Abstract
Purpose
To determine the temporal evolution, clinical correlates, and prognostic significance of electroencephalographic (EEG) patterns in post-cardiac arrest comatose patients treated with hypothermia.
Methods
Prospective cohort study of consecutive post-anoxic patients receiving hypothermia and continuous EEG monitoring between May 2011 and June 2014 (n = 100). In addition to clinical variables, 5-min EEG clips at 6, 12, 24, 48, and 72 h after return of spontaneous circulation (ROSC) were reviewed. EEG background was classified according to the American Clinical Neurophysiological Society critical care EEG terminology. Clinical outcome at discharge was dichotomized as good [Glasgow outcome scale (GOS) 4–5, low to moderate disability] vs. poor (GOS 1–3, severe disability to death).
Results
Non-ventricular fibrillation/tachycardia arrest, longer time to ROSC, absence of brainstem reflexes, extensor or no motor response, lower pH, higher lactate, hypotension requiring >2 vasopressors, and absence of reactivity on EEG were all associated with poor outcome (all p values ≤0.01). Suppression-burst at any time indicated a poor prognosis, with a 0 % false positive rate (FPR) [95 % confidence interval (CI) 0–10 %]. All patients (54/54) with suppression-burst or a low voltage (<20 µV) EEG at 24 h had a poor outcome, with an FPR of 0 % [95 % CI 0–8 %]. Normal background voltage ≥20 µV without epileptiform discharges at any time interval carried a positive predictive value >70 % for good outcome.
Conclusions
Suppression-burst or a low voltage at 24 h after ROSC was not compatible with good outcome in this series. Normal background voltage without epileptiform discharges predicted a good outcome.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Prognostication in patients with post-anoxic brain injury continues to remain a challenge, and our limitation in addressing “the self-fulfilling prophecy” of early (and perhaps premature) withdrawal of treatment only compounds this challenge [1]. Absence of brainstem reflexes, bilateral absence of N20 responses on somatosensory evoked potentials (SSEPs), and high serum neuron specific enolase (NSE) values have all been shown to assist in prognostication, albeit with variable accuracy [2–6].
The negative prognostic value of an EEG that is unreactive to external stimulation and malignant patterns (e.g., suppression, suppression-burst, and periodic discharges) has been demonstrated in several series [4, 5, 7, 8]. However, the generalizability of these findings is compounded by the lack of standardization of the stimulation protocol used to assess reactivity, the time at which they are assessed, and the possible effects of sedation [9] and hypothermia. In addition, the prior lack of consensus in defining various EEG patterns further limited generalizability. For example, there are discrepancies between studies about the prognostic value of a suppression-burst pattern [5, 8, 10, 11]. Another controversy relates to the evolution of post-anoxic EEG findings that were found to be prominent in some studies [10], and almost absent in others [8]. This latter point is important, as the superiority and cost-effectiveness of continuous EEG (cEEG) monitoring over intermittent EEG to assess prognosis after cardiac arrest (CA) is currently debated [8, 12]. The aim of this study was to assess the prognostic significance of EEG findings scored at predetermined time intervals after ROSC using standardized terminology developed for critical care [13].
Methods
Study population
We maintain separate prospective databases of all patients undergoing cEEG and those admitted with CA. We cross-checked both databases to identify all patients between May 2011 and June 2014 who suffered from CA and underwent therapeutic hypothermia (TH) protocol and cEEG. This study was approved by the Yale Institutional Review Board, which granted a waiver of consent, given the observational nature of this study.
Standard of care
At our institution, after successful resuscitation from CA, eligible patients are placed on a TH protocol with a target core body temperature of 32–34 °C. Analgesia and sedation are provided by continuous infusion of fentanyl and midazolam. Propofol is seldom used and vecuronium is administered continuously. All patients are seen by the neurology consult team at least twice during the admission, once prior to initiation of TH protocol and later during the rewarming phase. cEEG is initiated as soon as possible upon the patient’s arrival to the ICU and maintained for at least 72 h unless the patient regains consciousness, is pronounced dead, or the decision is made to withdraw life-sustaining therapy (WLST). EEG recordings are reviewed twice daily and findings are communicated to the primary team, who makes the decisions regarding patient care in conjunction with the neurology consult team. The 72-h clinical examination is primarily used to guide prognosis. NSE, SSEPs, and imaging are not routinely obtained. The primary purpose of cEEG is to detect and treat seizures. However, this being an observational study, those making decisions to WLST were not blinded to the EEG results, so we could not mandate that it not be part of the decision-making process.
