Abstract
Background
Reactive electroencephalography (EEG) background during therapeutic hypothermia (TH) is related to favorable prognosis after cardiac arrest (CA), but its predictive value is not 100 %. The aim of this study was to investigate outcome predictors after a first reactive EEG recorded during TH after CA.
Methods
We studied a cohort of consecutive comatose adults admitted between February 2008 and November 2012, after successful resuscitation from CA, selecting patients with reactive EEG during TH. Outcome was assessed at three months, and categorized as survivors and non-survivors (no patient was in vegetative state). Demographics, clinical variables, EEG features, serum neuron-specific enolase (NSE) and procalcitonin, were compared using uni- and multivariable analyses.
Results
A total of 290 patients were treated with TH after cardiac arrest; 146 had an EEG during TH, which proved reactive in 90 of them; 77 (86 %) survived and 13 (14 %) died (without recovery from coma). The group of non-survivors had a higher occurrence of discontinuous EEG (p = 0.006; multivariate analysis p = 0.026), and a higher serum NSE peak (p = 0.021; multivariate analysis p = 0.014); conversely, demographics, and other clinical variables including serum procalcitonin did not differ.
Conclusions
A discontinuous EEG and high serum NSE are associated with mortality after CA in patients with poor outcome despite a reactive hypothermic EEG. This suggests more severe cerebral damage, but not to higher extent of systemic disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hypoxic ischemic encephalopathy following cardiac arrest (CA) represents a leading cause of mortality [1]. The introduction of therapeutic hypothermia (TH) in the last decade has contributed to the improvement of survival and functional outcome [2–5], leading to increased attention on issues related to prognostication. According to current knowledge, lack of brainstem reflexes [6, 7], high serum neuron-specific enolase (NSE) [8, 9], and non-reactive or discontinuous electroencephalography (EEG) background activity [6, 10] are considered to be relatively robust predictors of poor outcome after CA and TH. Moreover, we recently found that the EEG background reactivity during TH represents a reliable outcome predictor in this setting; furthermore, hypothermic EEG features seem to correlate with the extent of neurological damage [11]. In that study, while the predictive value for mortality in patients without EEG reactivity during TH was very high (23/23, 100 %), 4/38 (11 %) subjects died despite a reactive EEG.
We undertook the present analysis to identify clinical variables correlating with poor outcome despite a reactive hypothermic EEG. The main objective was to assess if non-survivors with a reactive EEG had a higher extent of cerebral damage (i.e., higher NSE, other malignant EEG signs) and/or a greater severity of systemic injury (i.e., higher procalcitonin (PCT)) [12, 13].
Methods
Patients and Procedures
We included all patients with a reactive EEG during TH from our prospective registry of consecutive comatose adults admitted between February 2008 and November 2012 to the Department of Intensive Care Medicine, after successful resuscitation from CA and treated with TH, according to our protocol (in line with current recommendations [6]). Briefly, all patients received mild TH to 33 °C for 24 h, using ice packs and IV ice-cold fluids, together with a surface cooling device (Arctic Sun System, Medivance, Louisville, CO). In a standardized approach, midazolam (0.1 mg/kg/h) and fentanyl (1.5 μg/kg/h) were given for sedation-analgesia, and vecuronium (0.1 mg/kg boluses) to control shivering. Serum NSE was sampled at 24 and 48 h after CA, and analyzed with an automated immunofluorescent assay (Thermo Scientific Brahms NSE Kryptor Immunoassay); for the present study, the peak level was recorded. Serum PCT was sampled at 24 h after CA, during TH, using the ELFA method (Vidas Brahms PCT assay, bioMerieux Inc., Geneva, Switzerland) [13], with a detection limit of 0.05 μg/ml and an inter-plate variance of under 20 % (normal values in our laboratory: <0.25 μg/ml for detection of bacterial infections).
Decisions upon withdrawal of care were based on multidisciplinary decisions according to our local protocol [6]: occurrence of two or more of four criteria (1. unreactive EEG background in normothermia, 2. treatment-resistant myoclonus, 3. bilateral absence of N20 in somatosensory evoked potentials in normothermia, and 4. incomplete return of brainstem reflexes) after more than 48–72 h after CA, in normothermia and off sedation lead to consideration of interruption of supportive care. Importantly, the EEG during hypothermia and NSE values were not taken into consideration for the withdrawal of care. Outcome at 3 months was assessed with a semistructured interview using the Cerebral Performance Categories (CPC): CPC 1 indicates full recovery; CPC 2 indicates moderate disability (able to work at least part-time, and independent for activities of daily living, with or without neurological manifestations such as hemiplegia, seizures, ataxia, dysarthria, dysphasia, or permanent memory or mental changes); CPC 3 indicates severe disability (conscious, but fully dependent for daily support because of severely impaired cognitive function). Patients with CPC 4 are comatose or in a persistent vegetative state, and those with CPC 5 have died [12]. These variables were collected prospectively according to the Utstein style [14].
