [2, 7, 15]. Furthermore, it has been found that CK2 is associated with neurofibrillary tangles and the hyperphosphorylation of the tau protein [3]. Additional information shows that CK2 is present in tangles and neuronal inclusions in other neurodegenerative diseases, including Parkinson dementia complex, chromosome 18 deletion syndrome, progressive supranuclear palsy, Kuf′s disease and Pick’s disease [4]. These data indicate that changes of CK2 activity and content might be a common phenomenon in neurodegenerative diseases.
In the present study, the relative quantity of CK2, in the context of total CK2 and its subunits, in the brain tissues of hamsters infected with scrapie agent 263K or 139A as well as mice infected with agent 139A were evaluated. We found that the amount of CK2α and CK2β decreased, while that of CK2α′ and/or CK2α′′ increased, in the terminal stage of experimental scrapie. Furthermore, decreases of CK2α and CK2β and increases of CK2α′/CK2α′′ were observed in cerebella homogenates from one familial CJD (fCJD) case and one fatal familial insomnia (FFI) case. The changes of CK2 level and pattern in scrapie-infected animals and naturally occurring human TSE cases may indicate a potential linkage between CK2 and prion diseases.
Materials and methods
Animal brain samples infected with scrapie agents
Seven Syrian golden hamsters inoculated intracerebrally with hamster-adapted scrapie strain 263K, five C57BL mice inoculated intracerebrally with mouse-adapted scrapie strain 139A, and four hamsters inoculated intracerebrally with agent 139A were included in this study. Previous studies confirmed that the incubation time of 263K-infected hamsters was 79.1 ± 8.6 days [13], while that of 139A-infected mice was 153 ± 4 days [18] and 139A-infected hamsters was 395 ± 8.5 days [35]. The brains were removed surgically at the moribund stage and immediately dissected. They were then frozen and stored at −80°C until use. For healthy controls, brains of 80-day-old hamsters and 150-day-old C57BL mice were collected.
Human brain samples
A whole-brain protein sample from normal adult donors was purchased from Sigma (St. Louis, MO, USA). Human brains with TSE were collected from a patient who was definitely diagnosed with fCJD, with the PRNP gene containing seven extra octarepeats [31], and from a patient definitely diagnosed with FFI, with the PRNP gene containing a D178 N mutation while homozygous for methionine at codon 129 (Han et al. in preparation). Brain samples of two clinical patients were taken with informed consent. The protocol of the study adhered to the tenets of the Declaration of Helsinki and was approved by the local ethics committee.
The fCJD patient was a 48-year-old Chinese female with a 4-year-long clinical course. The main clinical manifestations were progressive dementia and ataxia. Neuropathological analysis revealed typical spongiform degeneration, and Western blots identified proteniase K (PK)-resistant PrP signals in brain tissues [31]. The FFI case was a 48-year-old Chinese male with a roughly ten-month-long clinical course. The main clinical manifestations were progressive and untreatable insomnia, dysautonomia, disruption of circadian rhythms, motor dysfunction and progressive sympathetic overactivity. Spongiform degeneration was observed in the region of the thalamencephalon, but a PK-resistant PrP signal was not observed by Western blot analysis.
Preparation of brain tissue samples
Brain samples from scrapie-infected and healthy hamsters or mice and fCJD or FFI patients were washed three times with TBS (10 mM Tris HCl, 133 mM NaCl, pH 7.4), and 10% (w/v) brain homogenates were prepared in lysis buffer [33] containing a mixture of protease inhibitors (Sigma, St. Louis, MO, USA). The tissue debris was removed by low-speed centrifugation at 2,000g for 10 min, and the supernatants were used for further experiments.
Protease resistance assay
For detection of PrPSc in brain tissues, all tested brain homogenates were digested with a final concentration of 50 μg/ml proteinase K (PK) at 37°C for 60 min prior to Western blots, and digestion was terminated by addition of PMSF to a final concentration of 5 mM.