EEG scoring
Five-minute EEG clips taken at 6 (±1), 12 (±2), 24 (±2), 48 (±2), and 72 (±2) h after ROSC were reviewed. Clips were scored independently by two authors blinded to the outcome (AS and NG). Disagreements were resolved by discussion. EEG background and specific epileptiform patterns, namely periodic discharges (PD), rhythmic delta activity (RDA), spike-and-wave or sharp-and-wave (SW), and sporadic epileptiform discharges (sED), were scored according to the latest version of the American Clinical Neurophysiological Society (ACNS) critical care EEG terminology [13], which has been recently validated [14]. EEG background was divided into four categories: (a) normal voltage (>20 µV) without any epileptiform patterns; (b) normal voltage (>20 µV) with epileptiform patterns, i.e., PDs, RDA, SW, or sED (normal voltage plus); (c) suppression-burst or attenuation-burst (SB); and (d) suppression/low voltage: most or all activity ≤20 µV (LV). Additional details of the background descriptors are given in Table 1.
Reactivity
Reactivity of EEG background is formally assessed at least once daily, and is defined as any clear change in amplitude or frequency following auditory (calling the patient’s name quietly, then loudly), followed by gentle tactile stimulation, followed by noxious stimulation (nail bed pressure).
Reactivity was classified as present or absent. For the purpose of this study, patients with unclear reactivity were classified as present and those with stimulation-induced rhythmic, periodic discharges or ictal discharges (SIRPIDS) were analyzed separately.
Quantitative analysis of identical bursts
The detection of identical bursts was carried out as previously described [15]. Briefly, the onset of 50 consecutive bursts was automatically detected using an amplitude threshold in the Cz-Pz derivation of an EEG clip at 24 h after ROSC. Correlations between the shape of the first 500 ms (128 samples) of the bursts were calculated using the cross-correlation over a range of lags from −127 to 127 samples between each possible pair of bursts. The maximum value of the correlation coefficient was determined for each pairwise cross-correlation, and the mean correlation coefficient per patient was determined. All routines were implemented in Matlab (The Mathworks, Natick, MA, USA).
Clinical variables
The following variables were collected retrospectively: age, sex, time to ROSC, time from ROSC to cEEG, initial heart rhythm, brainstem reflexes, motor response of the Glasgow coma scale at 24 and 72 h after ROSC, myoclonus, lowest pH, lowest Pa/FiO2 ratio, lowest platelet count, lowest mean arterial pressure (MAP), highest lactate, creatinine and bilirubin levels, need for vasopressors, and type of sedation administered. Our primary outcome measure was best functional outcome during hospital stay up to discharge, dichotomized as good [Glasgow outcome scale (GOS) 4–5, low to moderate disability] vs. poor (GOS 1–3, severe disability to death).
Cause of death and WLST
Charts of patients with poor outcome were retrospectively and independently reviewed by two authors (DG and EG), both board-certified in neurology and neurocritical care, who were not directly involved in the care of the study patients. They ascertained whether a decision to limit treatment was taken and determined the reason for WLST, which was classified as medical, neurological, or both. Disagreements were resolved by discussion.
Statistical analysis
Variables were analyzed using the Fisher’s exact test, the χ 2 test, or the Mann–Whitney test, as appropriate. P values less than 0.05 were considered significant. We used a classification tree approach [16] to establish the multivariate model with the best accuracy to predict outcome, including predictors that showed the highest accuracy in univariate analysis. Statistical analyses were performed with R (The R Foundation, Vienna, Austria).
