Abstract
Intravitreal injection of α-crystallin can promote axons from optic nerve regeneration after crushing in rats. We have previously demonstrated that α-crystallin can counteract the effect of myelin inhibitory factors and stimulate neurite growth. And a common crucial signaling event for myelin inhibitory factors is the activation of RhoA. To investigate whether α-crystallin counteracts the inhibitory effect of myelin inhibitory factors through regulation of RhoA/Rock signaling pathway, α-crystallin (10−4 g/L) was injected into rat vitreous at the time the optic nerve crushed. The RhoA protein activity and the expression of RhoA and Rock were evaluated after 3 days of optic nerve axotomy. Rock downstream effectors, phosphorylated cofilin, and phosphorylated myosin light chain were detected when retinal neurons were cultured for 3 days. Axonal regeneration and neurites growth of cultured cells were observed also. Our results showed that α-crystallin decreased the RhoA protein activity and the phosphorylation of both cofilin and myosin light chain, and promoted the axonal growth. However, the expression of RhoA and Rock was not affected by α-crystallin. These findings indicated that α-crystallin could counteract the effect of myelin inhibitory factors through the regulation of RhoA/Rock signaling pathway.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Retinal ganglion cells (RGCs) fail to spontaneously regenerate their axons after optic nerve injury. The inhibitory microenvironment plays an important role in the failure of regeneration (Yiu and He 2003). In addition to scar-associated inhibitory molecules at injury sites, for example chondroitin sulfate proteoglycans (CSPGs), myelin-associated proteins are the important growth inhibitors, such as Nogo-A, myelin-associated glycoprotein (MAG), and oligodendrocyte myelin glycoprotein (Morgenstern et al. 2002; Tang 2003; Yiu and He 2003). They are thought to inhibit axon growth via binding to Nogo receptors that require a variety of transmembrane co-receptors for intracellular signaling. And a common crucial signaling event for these axonal inhibitors is the activation of RhoA, changing GDP-binding RhoA to GTP-binding RhoA (Monnier et al. 2003; Yiu and He 2006; Kubo et al. 2007). Rock is the effector of GTP-RhoA. After the activation of Rock, its downstream effectors: the myosin light chain (MLC) and cofilin are activated by phosphorylation. Phosphorylated MLC stimulates the binding of myosin to actin and subsequent actomyosin contraction. While phosphorylated cofilin (p-cofilin) results in an increase in actin filament assembly (Kubo et al. 2007; Lu et al. 2009). At last, it leads to growth cone collapse and regeneration inhibition.
There are several lines of evidence suggesting that lens injury can promote RGC survival and stimulate axonal regeneration either in vivo or in vitro (Fischer et al. 2000, 2001; Lorber et al. 2002). Lens-derived factors are thought to contribute to the regeneration-promoting effects (Lorber et al. 2005; Yin et al. 2006). Recently, it is shown that crystallins of the β/γ superfamily can elongate the length and increase the number of regenerating axons (Liedtke et al. 2007; Fischer et al. 2008), and their effects might be mediated by astrocyte-derived CNTF (Fischer et al. 2008). In addition to β/γ superfamily, α-crystallin is another lens-derived protective factor.
α-Crystallin has a high antiapoptotic activity. It has been found that α-crystallin can stall the maturation of caspase-3 (Kamradt et al. 2005), bind to proapototic proteins such as P53 (Jolly and Morimoto 2000), and play a beneficial role in preventing stress-induced cell death (Andley 2007). α-Crystallin has also been found to be a potent negative regulator in several inflammatory pathways for both the immune system and the central nervous system (Masilamoni et al. 2006). In the optic nerve, Munemasa reported that α-crystallins could increase the survival of RGC after optic nerve axotomy (Munemasa et al. 2009). Presently, studies showed that axonal density distal to the crush site was significantly higher than in untreated controls up to 4 weeks after a single intravitreal administration of α-crystallin (Ying et al. 2008). These results suggest that α-crystallin assists RGCs survival and axonal growth in the inhibitory microenvironment after optic nerve injury.