Western blot analysis
Samples were separated by 15% SDS-PAGE and electroblotted onto a nitrocellulose membrane using a semi-dry blotting system. Membranes were blocked with 5% (w/v) non-fat milk powder (NFMP) in 1× Tris-buffered saline containing 0.1% Tween 20 (NFMP–TBST) at room temperature for 1 h. Blots were incubated overnight at 4°C in primary antibody solutions, i.e. 1:4,000-diluted polyclonal antibodies against CK2α, CK2α′ and CK2α′′ (Santa Cruz, CA, USA), monoclonal antibody (mAb) against CK2β (Santa Cruz, CA, USA), mAb 3F4 against PrP from hamster and human (Dako, Ely, UK), mAb 1E4 against PrP from mouse (Cell Sciences, Inc., Canton, MA) and mAb against β-actin (Santa Cruz, CA, USA). After washing with TBST, membranes were incubated in secondary antibody solution, i.e. 1:5,000-diluted HRP-conjugated goat anti-rabbit IgG or goat anti-mouse IgG (Santa Cruz, CA, USA) at room temperature for 2 h. Immunoreactive bands were visualized by the ECL method (Amersham Life Sciences, Buckinghamshire, UK).
Quantitative and statistical analysis
Quantitative analysis of immunoblot images was carried out using Image Total Tech software (Pharmacia). Briefly, the image of immunoblot was scanned with Typhoon (Pharmacia) and digitalized, saved in TIF format. The values of each target blot were evaluated. All data are presented as the mean ± SD. Student’s unpaired t test for comparison of means was used to compare groups. Differences were considered significant at a P value < 0.05.
RNA isolation and semi-quantitative RT-PCR
To measure transcription of CK2-subunits in brain samples from hamsters and mice, CK2 specific semi-quantitative RT-PCR was performed. Using TRIzol reagent, total cellular RNA was isolated from brain tissues according to the manufacturer’s instructions. Reverse transcription was performed using SuperScript™ III First-Strand Synthesis System (Invitrogen). Briefly, 2 μg of total RNA was mixed with 200 U of MMLV reverse transcriptase and 50 pM oligo (dT20) in a volume of 20 μl. The mixtures were maintained at 50°C for 50 min and inactivated by heating at 85°C for 5 min. To remove the RNA from the cDNA, 1 μl E. coli RNase H was added to the mixture and incubated at 37°C for 20 min. Aliquots (2 μl) of RT reaction products were amplified by PCR in a volume of 50 μl under the following conditions: 94°C for 30 s, 51°C for 30 s and 72°C for 30 s. The primers for CK2α, CK2α′, CK2α′′ and CK2β were synthesized based on the CK2 cDNA sequences in Genbank (CK2α: L15618; CK2α′: BC057862; CK2α′′: DQ354583 and CK2β: BC078807), including CK2α-sense (5-GGGAAATCAAGATGA-3, nt.99–113) and CK2α-anti-sense (5-ATTGCTTGAAGTCTGT-3, nt.355–370), CK2α′-sense (5-GAGAACCTTCGTGGTG-3, nt.259–274) and CK2α′-anti-sense: (5-GATTCCCTTGCTGTG-3, nt.445–459), CK2α′′-sense (5- AGGCAATGGAGCA-3, nt.950–962) and CK2α′′-anti-sense (5-GCAACACGGCAAA-3, nt.1070–1082), CK2β-sense (5-GGCTCCGTGGTAATG-3, nt.44–55) and CK2β-anti-sense (5-GGGATGTCCGAAAGG-3, nt.369–383), respectively. For the internal control, β-actin-specific RT-PCR was performed using the same amplifying conditions as for CK2α with the primers β-actin-sense (5-TGCTGTCCCTGTATGCCTCT-3) and β-actin-anti-sense (5-CTCGTTGCCAATGGTGAT-3). The PCR products were analyzed by 2% agarose gel electrophoresis, and positive CK2-related bands were further confirmed by sequence analysis.
Results
Brain tissues from seven hamsters infected with agent 263K, five mice infected with agent 139A, and five hamsters infected with 139A were used in this study. Western blots with PrP-specific monoclonal antibody 3F4 for hamsters and 1E4 for mice identified PK-resistant PrPSc in all tested infected brains (Fig. 1). In line with the previous study [12], the intensity of the total PrP signal without PK treatment in animals infected with agent 263K was much higher than that in the normal control (Fig. 2a). The raised level of PrP in the late stage of scrapie 263K-infected hamsters was further verified by Western blots with other commercial PrP monoclonal antibodies, including 1E4, 6G3 and 6H4 (data not shown). To evaluate the protein status, β-actin in the brain tissues was measured by Western blot using equal amounts of homogenate. No significant difference was identified between the stored infected samples and relatively freshly prepared normal samples (Figs. 2a, 3a, 4a), indicating no significant degradation in the stored samples.
