Abstract
Background
Lack of electroencephalography (EEG) background reactivity during therapeutic hypothermia (TH) has been associated with poor outcome in post-anoxic comatose patients. However, decision on intensive care withdrawal is based on normothermic (NT) evaluations. This study aims at exploring whether patients showing recovery of EEG reactivity in NT after a non-reactive EEG in TH differ from those remaining non-reactive.
Methods
Patients with non-reactive EEG during TH were identified from our prospective registry of consecutive comatose adults admitted after successful resuscitation from CA between April 2009 and June 2014. Variables including neurological examination, serum neuron-specific enolase (NSE), procalcitonin, and EEG features were compared regarding impact on functional outcome at 3 months.
Results
Seventy-two of 197 patients (37 %) had a non-reactive EEG background during TH with thirteen (18 %) evolving towards reactivity in NT. Compared to those remaining non-reactive (n = 59), they showed significantly better recovery of brainstem reflexes (p < 0.001), better motor responses (p < 0.001), transitory consciousness improvement (p = 0.008), and a tendency toward lower NSE (p = 0.067). One patient recovering EEG reactivity survived with good functional outcome at 3 months.
Conclusions
Recovery of EEG reactivity from TH to NT seems to distinguish two patients’ subgroups regarding early neurological assessment and transitory consciousness improvement, corroborating the role of EEG in providing information about cerebral functions. Understanding these dynamic changes encourages maintenance of intensive support in selected patients even after a non-reactive EEG background in TH, as a small subgroup may indeed recover with good functional outcome.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Coma after cardiac arrest (CA) represents a severe condition with very uncertain outcome. In order to assist the clinician to perform early prediction on chances of survival and help in the decision of maintaining intensive life support, multimodal evaluations including brainstem reflexes. Motor response to painful stimuli, early myoclonus, electroencephalography (EEG), somatosensory evoked potentials (SSEP), and serum biomarkers [especially neuron-specific enolase (NSE)] are part of current recommendations [1–5]. Furthermore, serum procalcitonin (PCT) has been recently described as a marker of post-resuscitation illness correlating with final outcome [6].
In the last decade, therapeutic hypothermia (TH) has been increasingly used in this clinical setting [7–9], although the exact parameters of temperature management have been recently challenged [10]. While it is important to try to predict as soon as possible the patient’s outcome, TH itself and sedation have been shown to impact on the prognostic assessment [11, 12]. Current guidelines taking into account TH treatment recommend to assess prognosis multimodally at more than 72 h after CA onset, once the patient is back in normothermia (NT) and off sedation [13, 14].
Although clinical evaluation may be delayed with TH treatment, EEG is widely available, non-invasive, and seems to represent a relatively robust predictor, especially if recorded after at least 9–12 h following CA, particularly in terms of background reactivity [15–17]. Recently, we showed that despite a reactive EEG in TH, a discontinuous EEG and high NSE [but not serum procalcitonin (PCT)] correlate with mortality [18].
In order to further explore the dynamic role of EEG as a biological and prognostic marker in this clinical setting, we assessed the evolution of EEG features and clinical outcome in patients lacking background reactivity in TH. We hypothesized that return of EEG reactivity over time would define a subgroup of patients with a different clinical profile.
Methods
Patients and Procedures
We considered all patients from our prospective registry of consecutive comatose adults admitted between April 2009 and June 2014 to the Department of Intensive Care Medicine after successful resuscitation from CA. We included patients with both EEG recordings during TH and NT, selecting only those with a non-reactive EEG background in TH. All subjects were treated using a standardized protocol in agreement with current guidelines [9, 15] with mild TH to 33 °C maintained for 24 h; midazolam (0.1 mg/kg/h) and fentanyl (1.5 µg/kg/h) were given for sedation-analgesia, and vecuronium (0.1 mg/kg boluses) to control shivering. All data were collected prospectively. This study received full approval from the Ethic Commission of our hospital.
Clinical and Laboratory Variables
Neurological examination testing brainstem reflexes (pupillary, oculocephalic, corneal; all present vs. one or more absent), motor response to painful stimuli (flexion posturing or better vs. extension or no response) and myoclonus occurrence was performed repetitively after rewarming and up to 72 h after CA; the best evaluation was considered for this analysis. Serum NSE at 24 h and/or 48 h after CA was analyzed with an automated immunofluorescent assay (Thermo Scientific Brahms NSE Kryptor Immunoassay); the highest value was selected for this analysis. Serum PCT was sampled during TH using the ELFA method (Vidas Brahms PCT assay, bioMerieux Inc., Geneva, Switzerland; see [6]). Response to median-nerve SSEP was recorded 24–72 h after CA, after rewarming [15].