We previously demonstrated that α-crystallin significantly stimulated neurite initiation and outgrowth when retinal neurons were cultured on myelin-coated dishes. It indicated that α-crystallin could counteract myelin inhibitory factors and promote neurite growth (Wang et al. 2011). However, the mechanism is unknown. In this work, we studied the regulation effect of α-crystallin on RhoA/Rock signaling pathway, through which myelin-associated and scar-associated inhibitory molecules interfere nerve regeneration.
Materials and Methods
Animals
Newborns from postnatal day 0 to day 2 (P0 to P2) and adult (200–250 g) Long Evans rats were used for the experiments. All experimental protocols were approved by the Institutional Animal Care and Use Committee of Third Military Medical University. And all studies were conducted according to the Declaration of the NIH Statement for the Use of Animals in Ophthalmic and Vision Research.
Surgical Procedures
The right optic nerves were crushed (ONC) intraorbitally using a standard protocol (Ying et al. 2008). In brief, adult animals were anesthetized by intraperitoneal injections of chloral hydrate (3.5 mg/kg). A 1-cm incision was made in the conjunctiva at the temporal side of each eye. The optic nerve was exposed under an operating microscope, and its dura was opened longitudinally. Using the blood vessel forceps, the optic nerve was crushed 2 mm behind the eye for 10 s, avoiding injury to the ophthalmic artery. Nerve injury was verified by the appearance of a clearing at the crush site, and the vascular integrity of the retina was verified by funduscopic examination. α-Crystallin (10−4 g/L; 5 μL), which was prepared as previously described (Wang et al. 2009, 2011), was injected into vitreous space by posterior approach in the right eyes, taking care not to damage the lens. In two control groups, the same volume of C3 transferase (1 × 10−1 g/L, Upstate, USA), a specified inactivator of RhoA (Kubo et al. 2007; Lu et al. 2009), or bovine serum albumin (BSA, 10−4 g/L) was injected into right eyes after optic nerve crushed. RhoA protein activity and the expression of RhoA and Rock were evaluated on 3 days after the optic nerve was crushed and intravitreal injections of BSA, α-crystallin, or C3 transferase.
Expression of RhoA and Rock Protein
Retinas were lysed in lysis buffer [50 mM Tris–HCl (pH 7.2), 500 mM NaCl, 10 mM MgCl2, 1% Triton X-100, 0.5% sodium deoxycholate, 0.1% SDS, and 1 mM PMSF], and centrifuged at 15,000×g for 15 min at 4°C. Protein concentrations were determined using bicinchoninic acid protein assay kit. Equal amounts of protein were loaded into each lane of SDS-12% polyacrylamide gels. The gels were then electrophoresed, transferred to a polyvinylidene difluoride membrane, and finally analyzed by Western blotting using mouse monoclonal anti-RhoA (Upstate) or rabbit monoclonal anti-Rock (Upstate). As an internal control, anti-glyceraldehyde-3-phosphate dehydrogenase polyclonal antibody (GAPDH; Santa Cruz Biotechnology) was blotted in the same membrane. The immune complex was visualized with an ECL detection system.
RhoA Activity Assay
RhoA pull-down assay was carried out as previously reported using immobilized GST-RBD (Upstate; Chen et al. 2005). Retinas were lysed in Rho-binding lysis buffer [50 mM Tris–HCl (pH 7.2), 500 mM NaCl, 10 mM MgCl2, 1% Triton X-100, 0.5% sodium deoxycholate, 0.1% SDS, and 1 mM PMSF]. Retina lysates were cleared by centrifugation at 10,000×g for 10 min at 4°C, and protein extracts (200 μg) were incubated with 20 μg of immobilized GST-RDB for 45 min at 4°C. The beads were washed four times with buffer [50 mM Tris–HCl (pH 7.2), 150 mM NaCl, 10 mM MgCl2, 1% Triton X-100, and 1 mM PMSF]. The bound RhoA GTPases were eluted by boiling in Laemmli SDS sample buffer and were subjected to Western blot with antibody against RhoA.