Detection of PrPSc in brain homogenates of scrapie-infected experimental animals. Brain homogenates of infected animals were digested with a final concentration of 50 μg/ml PK before loading to SDS-PAGE. For Western blots, PrP mAb 3F4 was used for hamster samples, while mAb 1E4 was used for mouse specimens. The scrapie agents and experimental animals are indicated at the top of each picture. PK treatments is indicated at the bottom, + with PK, − without PK. Molecular mass markers are indicated at the left
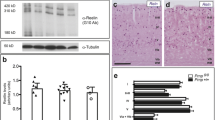
Western blot analyses of PrP, CK2α, CK2β and β-actin in the brain tissues of normal hamsters and those infected with scrapie agent 263K. a Western blots. Lanes 1–4 normal controls, lanes 5–8 infected hamsters. Identical amounts of individual brain homogenate were separated by 15% SDS-PAGE. Various specific bands are indicated by arrows on the right. b Quantitative analysis of CK2α or CK2β band density versus that of β-actin. The average values were calculated from seven individual animals and are presented as mean ± SD. Statistical differences compared with controls are illustrated as P < 0.01 (double asterisks) and P < 0.001 (triple asterisks)
Western blot analysis of CK2α, CK2β, and β-actin in the brain tissues of normal mice and those infected with scrapie agent 139A C57BL. a Western blots. Lanes 1–5 normal controls, lanes 6–10 infected mice. Identical amounts of individual brain homogenate were separated by 15% SDS-PAGE. Various specific bands are indicated by arrows on the right. b Quantitative analysis of CK2α or CK2β band density versus that of β-actin. The average values were calculated from five individual mice and are presented as mean ± SD. Statistical differences compared with controls are illustrated as P < 0.001 (triple asterisks)
Western blot analysis of CK2α, CK2β and β-actin in the brain tissues of normal hamsters and those infected with scrapie agent 139A. a Western blots. Lanes 1–3 normal controls, lanes 4–6 infected hamsters. Identical amounts of individual brain homogenate were separated by 15% SDS-PAGE. Various specific bands are indicated by arrows on the right. b Quantitative analysis of CK2α or CK2β band density versus that of β-actin. The average values were calculated from four individual hamsters and are presented as mean ± SD. Statistical differences compared with controls are illustrated as P < 0.05 (asterisk) and P < 0.01 (double asterisks)
CK2 alteration in the brain tissues of hamsters infected with scrapie agent 263K
To assess possible changes of CK2 in TSEs, total amounts of CK2α and CK2β in scrapie 263K-infected and healthy hamsters were evaluated by Western blot. A roughly 44-kDa CK2α-specific signal was detected in all tested animals, but the signal intensities observed with samples from infected hamsters were obviously lower than those from normal ones (Fig. 2a). To obtain more detailed data, the gray value of the CK2α signal in each sample was collected after scanning the image, and the relative quantity was calibrated against the gray value of the β-actin band. The results showed that the mean relative quantity of CK2α in the group of infected hamsters was markedly lower than that of the control group (P < 0.001, Fig. 2b). Interestingly, another CK2α-specific band at the position of Mr 38,000 was repeatedly observed in the preparations from infected hamsters, while this signal was almost undetectable in the healthy animals (Fig. 2a). It could be the CK2α′, which represents another form of CK2α, considering the electrophoretic position of this CK2α-specific band. Quantitation of the relative band intensities revealed that the CK2α′ in the scrapie-infected hamsters was significantly higher than in the normal control (P < 0.001, Fig. 2b).
Using an anti-CK2β mAb, specific reactive bands, migrating at the position of Mr 28,000, were detected in the brain homogenates by Western blots (Fig. 2a). Like the α-subunit, the amount of CK2β in the group of infected hamsters was remarkably decreased. The relative amount of CK2β in infected animals was reduced to only 46.5% of that in healthy animals, and this was statistic significant (P < 0.01, Fig. 2b).