Results
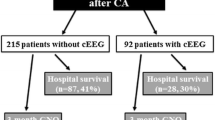
Of the 116 consecutive survivors of cardiac arrest who benefited from hypothermia, 100 patients were included in the study. Sixteen patients were excluded from analysis because of lack of EEG data at all investigated time points within 48 h; most of those were transferred from outside hospitals after that time window. Three patients had in-hospital arrests.
Clinical characteristics
Poor outcome was associated with non-ventricular fibrillation/tachycardia arrest, longer time to ROSC, absence of brainstem reflexes, extensor or no motor response, lower pH, higher lactate, lower PaO2/FiO2 ratio, and the need for more than two vasopressors (all with p values no greater than 0.01 on univariate analysis; Table 2). Of all brain stem reflexes, absence of corneal reflex at 48–72 h was best at predicting poor outcome, with 100 % specificity (95 % CI 85–100 %), 67 % sensitivity (95 % CI 53–79 %), 100 % positive predictive value (PPV) (95 % CI 88–100 %), 61 % negative predictive value (NPV) (95 % CI 45–75 %), and 0 % false positive rate (FPR) (95 % CI 0–12 %). Myoclonus was observed in 27 patients; 23 had poor outcome and all had either SB or low voltage background. Of these 23, 12 had an SB background with highly epileptiform bursts time locked to myoclonus. Four patients (15 %) had a good outcome, and all four had a normal voltage or normal voltage-plus background; 1/4 had generalized periodic discharges on a normal voltage background, which were time locked to the myoclonus. Also, 4/28 patients in the good outcome group had extensor or no motor response and all four had normal voltage or normal voltage-plus background.
Evolution of EEG patterns
SB pattern was more prevalent during the early phases of EEG monitoring (up to 24 h after ROSC) (Fig. 1). Low voltage backgrounds were more common at 6 and 72 h after ROSC, while a normal voltage-plus background was seldom seen during the early phases (less than 48 h after ROSC). These categories (low voltage and normal voltage-plus) became more frequent at 48 h or later, mostly because of the evolution of SB patterns (as shown in Supplemental Fig. 4).
EEG reactivity
Lack of background reactivity was more common in the poor outcome group; 48/61 (79 %) patients vs. 4/28 (14 %) patients in the good outcome group (p < 0.001), with 86 % specificity (95 % CI 66–95 %), 79 % sensitivity (95 % CI 66–88 %), 92 % PPV (95 % CI 81–98 %), 65 % NPV (47–79 %), and 8 % FPR (95 % CI 2–19 %) for poor outcome. Six patients had SIRPIDS, and all of them had a poor outcome.
Prognostic significance of different EEG patterns
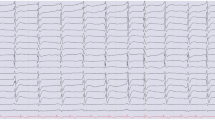
An SB pattern at any time point (17/17 at 6 h, 31/31 at 12 h, 35/35 at 24 h, 18/18 at 48 h, and 5/5 at 72 h) after ROSC was invariably associated with poor outcome (Fig. 2). A low voltage background could be associated with a good outcome (8/19 at 6 h and 2/20 at 12 h) only if present within 12 h after ROSC. All patients (19/19 at 24 h, 19/19 at 48 h, and 16/16 at 72 h) with a suppression/low voltage background at 24 h after ROSC (i.e., during and after the rewarming period) had a poor outcome. Three patients had non-convulsive status epilepticus (NCSE) and three had non-convulsive seizures; all had a poor outcome. Patients with NCSE were treated with escalating doses of midazolam in addition to three antiepileptic drugs (AED) and those with seizures were placed on an AED.
Prognostic significance of a pattern based on when it is seen after return of spontaneous circulation (ROSC). For example, if suppression/low voltage (LV) is seen at 6 h, the probability of poor outcome is 58 %; but if the same pattern is present at 24 h or later, then the chance of a poor outcome is 100 %. The total number of patients at each time point is indicated at the bottom of the figure
A normal voltage background without epileptiform discharges, even if discontinuous, was associated with a good outcome in more than 70 % of patients (8/11 at 6 h, 23/27 at 12 h, 23/32 at 24 h, 15/19 at 48 h, and 5/6 at 72 h), regardless of when it was noted after ROSC. A majority of patients in the normal voltage group had a continuous background (10/11 at 6 h, 25/27 at 12 h, 30/32 at 24 h, 18/19 at 48 h, and 6/6 at 72 h). A normal voltage-plus background was more common at 48 h and was associated with a poor outcome in most patients, if observed for the first time after 24 h (13/16 at 48 h and 7/9 at 72 h). In the 13/16 patients with poor outcome at 48 h, an SB pattern was present at 12 and 24 h in ten patients, and none of them previously had a normal voltage background without epileptiform discharges.