Retinal Neuron Cultures
Retinal cell cultures were prepared as previously described (Wang et al. 2009, 2011). P0 to P2 rats were killed by decapitation and neural retinas dissected at 4°C. The tissue was incubated in 0.125% trypsin and 0.2% atidase for 30 min at 37°C. Activity of exogenous proteases was eliminated by washing the tissue with Dulbecco’s modified eagle medium (DMEM) containing 10% FBS. Retinal cells were mechanically dissociated using a pipette then seeded onto 24-well culture plates. Cells were initially seeded at 1 × 106 cells/mL. The cells were cultured in DMEM supplemented with 10% FBS, glucose (4.5 g/L), streptomycin (100 μg/mL), penicillin (100 U/mL), and HEPES (15 mmol/L). After 24 h in culture, 5-bromium-2′deoxyuridine (20 μg/mL) was added to culture medium to restrict the growth of non-nerve cells. Each independent culture was derived from three animals (six retinas). Treatment groups were: (1) α-crystallin (10−4 g/L) dissolved in DMEM; (2) control, C3 transferase, 10−1 g/L dissolved in DMEM; and (3) control, BSA, 10−4 g/L dissolved in DMEM. α-Crystallin, C3 transferase, or BSA was added to the cell cultures immediately when the cells were seeded onto culture plates. Each experiment was repeated three times, and the cells were continuously cultured under the experimental conditions until cell death.
Phosphorylated MLC and Cofilin Assay
Myelin solution (0.1 mg/mL) was added into culture medium when cells were cultured for 3 days. After 1 h, as the evaluation of RhoA and Rock protein expression, Western blot was used to detect the phosphorylation of MLC and cofilin. The antibodies were goat monoclonal anti-phospho-MLC (Santa), mouse monoclonal anti-MLC (Santa), rabbit monoclonal anti-phospho-cofilin (Santa), goat monoclonal anti-cofilin (Santa), and mouse polyclonal anti-GAPDH. The immune complex was visualized with an ECL detection system.
Evaluating Axonal Regeneration
In order to anterogradely label regenerating axons, cholera toxin B subunit (CTB; Molecular Probes, USA) was intravitreally injected 2 days before sacrificing the animals. Regenerating axons were visualized in longitudinal sections of the optic nerve at 14 days after ONC. Neurite outgrowth assays were also performed in vitro as previously described (Wang et al. 2009, 2011). Briefly, retinal neurons were cultured on culture plates coated with the myelin solution (0.1 mg/mL) which was prepared as previously described (Wang et al. 2011). As previously described (Wang et al. 2011), the numbers of retinal neurons with neurites were counted and the longest neurite was measured on days 1, 3, and 5 by tracing the neurite from the cell body to its farthest tip. Measurements were obtained using a computerized image analysis system (Leica QWin).
Statistics
The data are expressed as the mean±SD. Differences among groups of Western blot results were analyzed by one-way ANOVA, followed by LSD method. The numbers of retinal neurons with neurites and the longest neurite were analyzed using paired t tests. Differences were considered to be significant when P < 0.05.
Results
The Effect of α-Crystallin on the Expression of RhoA and Rock Protein
The previous study indicated that the expression of RhoA was enhanced after the optic nerve crushed (Wang et al. 2007). Here, we examined the effect of α-crystallin on the expression of RhoA and its downstream effector, Rock protein, using Western blot. The result showed that α-crystallin did not induce a decrease in RhoA and Rock protein levels in the retina (Fig. 1).