CK2 alteration in the brain tissues of C57BL mice infected with scrapie agent 139A
In order to elucidate whether the above results are a common feature in TSEs or just a special case in scrapie 263K-inoculated hamsters, the presence of CK2 subunits in brain tissues of mice challenged with scrapie strain 139A were analyzed. Consistent with the observations in the scrapie 263K experimental hamsters, the signals of CK2α and CK2β weakened, while CK2α′ was enhanced, in the C57BL mice inoculated with scrapie agent 139A (Fig. 3a). The relative intensities of the CK2 signals revealed that the amounts of both CK2α (P < 0.001) and CK2β (P < 0.001) decreased prominently in the group of infected mice (Fig. 3b). Additionally, another CK2α-specific band that migrated at the position of Mr 41,000 was repeatedly identified in the brain tissues of infected mice, and this may represent CK2α′′ (Fig. 3a). In contrast, the signals of CK2α′ and CK2α′′ isoforms were almost not detected in the normal C57BL mice (Fig. 3a).
CK2 alteration in the brain tissues of hamsters infected with scrapie agent 139A
To address whether the different patterns of CK2α in the scrapie-infected animals were species-related or scrapie-strain-related phenomenon, five hamster brains infected with agent 139A were subjected to CK2-specific Western blots. Figure 4 showed that the signals of the CK2α isoform and CK2β isoform weakened markedly in the preparations from infected hamsters, revealing a statistically significant difference (P < 0.001) compared with the normal control. Similar to the pattern in 263K-infected hamsters, only two CK2α reactive bands were visible in the preparations of 139A-infected hamsters, which represented CK2α and CK2α′ (Fig. 4). These results imply that the different patterns of CK2α subunits appearing in hamsters and mice are species-related.
Changes of mRNA transcripts of CK2 subunits in rodent brains infected with scrapie agents
To confirm the data from the Western blots, semi-quantitative RT-PCR analysis was performed for evaluating mRNA transcripts of CK2 subunits in the rodent brains infected with scrapie agents 139A or 263K. In addition, the transcription of the housekeeping gene encoding β-actin was also evaluated as an internal control. Analysis of five 263K-infected and five 139A-infected hamsters revealed that the levels of CK2α- and CK2β-subunit-specific mRNA in the brain samples were considerably lower than that of the normal ones, while the amounts of CK2α′ mRNA were significantly higher than that of the control (Fig. 5a, b). In line with the observations with scrapie-infected hamsters, the semi-quantitative RT-PCR analysis of CK2 subunits mRNA showed that the levels of CK2α- and CK2β-specific mRNAs were lower, while that of CK2α′ was higher in the brains of five scrapie-139A-inoculated C57BL mice, compared with the normal controls (Fig. 5c). Furthermore, the transcripts of CK2α′′ in the brain tissues of 263K-infected and agent-139A-infected rodents were evaluated. As expected, the mRNA levels of CK2α′′ in the brain tissues of 139A-infected mice were higher than those of the normal mice (Fig. 5c). However, the CK2α′′ transcripts in brains from both normal and scrapie-infected hamsters were almost undetectable (Fig. 5a, b). To confirm the absence of CK2α′′ in the hamster brains, another pair of CK2α′′-specific primers was synthesized and employed in PCR. It failed to amplify positive bands or gave only disperse signals from hamster brains, but yielded clear amplified bands from mouse brains (data not shown). All amplified bands with different CK2 primers were verified by sequence analysis to be the individual CK2 subunit sequences.
Analysis of the mRNA transcripts of various CK2 subunits by RT-PCR. Two μg of total RNA extracted from each brain sample was employed in RT-PCR, and the PCR products were analyzed by 2% agarose electrophoresis. a Scrapie-263K-adapted hamsters. b Scrapie-139A-adapted hamsters. c Scrapie-139A-adapted mice. Samples of normal and infected animals are indicated at the top. M represents the DNA marker DL2000 (2,000, 1,000, 750, 500, 250 and 100 bp). RT-PCR of each CK2 subunit and β-actin are indicated at the left
CK2 alteration in brain tissues of human TSEs
To see possible changes of CK2 subunits in human TSE cases, brain homogenates were prepared from cerebellar tissues of one fCJD and one FFI case. Immunoblot results showed that the signals of CK2α and CK2β in both fCJD and FFI brains were obviously weaker than that of normal control (Fig. 6). Consistent with the observations in the 139A-infected mice, two CK2α-specific signals were visualized in the preparations of human fCJD and FFI, but not in that of normal human brains, which may represent CK2α′ and CK2α′′ (Fig. 6). This indicates that human TSE cases show similar alterations of CK2α subunits as scrapie experimental rodents.