EEG Recordings
Video-EEGs (Viasys Neurocare, Madison, WI, USA) were recorded for 20–30 min using a 21-electrodes montage according to the international 10–20 system. For each patient, two EEG recordings were performed, the first time early after coma onset, during TH (range 2–36 h after CA; temperature at 33–34 °C), the second time after rewarming over 35 °C and most often after sedation weaning (range 24–72 h after CA). EEG activity was visually interpreted, before knowing the clinical outcome, by one experienced EEG-certified neurologist (AOR or JN) on three dimensions (see [18] for details): (1) background reactivity, categorized as present if clear and reproducible change in amplitude or frequency in the background occurred in reaction to stimulations, excluding muscle artifacts or stimulus-induced rhythmic, periodic, or ictal discharges (SIRPIDs); (2) spontaneous discontinuous pattern, defined as an interruption of the EEG background by flat periods during >10 % of the recording [19]; (3) epileptiform activity. In case of doubt about the qualification of the signal, the second EEG-certified neurologist was consulted and a consensus found.
Decisions on Intensive Care Withdrawal and Outcome Assessment
Intensive care withdrawal was discussed interdisciplinary within 7 days of CA, and based on the occurrence of two or more of: (1) unreactive EEG background in normothermia, (2) incomplete recovery of brainstem reflexes, (3) early myoclonus resistant to treatment, (4) bilateral absence of N20 cortical potential in SSEP tested in normothermia [20]. Importantly, EEG evaluation in hypothermia, NSE, and PCT values were not considered for this decision. Outcome was assessed at 3 months by a semi-structured phone interview and categorized according to the Cerebral Performance Categories (CPC; [21]).
Statistical Analysis
Two-sided Fisher and Wilcoxon tests were used to explore relationships between patients recovering EEG reactivity after return to NT versus those remaining without reactivity. Significance was set at p < 0.05, without correction for multiple comparisons, given the exploratory character of this study. Calculations were performed with Stata software, version 12 (College Station, TX).
Results
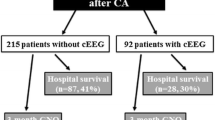
During the study period, 246 patients were admitted for CA and treated with TH but 49 were excluded for lack of EEG evaluation during TH (Fig. 1). Among the resulting 197 patients, 72 (37 %) had a non-reactive EEG in TH, with 59 (82 % of them) remaining non-reactive during NT and 13 (18 %) turning to be reactive in NT. Overall, outcome was poor, as 71/72 patients died (CPC 5). Demographic and clinical variables are summarized in Table 1. Groups did not differ according to age, sex, etiology, and latency to the first EEG in TH. Significant differences between groups with non-reactive EEG (NTnrEEG) versus reactive EEG (NTrEEG) in NT were found on the proportion of patients having absent brainstem reflexes (p < 0.001) and absent motor response (p < 0.001). Although not significant, there was a strong tendency concerning lower NSE values in the NTrEEG group (p = 0.067). The proportion of patients recovering partial consciousness (vegetative state or better) at least transiently was significantly higher in the NTrEEG group compared to the NTnrEEG group (p < 0.01).
Latency between cardiac arrest and death was longer in the NTrEEG group (p < 0.001), but the proportion of patients having residual pharmacological sedation during EEG recording in NT was not significantly different between both groups. In order to assess whether sedation had an influence on these results, we repeated calculations without the four patients sedated in NTnrEEG group (n = 55) and the three sedated in the NTrEEG group (n = 10). Concerning brainstem reflexes, the difference was still significant (respectively, 87 % for NTnrEEG group and 30 % for NTrEEG group; p < 0.001), as well as for motor response (respectively, 98 and 60 %; p = 0.001), the best level of consciousness achieved (respectively, 4 and 30 %; p = 0.02), and death latency (respective medians [range] in days: 3 [1–12] and 7 [4–11]; p < 0.001).
The only survivor of this cohort without a reactive EEG in TH belonged to the NTrEEG group. This 80-year-old man had CA caused by ventricular fibrillation of a cardiac etiology, and 25 min to a return of spontaneous circulation (ROSC); his EEG in TH was non-reactive, discontinuous, without epileptiform transients, and became reactive but still discontinuous in NT; brainstem reflexes were all present, motor response was obtained and no myoclonus was observed; NSE peak value was 17.3 µg/l and PCT 0.06 µg/l. At 3 months, this man was considered having a good functional outcome (CPC 2: moderate disability).