Western blot analysis of the effect of α-crystallin on the expression of RhoA and Rock protein. Immunnoreactive bands of a RhoA and b Rock in α-crystallin, C3 transferase, or BSA group, respectively, at 3 days after the optic nerve crushed. Bands of RhoA and Rock were detected in samples from the retina. In the same time, GAPDH protein was detected by its antibody. c, d Densitometry of the immunoreactive bands. Data are expressed as a ratio of RhoA or Rock to GAPDH. Compared to BSA, α-crystallin and C3 transferase did not change the expression of both c RhoA and d Rock (n = 6; mean±SD, P > 0.05 compare with BSA)
The Effect of α-Crystallin on RhoA Protein Activity
After the optic nerve injury, RhoA is activated, changing GDP-binding RhoA to GTP-binding RhoA, and plays an important role in the failure of axonal regeneration (Monnier et al. 2003; Yiu and He 2006; Kubo et al. 2007). To examine the effect of α-crystallin on RhoA protein activity, GTP-binding RhoA was detected. RhoA pull-down assay showed that α-crystallin decreased GTP-binding RhoA significantly. However, the effect of α-crystallin (reduced to 86% of BSA values) was slighter than that of C3 transferase (reduced to 79% of BSA values; Fig. 2).
Western blot analysis of the effect of α-crystallin on RhoA protein activity. a Immunoreactive bands of GTP-RhoA and total RhoA in α-crystallin, C3 transferase, or BSA group, respectively, at 3 days after the optic nerve crushed. b Densitometry of the immunoreactive bands. Data are expressed as a ratio of GTP-RhoA to total RhoA (n = 6; mean±SD, *P < 0.05 compare with BSA; **P < 0.01 compare with BSA; #P < 0.05 compare with C3)
The Effect of α-Crystallin on the Phosphorylation of Cofilin and MLC
RhoA stimulates accumulation of stress fibers through activation of Rock. LIM kinase and MLC kinase are downstream effectors of Rock responsible for the phosphorylation of cofilin and MLC, respectively (Kubo et al. 2007; Lu et al. 2009). We found that compared to BSA, α-crystallin treatment decreased the phosphorylation of both cofilin (reduced to 71% of BSA values) and MLC (reduced to 73% of BSA values). C3 transferase also decreased the phosphorylation of both cofilin and MLC. However, there was no great contrast between α-crystallin and C3 transferase (Fig. 3).
Western blot analysis of the effect of α-crystallin on the phosphorylation of cofilin and MLC. Immunoreactive bands of a phosphorylated cofilin (p-cofilin) and total cofilin and b phosphorylated MLC (p-MLC) and total MLC in α-crystallin, C3 transferase, or BSA group, respectively, at cell culture for 3 days. c, d Densitometry of the immunoreactive bands. Data are expressed as a ratio of c p-cofilin to total cofilin or d p-MLC to total MLC (n = 6; mean±SD, *P < 0.01 compare with BSA)
The Effect of α-Crystallin on Axonal Regeneration
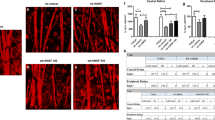
To examine the effect of α-crystallin on axonal regeneration, α-crystallin or BSA was injected into vitreous after OPN. Two weeks later, regenerating axons were visualized and anterogradely labelled with CTB. In the BSA experiments, several axons were found in the crush site, but no axons were beyond the crush site. In α-crystallin injection, many axons regenerated beyond the crush site and extended toward the central direction (Fig. 4a, b). We also analyzed axonal outgrowth by retinal neurons cultured on culture plates coated with the myelin. The numbers of neurons with neurites were counted, and the longest neurite was measured on days 1, 3, and 5. Most cells initiated neurite in α-crystallin group. Whereas, only several cells initiated neurite in BSA group (Fig. 4c–e). And the neurite length was longer significantly in α-crystallin administration than in BSA administration (Fig. 4c, d, f).