Alteration of the patterns of CK2α and CK2β in human brains with FFI and fCJD. A homogenate from whole brains of normal human adult donors (Sigma) and homogenates of cerebellar tissues from individuals with FFI and a fCJD were separated by 15% SDS-PAGE, and CK2α- and CK2β-specific signals were visualized in Western blots with CK2α- or CK2β-specific antibodies. The electrophoretic positions of CK2α, CK2α′, CK2α′′ and CK2β are indicated by arrows
Discussion
In this study, we found that the levels of CK2α and CK2β in the brain tissues of scrapie-infected hamsters and mice were significantly decreased at their terminal stages, while the amounts of CK2α′ in 263K- or 139A-infected hamster brains and CK2α′/CK2α′′ in 139A-infected mouse brains notably increased. The same feature was also observed in human fCJD and FFI patients. Meanwhile, the levels of the specific mRNAs of CK2α and CK2β were obviously decreased, whereas that of CK2α′ and/or CK2α′′ increased in brains of scrapie experimental rodents. As a ubiquitously expressed kinase, CK2 is widely distributed in the CNS, e.g. in large striatal neurons, Purkinje cells and some scattered cells in the subiculum of the hippocampus [11, 22]. In prion diseases, loss of neuron cells is common feature, particularly in the late stage of their clinical course [19]. The strong association between the reduction of CK2 and the loss of neurons suggests that the observation of a low level of CK2 in brain tissues may largely result from the destruction of neurons during spongiform degeneration and deposits of PrPSc in the CNS.
It has been widely considered that CK2 activity is consistently enhanced in many human cancers as well as in experimental tumors [17, 28]. Overexpression of CK2α has been observed in numerous benign proliferative disorders, e.g. the proliferative glomerular lesions in rat glomerulonephritis (GN) models and renal biopsy specimens from lupus nephritis or IgA nephropathy patients. Down-regulation of CK2 leads to cell apoptosis, which is being used as a potential methodology for cancer therapy [14, 30, 34]. In contrast, the level and activity of CK2 in the degenerative illnesses have usually declined [4]. Male mice lacking CK2α display a series of abnormalities in their spermatids, probably resulting from a particular death pathway similar to apoptosis [10]. Apoptosis in brains has been repeatedly described in various TSEs, while PrPC is believed to have anti-apoptotic activity for neuron cells [6, 26]. Previous studies have illustrated that recombinant bovine PrP protein forms a complex with CK2α, resulting in up-regulation of CK2 catalytic activity [20, 23]. Our unpublished data also demonstrate that recombinant human PrP is able to interact with CK2α in vitro (Chen et al. in preparation). These results highlight the possibility that the anti-apoptosis activity of PrPC may be mediated through an interaction with CK2. Although the molecular and cellular mechanism of PrPSc-induced neuropathogenesis is not yet fully understood, increasing evidence supports the view that PrPSc accumulation interferes with normal function(s) of PrPC in neurons. A loss of normal PrPC function and a decrease in CK2 content or activity may also contribute to neuron death in prion diseases.
The CK2α subunit mainly consists of two isoforms, CK2α and CK2α′. Recently, a novel isoform of CK2α, named CK2α′′, was found in the human hepatoma cell line HuH-7 [29]. These three catalytic isoforms of CK2 are the products of distinct genes localized to different chromosomes [1]. The expression and localization of CK2 catalytic subunits occur along with neuronal development, in which the CK2α subunit appears early during rat brain development, whereas CK2α′ appears within mature rat neurons at the time of dendritic maturation and synaptogenesis [4]. Consistent with the various roles proposed for CK2, the distribution of this protein kinase in the nucleus, cytosol and membranes appears to be dependent on the tissue type and the state of differentiation or stage of the cell cycle [4]. Although there is little documentation describing the proportion of various CK2α subunits (CK2α, CK2α′ and CK2α′′) in various kinds of tissues, the data of this study indicate that the CK2α isomer is the predominant one in the brain tissues of healthy adult rodents, while CK2α′ and CK2α′′ seem to be disease-associated. The down- and up-regulation of certain subunits, found to be similar in all of the situations examined (i.e. 4 prion strains) may indicate changes in composition of the CK2 hetero-tetramers during the pathogenesis of TSEs. Additionally, the two smaller CK2α-specific bands present in infected brain samples are probably not be the breakdown products of CK2α by proteolysis during storage, since such smaller CK2α-specific signals were never observed in normal brain tissues that have been stored for years. The different distribution of various CK2α isomers in brain tissues at different physiological and pathological periods again indicates that the subunit constitution of CK2 may vary according to the different state or stage of the cells.