The EEG findings at 24 h after ROSC (N = 89 patients) showed the best accuracy for the prediction of outcome, with all (54/54) patients showing SB or low voltage on EEG having a poor outcome at discharge (as shown in Supplemental Table 3); this had a specificity of 100 % (95 % CI 84–100 %), 84 % sensitivity (95 % CI 73–92 %), 100 % PPV (95 % CI 92–100 %), 72 % NPV (95 % CI 53–85 %), and 0 % FPR (95 % CI 0–8 %). A normal voltage background without epileptiform patterns was associated with a good outcome in 23/32 (73 %) cases vs. 2/57 (4 %) in patients with other patterns, giving a specificity of 97 % (95 % CI 87–99 %), 72 % sensitivity (95 % CI 53–86 %), 92 % PPV (95 % CI 72–99 %), 86 % NPV (95 % CI 74–93 %), and 8 % FPR (95 % CI 1–27 %). EEG findings at 24 h were associated with decision to WLST (but not specifically used for that purpose) with a sensitivity of 83 % (95 % CI 69–92 %) and specificity of 66 % (95 % CI 49–80 %) but so were extensor or worse motor response [sensitivity of 88 % (95 % CI 73–95 %) and specificity of 54 % (95 % CI 37–70 %)] and absence of corneal reflex [sensitivity of 59 % (95 % CI 41–76 %) and specificity of 73 % (95 % CI 54–86 %)] to predict WLST. Of 33 patients at 24 h with poor neurological prognosis as the reason for WLST and low voltage or suppression-burst pattern, only one patient had normal brainstem reflexes and motor response better than extensor. Her brain imaging showed signs of diffuse anoxic injury.
Identical bursts
Of the 29 patients with a suppression-burst pattern at 24 h after ROSC, all of whom had poor outcome, 27 showed identical bursts with a mean correlation coefficient greater than 0.75. The two others had a correlation coefficient of 0.65 and 0.58, respectively. Six of 35 patients in the SB group at 24 h showed generalized periodic discharges on a suppressed background and were excluded from this analysis.
Clinical correlates
At 24 h after ROSC, when compared with patients having normal voltage or normal voltage-plus background, patients with low voltage or SB background had a lower PaO2/FiO2 [114 (85–195) vs. 179 (148–277) mmHg; p < 0.001], a lower pH [7.17 (7.08–7.26) vs. 7.30 (7.22–7.37); p < 0.001], higher lactate levels [6.95 (3.5–10.3) vs. 3.6 (2.2–7.3); p = 0.02], and a lower MAP [51 (43–64) vs. 66 (58–75) mmHg; p < 0.001]. This pattern was also associated with absence of brainstem reflexes on admission [29/52 (56 %) vs. 4/34 (12 %); p < 0.001] and at 48–72 h after ROSC [27/43 (63 %) vs. 0/31 (0 %); p < 0.001], and with extensor or no motor response at 48–72 h post ROSC [48/52 (92 %) vs. 9/34 (26 %); p < 0.001].
Mortality adjudication
In patients with poor outcome (n = 71), seven were pronounced brain dead, six patients re-arrested and could not be resuscitated, and the cause of death could not be ascertained because of insufficient information in ten patients. Therapeutic limitations were undertaken in 48 patients; treatment was limited because of a poor neurological prognosis in 35 patients, poor medical prognosis in seven patients, and a combination of poor medical and poor neurological prognosis in six patients. Of these 48 patients, 25 died within 72 h from the time of ROSC; 15/25 with poor neurological prognosis, 6/25 with poor medical prognosis, and 4/25 with poor medical and neurological prognosis. Clinical examination findings at 72 h were available in 9/15 patients with poor neurological prognosis, out of which only one patient had normal brainstem reflexes and motor response better than extensor. Her brain imaging showed signs of diffuse anoxic injury.