The effect of α-crystallin on axonal regeneration. Two weeks after ONC and intravitreal injection of BSA, several CTB-labelled axons regenerated into the crush site (arrow), but not beyond the site (a). Whereas, in α-crystallin injection, much more axons distal to the injury site (b). The culture photographs show the neurite initiation and outgrowth in α-crystallin (c) and BSA (d) groups after retinal neurons were cultured for 3 days on plates coated with the myelin. Cell cultures treated with α-crystallin had significantly more retinal neurons with neurites compared with cultured containing BSA (e). The mean length of the longest neurites in the α-crystallin group were significantly longer than those of the BSA group (f; mean±SD, *P < 0.01; n = 30 fields for e, 150 cells for f, respectively. Scale bar: 50 μm for a, b, c, and d)
Discussion
It has been found that α-crystallin can promote RGC survival and axonal growth after optic nerve injury (Ying et al. 2008; Munemasa et al. 2009). Our further studies demonstrated that α-crystallin significantly stimulated neurite initiation and outgrowth when retinal neurons were cultured on myelin-coated dishes (Wang et al. 2011). Since myelin-associated and scar-associated inhibitory molecules interfere nerve regeneration through activating RhoA, we studied the regulation effect of α-crystallin on RhoA/Rock signaling pathway in this work. And the results showed that α-crystallin decreased the RhoA protein activity, the phosphorylation of both cofilin and MLC, and promoted the axonal growth, but did not affected the expression of RhoA and Rock protein.
RhoA is abundantly expressed in various types of cells, including those in the central nervous system and retina (Nakayama et al. 2000; Kitaoka et al. 2004). Nerve injury can change the expression of RhoA and Rock. It was reported that RhoA mRNA and protein expressions were enhanced significantly in the injured spinal cord 1 week after surgery (Sung et al. 2003). RhoA and ROCK were upregulated in NMDA-induced retinal neurotoxicity (Kitaoka et al. 2004). Our recent studies also found that the expression of RhoA in retina were extended and enhanced after the optic nerve crushed (Wang et al. 2007). In this work, we detected the expression of RhoA and Rock protein, and found that α-crystallin did not change the expression of both RhoA and Rock after the optic nerve injury.
In addition to the expression of RhoA, the activation of RhoA plays an important role in inhibition nerve regeneration. After optic nerve injury, the regeneration inhibitors, such as CSPGs, Nogo-A, MAG, and so on, activate RhoA by mediating the transition from an inactive GDP bound state to an active GTP bound state (Monnier et al. 2003; Yiu and He 2006; Kubo et al. 2007; Lu et al. 2009). Our results showed that as C3 transferase, α-crystallin decreased GTP-binding RhoA significantly. It indicated that α-crystallin could interfere the activation of RhoA after optic nerve injury. We also examined the effect of α-crystallin on the phosphorylation of cofilin and MLC which are the downstream effectors of Rock (Kubo et al. 2007; Lu et al. 2009). The result was that α-crystallin downregulated the phosphorylation both of cofilin and of MLC, the same as C3 transferase. And at the same time, α-crystallin promoted axonal regeneraton not only in vivo optic nerve injury model, but also in neuron culture on myelin-coated dishes. These results indicated that α-crystallin could counteract the effect of regeneration inhibitory factors and stimulate axonal regeneration by mediating the RhoA/Rock signaling pathways.