References
Ackermann K, Neidhart T, Gerber J, Waxmann A, Pyerin W (2005) The catalytic subunit alpha’ gene of human protein kinase CK2 (CSNK2A2): genomic organization, promoter identification and determination of Ets1 as a key regulator. Mol Cell Biochem 274:91–101
Aksenova MV, Burbaeva GS, Kandror KV, Kapkov DV, Stepanov AS (1991) The decreased level of casein kinase 2 in brain cortex of schizophrenic and Alzheimer’s disease patients. FEBS Lett 279:55–57
Avila J, Ulloa L, Gonzalez J, Moreno F, Diaz-Nido J (1994) Phosphorylation of microtubule-associated proteins by protein kinase CK2 in neuritogenesis. Cell Mol Biol Res 40:573–579
Blanquet PR (2000) Casein kinase 2 as a potentially important enzyme in the nervous system. Prog Neurobiol 60:211–246
Blanquet PR (2000) Identification of two persistently activated neurotrophin-regulated pathways in rat hippocampus. Neuroscience 95:705–719
Bounhar Y, Zhang Y, Goodyer CG, LeBlanc A (2001) Prion protein protects human neurons against Bax-mediated apoptosis. J Biol Chem 276:39145–39149
Chao CC, Chiang CH, Ma YL, Lee EH (2006) Molecular mechanism of the neurotrophic effect of GDNF on DA neurons: role of protein kinase CK2. Neurobiol Aging 27:105–118
Chiarini LB, Freitas AR, Zanata SM, Brentani RR, Martins VR, Linden R (2002) Cellular prion protein transduces neuroprotective signals. EMBO J 21:3317–3326
Collinge J, Clarke AR (2007) A general model of prion strains and their pathogenicity. Science 318:930–936
Escalier D, Silvius D, Xu X (2003) Spermatogenesis of mice lacking CK2alpha’: failure of germ cell survival and characteristic modifications of the spermatid nucleus. Mol Reprod Dev 66:190–201
Faust M, Montenarh M (2000) Subcellular localization of protein kinase CK2. A key to its function? Cell Tissue Res 301:329–340
Gao JM, Gao C, Han J, Zhou XB, Xiao XL, Zhang J, Chen L, Zhang BY, Hong T, Dong XP (2004) Dynamic analyses of PrP and PrP(Sc) in brain tissues of golden hamsters infected with scrapie strain 263K revealed various PrP forms. Biomed Environ Sci 17:8–20
Gao JM, Zhou XB, Xiao XL, Zhang J, Chen L, Gao C, Zhang BY, Dong XP (2006) Influence of guanidine on proteinase K resistance in vitro and infectivity of scrapie prion protein PrP(Sc). Acta Virol 50:25–32
Hamacher R, Saur D, Fritsch R, Reichert M, Schmid RM, Schneider G (2007) Casein kinase II inhibition induces apoptosis in pancreatic cancer cells. Oncol Rep 18:695–701
Iimoto DS, Masliah E, DeTeresa R, Terry RD, Saitoh T (1990) Aberrant casein kinase II in Alzheimer’s disease. Brain Res 507:273–280
Johnson RT (2005) Prion diseases. Lancet Neurol 4:635–642
Kim JS, Eom JI, Cheong JW, Choi AJ, Lee JK, Yang WI, Min YH (2007) Protein kinase CK2alpha as an unfavorable prognostic marker and novel therapeutic target in acute myeloid leukemia. Clin Cancer Res 13:1019–1028
Kim YS, Carp RI, Callahan SM, Wisniewski HM (1987) Incubation periods and survival times for mice injected stereotaxically with three scrapie strains in different brain regions. J Gen Virol 68(Pt3):695–702
Liberski PP, Sikorska B, Bratosiewicz-Wasik J, Gajdusek DC, Brown P (2004) Neuronal cell death in transmissible spongiform encephalopathies (prion diseases) revisited: from apoptosis to autophagy. Int J Biochem Cell Biol 36:2473–2490