Multimodality outcome prediction
We attempted to build a classification tree using EEG pattern at 24 h and corneal, pupillary reflexes and motor response at 72 h (N = 74). In this tree, EEG pattern was the parental node and the corneal reflex was selected as a daughter node (Fig. 3). The tree achieved a slightly better accuracy than EEG pattern alone, with a sensitivity of 86 % (95 % CI 73–94 %), a specificity of 100 % (95 % CI 83–100 %), and an FPR of 0 % (95 % CI 0–10 %) for poor outcome. Trees built using other combinations of variables showed lower accuracy (data not shown).
Classification tree for good outcome based on the best combination of predictors (EEG pattern at 24 h and presence of corneal reflexes at 72 h) in 74 patients with a full data set available. The tree achieved a slightly better accuracy than EEG pattern or corneal reflexes alone (see text for details)
Discussion
The key finding was that a low voltage or an SB pattern at 24 h was invariably associated with poor outcome. This confirms most previous studies and strengthens the value of these patterns as strong predictors of outcome [4, 5, 7, 8]. In particular, an SB pattern was invariably associated with poor outcome, regardless of the latency between ROSC and this EEG finding. This last point contradicts other studies that have shown that an early suppression-burst pattern (i.e., one that resolves within 24 h after ROSC) can still be associated with a good outcome [10, 17]. One possible explanation is that our definition of suppression-burst differed from that used in these prior studies [5, 8, 10]. Almost all patients in the literature with well-defined burst-suppression and good outcome [10] received propofol as a sedating agent, whereas almost all patients received midazolam in our series, as well as in other studies that also found suppression-burst associated with uniformly unfavorable outcome [5, 8]. This suggests that propofol may induce a suppression-burst pattern during hypothermia that does not necessarily imply an unfavorable prognosis. A variant of suppression-burst with identical bursts has been described [15] that is invariably associated with poor outcome, and propofol was used for sedation in this group. Most of the suppression-burst patterns in our series were characterized by identical bursts, confirming the association between severe anoxic brain injury and this potentially specific pattern. These findings support the hypothesis that suppression-burst with non-identical bursts is likely induced by sedation with propofol in this population and has less prognostic utility.
We also found that a low voltage background was not invariably associated with poor outcome if it resolved by 24 h after ROSC. This is consistent with prior studies [10, 11]. Our findings suggest that EEG patterns at 24 h after ROSC are most accurate for predicting outcome (although additional information could be gained from earlier recordings in a few cases) and performed better than predictors based on clinical examination in this series. However, combining EEG and clinical examination in the classification tree resulted in a slightly more accurate prediction.
Prognostication based on standardized classification of the background performed better in our series than using EEG reactivity to external stimulation, including having a lower FPR for poor outcome (0 vs. 8 %). This imperfect specificity of reactivity assessment is in line with prior studies [5, 18] and probably reflects both the lack of a clear definition of reactivity and the confounding effect of sedation [9] and hypothermia. The strengths of our study include the use of a standardized and validated EEG terminology, the systematic review of the EEG at fixed time intervals after ROSC by two independent readers blinded to the patients’ outcome, the assessment of therapeutic limitations by two authors who were not directly involved in the care of these patients, and the exploration of the relationship between EEG, physiological variables, and outcome.
The limitations of this study include retrospective collection of physiological variables and outcomes, leading to some missing data. We did not have data on SSEPs or NSE as those were not part of the protocol during this time period. Myoclonus was noted less than 72 h after ROSC, but the exact time of its appearance and further clinical description could not be ascertained. Reactivity was not assessed at specific time points; time to CEEG onset was variable and depended on the availability of EEG machines. Also, EEG data was unavailable at some time points in a few cases, as patients were transiently disconnected and some patients EEGs were discontinued within 72 h, mostly because they died or awakened. As with most studies, we were not able to fully account for the effect of self-fulfilling prophecy of early WLST; 25 patients died within 72 h from the time of ROSC and 15 of these patients were presumed to have a poor neurological prognosis. Of note, all patients with a low voltage or suppression-burst pattern at 24 h in which a decision to WLST was made had at least one other predictive factor of poor outcome (absent brainstem reflexes, extensor motor response, or worse or severely abnormal imaging), indicating that if EEG was used for prognostication, it was never in isolation.