α-Crystallin is a member of the mammalian sHsp superfamily. Except for the molecular chaperone function, as antiapoptotic proteins, α-crystallin can confer various types of cells including RGCs with the ability to resist cell stress (Andley 2007; Munemasa et al. 2009) by interfering with the activity of various apoptotic proteins, such as caspase-3, P53, and so on (Jolly and Morimoto 2000; Kamradt et al. 2005; Andley 2007). α-Crystallin can also prevent apoptosis by enhancing the expression of PKCα, suppressing the activation of the RAF/MEK/ERK pathway, and activating the AKT pathway (Liu et al. 2004). It may be one of the mechanisms that α-crystallin can promote axonal regeneration after optic nerve injury because the death of RGCs is the important reason of their axonal regeneration failure. Here, we first showed that another mechanism of α-crystallin in optic nerve regeneration was regulating RhoA/Rock signaling pathway. However, how α-crystallin downregulate the GTP-binding RhoA was still unknown. Recent studies indicated that α-crystallin interacted with α6 integrin receptor complexes and regulated cellular signaling (Sue and Andley 2010). α-Crystallin may prevent GDP-binding RhoA from changing to GTP-binding RhoA by binding to a membrane receptor, such as α6 integrin. And it may be one of the reasons why RhoA activation inhibition effect of α-crystallin was slighter than that of C3 transferase, since C3 transferase is a specified inactivator of RhoA which interferes the activation of RhoA directly (Kubo et al. 2007; Lu et al. 2009).
In summary, this study demonstrated that α-crystallin suppressed activation of RhoA/Rock signaling pathway and stimulated axonal regeneration after optic nerve injury. It adds to our knowledge of α-crystallin as promoter of optic nerve regeneration and provides insight into potential therapies for enhancing recovery from the optic nerve injury.
References
Andley UP (2007) Crystallins in the eye: function and pathology. Prog Retin Eye Res 26:78–98
Chen CJ, Liao SL, Huang YS, Chiang AN (2005) RhoA inactivation is crucial to manganese-induced astrocyte stellation. Biochem Biophys Res Commun 326:873–879
Fischer D, Pavlidis M, Thanos S (2000) Cataractogenic lens injury prevents traumatic ganglion cell death and promotes axonal regeneration both in vivo and in culture. Invest Ophthalmol Vis Sci 41:3943–3954
Fischer D, Heiduschka P, Thanos S (2001) Lens-injury-stimulated axonal regeneration throughout the optic pathway of adult rats. Exp Neurol 72:257–272
Fischer D, Hauk TG, Müller A, Thanos S (2008) Crystallins of the beta/gamma-superfamily mimic the effects of lens injury and promote axon regeneration. Mol Cell Neurosci 37(3):471–479
Jolly C, Morimoto RI (2000) Role of the heat shock response and molecular chaperones in oncogenesis and cell death. J Natl Cancer Inst 92:1564–1572
Kamradt MC, Lu M, Werner ME, Kwan T, Chen F, Strohecker A et al (2005) The small heat shock protein alpha B-crystallin is a novel inhibitor of TRAIL-induced apoptosis that suppresses the activation of caspase-3. J Biol Chem 280:11059–11066
Kitaoka Y, Kitaoka Y, Kumai T, Lam TT, Kuribayashi K, Isenoumi K et al (2004) Involvement of RhoA and possible neuroprotective effect of fasudil, a Rho kinase inhibitor, in NMDA-induced neurotoxicity in the rat retina. Brain Res 1018:111–118
Kubo T, Hata K, Yamaguchi A, Yamashita T (2007) Rho–ROCK inhibitors as emerging strategies to promote nerve regeneration. Curr Pharm Des 13:2493–2499
Liedtke T, Schwamborn JC, Schröer U, Thanos S (2007) Elongation of axons during regeneration involves retinal crystallin beta b2 (crybb2). Mol Cell Proteomics 6(5):895–907
Liu JP, Schlosser R, Ma WY, Dong Z, Feng H, Liu L (2004) Human aA- and aB-crystallins prevent UVA-induced apoptosis through regulation of PKCa, RAF/MEK/ERK and AKT signaling pathways. Exp Eye Res 79:393–403