Meggio F, Negro A, Sarno S, Ruzzene M, Bertoli A, Sorgato MC, Pinna LA (2000) Bovine prion protein as a modulator of protein kinase CK2. Biochem J 352(Pt1):191–196
Meggio F, Pinna LA (2003) One-thousand-and-one substrates of protein kinase CK2? FASEB J 17:349–368
Moreno FJ, Diaz-Nido J, Jimenez JS, Avila J (1999) Distribution of CK2, its substrate MAP1B and phosphatases in neuronal cells. Mol Cell Biochem 191:201–205
Negro A, Meggio F, Bertoli A, Battistutta R, Sorgato MC, Pinna LA (2000) Susceptibility of the prion protein to enzymic phosphorylation. Biochem Biophys Res Commun 271:337–341
Olsten ME, Litchfield DW (2004) Order or chaos? An evaluation of the regulation of protein kinase CK2. Biochem Cell Biol 82:681–693
Prusiner SB (1998) Prions. Proc Natl Acad Sci USA 95:13363–13383
Roucou X, Gains M, LeBlanc AC (2004) Neuroprotective functions of prion protein. J Neurosci Res 75:153–161
Safar J, Prusiner SB (1998) Molecular studies of prion diseases. Prog Brain Res 117:421–434
Seldin DC, Landesman-Bollag E, Farago M, Currier N, Lou D, Dominguez I (2005) CK2 as a positive regulator of Wnt signalling and tumourigenesis. Mol Cell Biochem 274:63–67
Shi X, Potvin B, Huang T, Hilgard P, Spray DC, Suadicani SO, Wolkoff AW, Stanley P, Stockert RJ (2001) A novel casein kinase 2 alpha-subunit regulates membrane protein traffic in the human hepatoma cell line HuH-7. J Biol Chem 276:2075–2082
Wang G, Unger G, Ahmad KA, Slaton JW, Ahmed K (2005) Downregulation of CK2 induces apoptosis in cancer cells–a potential approach to cancer therapy. Mol Cell Biochem 274:77–84
Wang XF, Guo YJ, Zhang BY, Zhao WQ, Gao JM, Wan YZ, Li F, Han J, Wang DX, Dong XP (2007) Creutzfeldt-Jakob disease in a Chinese patient with a novel seven extra-repeat insertion in PRNP. J Neurol Neurosurg Psychiatr 78:201–203
Westergard L, Christensen HM, Harris DA (2007) The cellular prion protein (PrP(C)): its physiological function and role in disease. Biochim Biophys Acta 1772:629–644
Yao HL, Han J, Gao JM, Zhang J, Zhang BY, Guo YJ, Nie K, Gao C, Wang XF, Dong XP (2005) Comparative study of the effects of several chemical and physical treatments on the activity of protease resistance and infectivity of scrapie strain 263K. J Vet Med B Infect Dis Vet Public Health 52:437–443
Yde CW, Frogne T, Lykkesfeldt AE, Fichtner I, Issinger OG, Stenvang J (2007) Induction of cell death in antiestrogen resistant human breast cancer cells by the protein kinase CK2 inhibitor DMAT. Cancer Lett 256:229–237
Zhang BY, Hou XS, Gao C, Gao JM, Zhou XB, Hong T, Dong XP (2002) Mouse-adapted scrapie strain 139A can overcome species barrier to induce spongiform encephalopathy in golden hamster. Chin J Virol 4:337–341 (In Chinese)
Acknowledgments
This work was supported by National Science and Technology Task Force Project (2006BAD06A13-2), National Basic Research Program of China (973 Program) (2007CB310505) and Chinese National Natural Science Foundation Grants 30571672, 30500018 and 30771914.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, JM., Gao, C., Shi, Q. et al. Different expression patterns of CK2 subunits in the brains of experimental animals and patients with transmissible spongiform encephalopathies. Arch Virol 153, 1013–1020 (2008). https://doi.org/10.1007/s00705-008-0084-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00705-008-0084-z