In conclusion, we found that (1) a suppression-burst or attenuation-burst pattern was invariably associated with poor outcome, regardless of when it was observed after ROSC; (2) suppression (<10 µV) or low voltage activity (<20 µV) was not always associated with poor outcome if seen at less than 24 h after ROSC but was invariably associated with a poor outcome if seen at 24 h or later; (3) a normal voltage background without any epileptiform discharges carries at least a 70 % chance of good outcome at all time points after ROSC; (4) severely abnormal EEG background (SB/low voltage) was associated with poor physiological variables; and (5) myoclonus, extensor, and no motor response were not always associated with poor outcome. Importantly, all eight patients with good outcome despite having myoclonus or extensor/no motor response had a normal voltage or normal voltage-plus EEG background at all time points, which suggests additional prognostic value of EEG findings over clinical exam.
This is a single-center study done at our institution, which has been using ACNS ICU EEG terminology and it has been our standard of reporting on ICU-EEG studies for the last 3 years. It is crucial to reproduce our findings, in a multicenter setting, with a larger sample size and using the standardized terminology.
References
Taccone F, Cronberg T, Friberg H, Greer D, Horn J, Oddo M, Scolletta S, Vincent JL (2014) How to assess prognosis after cardiac arrest and therapeutic hypothermia. Crit Care 18:202
Wijdicks E, Hijdra A, Young G, Bassetti C, Weibi S (2006) Practice parameter: prediction of outcome in comatose survivors after cardiopulmonary resuscitation (an evidence-based review): report of the quality standards subcommittee of the American Academy of Neurology. Neurology 67:203–210
Thenayan E, Savard M, Sharpe M, Norton L, Young B (2008) Predictors of poor neurologic outcome after induced mild hypothermia following cardiac arrest. Neurology 71:1535–1537
Rossetti A, Oddo M, Logroscino G, Kaplan P (2010) Prognostication after cardiac arrest and hypothermia: a prospective study. Ann Neurol 67:301–307
Rossetti A, Urbano L, Delodder F, Kaplan P, Oddo M (2010) Prognostic value of continuous EEG monitoring during therapeutic hypothermia after cardiac arrest. Crit Care 14:R173
Oddo M, Rossetti A (2011) Predicting neurological outcome after cardiac arrest. Curr Opin Crit Care 17:254–259
Rossetti A, Carrera E, Oddo M (2012) Early EEG correlates of neuronal injury after brain anoxia. Neurology 78:796–802
Crepeau A, Rabinstein A, Fugate J, Mandrekar J, Wijdicks EF, White R, Britton J (2013) Continuous EEG in therapeutic hypothermia after cardiac arrest: prognostic and clinical value. Neurology 80:339–344
Gilmore E, Gaspard N, Choi HA, Cohen E, Burkart KM, Chong DH, Claassen J, Hirsch LJ (2015) Acute brain failure in severe sepsis: a prospective study in the medical intensive care unit utilizing continuous EEG monitoring. Intensive Care Med 41:686–964
Cloostermans M, Meulen F, Eertman C, Hom H, Van Putten MJAM (2012) Continuous electroencephalography monitoring for early prediction of neurological outcome in postanoxic patients after cardiac arrest. Crit Care Med 40:2867–2875
Rundgren M, Westhall E, Cronberg T, Rosén I, Friberg H (2010) Continuous amplitude-integrated electroencephalogram predicts outcome in hypothermia-treated cardiac arrest patients. Crit Care Med 38:1838–1844
Alvarez V, Sierra-Marcos A, Oddo M, Rossetti AO (2013) Yield of intermittent versus continuous EEG in comatose survivors of cardiac arrest treated with hypothermia. Crit Care 17:R190