Lorber B, Berry M, Logan A, Tonge D (2002) Effect of lens lesion on neurite outgrowth of retinal ganglion cells in vitro. Mol Cell Neurosci 21:301–311
Lorber B, Berry M, Logan A (2005) Lens injury stimulates adult mouse retinal ganglion cell axon regeneration via both macrophage- and lens-derived factors. Eur J Neurosci 21:2029–2034
Lu Q, Longo FM, Zhou H, Massa SM, Chen YH (2009) Signaling through Rho GTPase pathway as viable drug target. Curr Med Chem 16:1355–1365
Masilamoni JG, Jesudason EP, Baben B, Jebaraj CE, Dhandayuthapani S, Jayakumar R (2006) Molecular chaperone alpha-crystallin prevents detrimental effects of neuroinflammation. Biochim Biophys Acta 1762:284–293
Monnier PP, Sierra A, Schwab JM, Henke-Fahle S, Mueller BK (2003) The Rho/ROCK pathway mediates neurite growth-inhibitory activity associated with the chondroitin sulfate proteoglycans of the CNS glial scar. Mol Cell Neurosci 22:319–330
Morgenstern DA, Asher RA, Fawcett JW (2002) Chondroitin sulphate proteoglycans in the CNS injury response. Prog Brain Res 137:313–332
Munemasa Y, Kwong JM, Caprioli J, Piri N (2009) The role of alphaA- and alphaB-crystallins in the survival of retinal ganglion cells after optic nerve axotomy. Invest Ophthalmol Vis Sci 50(8):3869–3875
Nakayama AY, Harms MB, Luo L (2000) Small GTPases Rac and Rho in the maintenance of dendritic spines and branches in hippocampal pyramidal neurons. J Neurosci 20:5329–5338
Sue MA, Andley UP (2010) αA-Crystallin associates with α6 integrin receptor complexes and regulates cellular signaling. Exp Eye Res 91:640–651
Sung JK, Miao L, Calvert JW, Huang L, Louis HH, Zhang JH (2003) A possible role of RhoA/Rho-kinase in experimental spinal cord injury in rat. Brain Res 959:29–38
Tang BL (2003) Inhibitors of neuronal regeneration: mediators and signaling mechanisms. Neurochem Int 42:189–203
Wang YH, Wang Y, Wang DW, Wu N, Liu DN (2007) Distribution and expression of RhoA in the rat retina after optic nerve crushed. Ophthalmic Res 39:174–178
Wang YH, Wang Y, Wang DW, Wu N, Liu DN, Yin ZQ (2009) In vitro study of the effects of lens extract on rat retinal neuron survival and neurite outgrowth. Ophthalmic Res 42:29–35
Wang YH, Wang DW, Wu N, Wang Y, Yin ZQ (2011) Alpha-crystallin promotes rat retinal neurite growth on myelin substrates in vitro. Ophthalmic Res 45:164–168
Yin Y, Henzl MT, Lorber B, Nakazawa T, Thomas TT, Jiang F et al (2006) Oncomodulin is a macrophage-derived signal for axon regeneration in retinal ganglion cells. Nat Neurosci 9:843–852
Ying X, Zhang J, Wang YH, Wu N, Wang Y, Yew DT (2008) Alpha-crystallin protected axons from optic nerve degeneration after crushing in rats. J Mol Neurosci 35:253–258
Yiu G, He Z (2003) Signaling mechanisms of the myelin inhibitors of axon regeneration. Curr Opin Neurobiol 13:545–551
Yiu G, He Z (2006) Glial inhibition of CNS axon regeneration. Nat Rev Neurosci 7:617–627
Acknowledgments
The study is supported by grants from National Key Basic Scientific Research Project (973 Project) of China (2007CB512203), National Natural Science Foundation of China(grant no. 30872833), the Military 11th Five-year Project of China (grant no. 06G072), and Youth Innovation Fund of the Third Military Medical University (no. 32009XQN29). The authors thank Dr. T. FitzGibbon for the comments on previous drafts of the manuscript.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Wang, Y.H., Wang, D.W., Wu, N. et al. Alpha-Crystallin Promotes Rat Axonal Regeneration Through Regulation of RhoA/Rock/Cofilin/MLC Signaling Pathways. J Mol Neurosci 46, 138–144 (2012). https://doi.org/10.1007/s12031-011-9537-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-011-9537-z