EEG
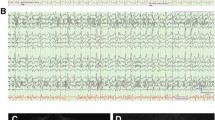
Video-EEGs were performed per protocol during TH and early after TH (during normothermia), with mostly 21 electrodes according to the international 10–20 system, for 20–30 min [6, 11]. The hypothermic EEG was performed at least 6 h after CA, at a temperature of 33–34 °C (in all patients). Normothermic EEG were performed up to 24 h after patients were at least at 35 °C, and (in the vast majority) after sedation weaning. Background reactivity was tested on site, using auditory and painful stimulations, at least 6 h after CA. EEG reactivity is defined as the presence of any clear and reproducible change in amplitude or frequency upon stimulation in the background, excluding stimulus-induced rhythmic, periodic or irritative discharges and muscle artifacts (SIRPIDs). A discontinuous pattern was defined as an EEG background interrupted by flat periods during >10 % of the recording [15]. Epileptiform activity was defined as any periodic or rhythmic spikes or waves, as well as recurrent sharp or spikes-waves.
Statistical Analysis
The cohort was analyzed according to the outcome (awakening versus death at 3 months; no patient remained in vegetative state). Fischer, Student t and χ 2 tests were used as needed. For statistical significant parameters, a stepwise multivariate logistic regression analysis was performed, and assessed using goodness-of fit (Hosmer–Lemeshow). Significance was set at p < 0.05. Calculations were performed with a Stata software, version 9 (College Station, TX).
Results
During the study period, 290 patients were treated with TH after CA; 146 had an EEG during TH (61 overlap with our previous study [11]). In 90 (62 %) of them (38 overlapping with [11]), this was reactive to stimulations: 77/90 (86 %) awoke (CPC: 1–3) and 13/90 (14 %) died (none recovered beyond a vegetative state).
Table 1 illustrates pertinent clinical and laboratory findings according to outcome (during TH and normothermia). While demographics, variables related to the CA and serum procalcitonin were not statistically different, the occurrence of a discontinuous EEG differed clearly between patients who awoke and those who died. NSE serum peak values during TH were measured in 82 patients (71/77 (87 %) survivors; 11/13 (13 %) non-survivors) and were notably higher in the non-survivors group. These two items were entered in the logistic regression using survival as dependent variable; both remained significant (discontinuous EEG: p = 0.026, OR 5.2, 95 % CI = 1.22–25.0; NSE: p = 0.014, OR 1.05, 95 % CI = 1.01–1.10) (Table 2). After return to normothermia, surviving patients versus deceased patients showed full return of brainstem reflexes (92 vs. 77 %) and motor response to pain (87 vs. 38 %); myoclonus was seen in 5 versus 23 %.
After return to normothermia, more deceased patients developed an epileptiform EEG, a discontinuous EEG, and lost EEG background reactivity.
Conclusions
Our study, which to the best of our knowledge represents the first attempt to address the issue of patients having a poor outcome despite an encouraging first EEG evaluation, shows that non-survivors with reactive EEG have greater extent of cerebral damage (higher NSE), but similar degree of systemic injury (PCT levels) as compared to survivors with reactive EEG.
Permanent neuronal damage following acute injury is either due to rapidly occurring necrosis, or to delayed apoptosis; it is generally assumed that necrosis and apoptosis are a part of a continuum of cell death [16]. Related to our findings, the apoptotic phenomenon can possibly underlie a somewhat delayed poor prognosis despite an initially encouraging (reactive) EEG. Serum procalcitonin in the acute postanoxic period is associated with the systemic post-resuscitation syndrome, independently of infections [12, 13]. This syndrome is related to a systemic inflammation, and seems not directly associated with neuronal damage [13]. Our results support these findings, and suggest that the post-resuscitation syndrome is not primarily implicated in the poor clinical evolution in patients with initially relatively preserved EEG.
EEG discontinuity correlates with cerebral damage after CA, but less importantly than the absence of background reactivity [11]. In our study, after return to normothermia, the EEG remained reactive though discontinuous in most of the deceased patients, while about one-third developed epileptiform transients. Even if mild hypothermia and sedation may exert an antiepileptic action [11, 17], this could in also part reflect the delayed apoptotic process.
NSE serum peak values during TH were significantly higher in the non-survivors; both groups median NSE values were beneath the cut-off of 33 μg/l. Nevertheless, several investigators pointed out that a reliable NSE cut-off after TH would be much higher (about 70–80 μg/l); in our cohort, no surviving patient and 1 deceased subject showed NSE >75 μg/l [18, 19].
This explorative, observational study has some limitations: first, despite being the first attempt to address this specific clinical problem, the number of patients is relatively small. This explains why the groups were separated according to survivorship (a very solid outcome) with no further subdivisions according to the functional status. Second, it is a single-center study, but this may enhance the internal validity. Third, our approach does not identify cut-off values for the serum NSE. Midazolam given during TH may impact on the EEG background reactivity, and given its prolonged half life may still be present in the blood of several patients after TH; however, this did not seem prominent in a previous study by our group [17].