Hirsch LJ, Laroche SM, Gaspard N, Gerard E, Svoronos A, Herman ST, Mani R, Arif H, Jette N, Minazad Y, Kerrigan JF, Vespa P, Hantus S, Claassen J, Young GB, So E, Kaplan PW, Nuwer MR, Fountain NB, Drislane FW (2013) American Clinical Neurophysiology Society’s standardized critical care EEG terminology: 2012 version. J Clin Neurophysiol 30:1–27
Gaspard N, Hirsch LJ, Laroche SM, Hahn CD, Westover MB (2014) Interrater agreement for critical care EEG terminology. Epilepsia 55:1366–1373
Hofmeijer J, Tjepkema-Cloostermans M, Putten M (2014) Burst-suppression with identical bursts: a distinct EEG pattern with poor outcome in postanoxic coma. J Clin Neurophysiol 125:947–954
Greer DM, Yang J, Scripko PD, Sims JR, Cash S, Kilbride R, Wu O, Hafler JP, Schoenfeld DA, Furie KL (2012) Clinical examination for outcome prediction in nontraumatic coma. Crit Care Med 40:1150–1156
Wennervirta JE, Ermes JM, Tianen MS, Salmi TK, Hynninen MS, Sarkela MOK, Hynynen MJ, Stenman UH, Viertio HE, Saastamoinen KP, Pettila VY, Vakkuri AP (2009) Hypothermia-treated cardiac arrest patients with good neurological outcome differ in early quantitative variables of EEG suppression and epileptiform activity. Crit Care Med 37:2427–2435
Oddo M, Rossetti A (2014) Early multimodal outcome prediction after cardiac arrest in patients treated with hypothermia. Crit Care Med 42:1340–1347
Conflicts of interest
The authors declare that they have no conflict of interest.
Disclosures
Adithya Sivaraju, MBBS, MHA: Dr. Sivaraju reports no disclosures. Emily Gilmore, MD: Dr. Gilmore is supported by Yale’s Center for Clinical Investigaton’s CTSA Grant (ULTR000142) and Yale’s Claude D. Pepper Older Americans Independence Center (P30AG021342 NIH/NIA). Charles R. Wira, MD: Dr. Wira reports no disclosures. Anna Stevens, MD, PhD: Dr. Stevens reports no disclosures. Nishi Rampal, MD: Dr. Rampal reports no disclosures. Jeremy J. Moeller, MD: Dr. Moeller has received royalties or payments from UpToDate, Inc., NeuroSeriesLive, QuantiaMD, and the Canadian Pharmacists’ Association. David M. Greer, MD: Dr. Greer reports no disclosures. Lawrence J. Hirsch, MD: Dr. Hirsch has received (a) research support to Yale for investigator-initiated studies from UCB-Pharma, Upsher-Smith, Lundbeck, Eisai, and Sunovion; consultation fees for advising from Lundbeck, Upsher-Smith, Neuropace, and Allergan; (b) honoraria for speaking from Natus and Neuropace; (c) royalties for authoring chapters for UpToDate-Neurology, and from Wiley for co-authoring the book Atlas of EEG in Critical Care, by Hirsch and Brenner, 2010.Nicolas Gaspard MD, PhD: Dr. Gaspard is a Clinical Master Specialist of the Belgian Fund for Scientific Research (FNRS) and received royalties for authoring chapters for UpToDate-Neurology. Dr. Sivaraju and Dr. Gaspard had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Selected findings of the manuscript were presented as a poster and platform presentation at the American Clinical Neurophysiological Society Meeting; February 7, 2015; Houston, Texas.
Author information
Authors and Affiliations
Corresponding author
Additional information
Take-home message: EEG findings at 24 h after resuscitation from cardiac arrest provide important prognostic informations: a suppression-burst pattern or a low-voltage background is irremediably associated with poor outcome, while a normal voltage background without periodic or epileptiform discharges is associated with good outcome.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sivaraju, A., Gilmore, E.J., Wira, C.R. et al. Prognostication of post-cardiac arrest coma: early clinical and electroencephalographic predictors of outcome. Intensive Care Med 41, 1264–1272 (2015). https://doi.org/10.1007/s00134-015-3834-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-015-3834-x