A prolongation of the TH duration may be taken into consideration in the subgroup of patients with early reactive but discontinuous EEG and high serum NSE during TH. Indeed, prolonged TH (72 h) improves the neurological outcome in newborns after neonatal anoxia [20] but it has not yet been investigated in adults. Furthermore, timely availability of NSE results would be critical in this context. Before this, our observations should be independently confirmed in larger cohorts.
References
Scirica BM. Therapeutic hypothermia after cardiac arrest. Circulation. 2013;127(2):244–50.
The Hypothermia After Cardiac Arrest Study Group. Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med. 2002;346(8):549–56.
Bernard SA, Timothy GW, Buist M, et al. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med. 2002;346(8):557–63.
Hörburger D, Testori C, Sterz F, et al. Mild therapeutic hypothermia improves outcomes compared with normothermia in cardiac-arrest patients—a retrospective chart review. Crit Care Med. 2012;40(8):2315–9.
Fugate JE, Brinjikji W, Mandekar JN, et al. Post-cardiac arrest mortality is declining: a study of the US National Inpatient Sample 2001 to 2009. Circulation. 2012;126:546–50.
Rossetti AO, Oddo M, Logroscino G, Kaplan PW. Prognostication after cardiac arrest and hypothermia: a prospective study. Ann Neurol. 2010;67(3):301–7.
Fugate JE, Wijdicks EFM, Mandrekar J, et al. Predictors of neurologic outcome in hypothermia after cardiac arrest. Ann Neurol. 2010;68:907–14.
Cronberg T, Rundgren M, Westhall E, et al. Neuron-specific enolase correlates with other prognostic markers after cardiac arrest. Neurology. 2011;77(7):623–30.
Rundgren M, Karrisonn T, Nielsen N, Cronberg T, Johnsson P, Friberg H. Neuron specific enolase and S-100B as predictors of outcome after cardiac arrest and induced hypothermia. Resuscitation. 2009;80(7):784–9.
Oh SH, Park KN, Kim YM, et al. The prognostic value of continuous amplitude-integrated electroencephalogram applied immediately after return of spontaneous circulation in therapeutic hypothermia-treated cardiac arrest patients. Resuscitation. 2013;84(2):200–5.
Rossetti AO, Carrera E, Oddo M. Early EEG correlates of neuronal injury after brain anoxia. Neurology. 2012;78(11):796–802.
Annborn M, Dankiewicz J, Erlinge D, et al. Procalcitonin after cardiac arrest—an indicator of severity of illness, ischemiareperfusion injury and outcome. Resuscitation. 2013;84(6):782–7.
Engel H, Hamouda NB, Portmann K, et al. K. et al. Serum procalcitonin as a marker of post-cardiac arrest syndrome and long-term neurological recovery, but not of early-onset infections, in comatose post-anoxic patients treated with therapeutic hypothermia. Resuscitation. 2013;84(6):776–81.
European Resuscitation Council, American Heart Association. Heart and Stroke Foundation of Canada, and Australian Resuscitation Council. Recommended guidelines for uniform reporting of data from out-of-hospital cardiac arrest (new abridged version). The “Utstein Style”. Br Heart J. 1992;67:325–33.
Hirsch LJ, Brenner RP, Drislane FW, et al. The ACNS Subcommittee on Research Terminology for Continuous EEG Monitoring: proposed Standardized Terminology for Rhythmic and Periodic EEG Patterns Encountered in Critically Ill Patients. J Clin Neurophysiol. 2005;22(2):128–35.
Banasiak KJ, Xia Y, Haddad GG. Mechanisms underlying hypoxia-induced neuronal apoptosis. Prog Neurobiol. 2000;62(3):215–49.
Rossetti AO, Urbano LA, Delodder F, Kaplan PW, Oddo M. Prognostic value of continuous EEG monitoring during therapeutic hypothermia after cardiac arrest. Crit Care. 2010;14(5):173.
Bouwes A, Binnekade JM, Kulper MA, et al. Prognosis of coma after therapeutic hypothermia: a prospective cohort study. Ann Neurol. 2012;71(2):206–12.
Steffen IG, Hasper D, Ploner CJ, et al. Mild therapeutic hypothermia alters neuron specific enolase as an outcome predictor after resuscitation: 97 prospective hypothermia patients compared to 133 historical non-hypothermia patients. Crit Care. 2010;14(2):R69.
Azzopardi VD, Strohm B, Edwards AD, et al. Moderate Hypothermia to treat perinatal asphyxia encephalopathy. N Engl Med. 2009;361:1349–58.
Acknowledgments
The authors thank Christine Stähli, RN, Dr. Alba Sierra, the EEG fellows and technologists, and the ICU fellows, for their help in data collection.
Disclosures
The Swiss National Science Foundation provides financial support to AOR (CR32I3_143780) and MO (320030_138191).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tsetsou, S., Oddo, M. & Rossetti, A.O. Clinical Outcome After a Reactive Hypothermic EEG Following Cardiac Arrest. Neurocrit Care 19, 283–286 (2013). https://doi.org/10.1007/s12028-013-9883-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-013-9883-5