Conclusions
This prospective study shows that patients with a reactive EEG in normothermia after a non-reactive hypothermic EEG tend to recover brainstem reflexes and better motor reactions to pain, than those remaining with a non-reactive recording. Outcome did not differ significantly in term of survival between the two groups; however, those with a reactive EEG background in NT had a greater chance to reach at least transiently a vegetative state, and one patient even awoke and survived at 3 months with good functional outcome.
Our results suggest that recovery of EEG background reactivity in NT parallels at least partial clinical recovery of cerebral functions. On the one side, motor response reflects the integrated function of brainstem and higher cerebral structures; in fact, the cut-off in the motor GCS scoring is extension posturing or worse, defining at best a decerebrate state [22, 23], while, on the other side, pupillary, oculocephalic, and corneal reflexes reveal activity of brainstem function. Since EEG signals arise from the cerebral cortex, and are more sensitive to cortico-cortical connections than SSEP [24], EEG reactivity may reflect the dynamic integration of the cortex with the underlying structures. Despite this, however, clinical improvement was limited and transitory in most cases, pointing to the fact that EEG reactivity during TH and under relatively standard conditions, including sedation, may inform more accurately than in NT on the general “tuning” resulting from neuronal injury [25].
Serum NSE values tended to be lower in the group becoming reactive in NT, suggesting that the extent of neuronal damage is somewhat lower in this group of patients [25, 26]. However, overall, the median values were higher as compared to the previous study focusing on patients with an initial reactive EEG background in TH [18], and clearly above the threshold of 33 µg/l [4], corroborating the hypothesis that the presently studied cohort (having a non-reactive EEG in TH) had a more severe brain damage from the beginning. PCT correlates with systemic post-resuscitation illness [6], but was not different here; again, it does not seem that EEG, at least in this clinical setting, mirrors the extent of systemic illness. SSEP, reflecting the integrity of thalamocortical projections using the N20 response, is an indicator of poor outcome [24]; however, knowing that its sensitivity is very low, the absence of difference in our setting is not surprising.
This study has some limitations: first, the number of patients included is relatively limited and only few belonged to the group with reactive EEG background in NT; however, these numbers reflect the analysis of a large cohort of 197 patients recorded consecutively over more than 5 years. Second, some few data were missing concerning PCT, NSE, and SSEP, but this reflects a common problem in observational studies related to availability of selected investigations during weekends and holidays. Third, this study was carried out in one single center, possibly limiting generalization of our results; however, we believe that this allows a better internal validity. Fourth, the fact that description of EEG activity was performed by two different neurologists could be a source of bias; however, we recently [27] showed that correlation among readers for reactivity has a reasonable agreement, which may be especially true in this particular setting as JN was trained by AOR and the two collaborate closely since many years. Moreover, the choice to use standard EEG and not continuous EEG should not impact on the validity of results, as the added value of the latter method was reported mainly for a marginal higher detection of epileptic seizures, without any change in outcome [28, 29] or assessment of reactivity, particularly during normothermia [16]. Conversely, the prospective nature of data ascertainment and the multimodal approach, including not only clinical and electrophysiological parameters but also biological markers, as well as the assessment of functional outcome at 3 months, strengthen in our view the results. Finally, self-fulfilling prophecy is always a risk in this type of studies, and as decision of intensive care withdrawal was based especially on reactive EEG background during NT, it seems logical that longer death latencies were found in the group converting to a reactive EEG background.
To our knowledge, this study represents the first attempt to better characterize the clinical profile of post-anoxic comatose patients having an initial poor electrophysiological assessment (non-reactive EEG in hypothermia). We show that although the initial clinical presentation of these patients seems homogenous, the evolution of EEG background reactivity in NT distinguishes two subgroups with different clinical profiles. By outlining differences between these two groups concerning both early clinical variables and outcome, our results encourage the maintenance of intensive life support in case of EEG background reactivity recovery in NT even after a non-reactive EEG background in TH, especially when this recovery appears along with improved clinical signs, as a few patients may indeed recover.
References
Friberg H, Cronberg T. Prognostication after cardiac arrest. Best Pract Res Clin Anaesthesiol. 2013;27(3):359–72.
Sandroni C, Cavallaro F, Callaway CW, et al. Predictors of poor neurological outcome in adult comatose survivors of cardiac arrest: a systematic review and meta-analysis. Part 2: Patients treated with therapeutic hypothermia. Resuscitation. 2013;84(10):1324–38.
Sandroni C, Cavallaro F, Callaway CW, et al. Predictors of poor neurological outcome in adult comatose survivors of cardiac arrest: a systematic review and meta-analysis. Part 1: Patients not treated with therapeutic hypothermia. Resuscitation. 2013;84(10):1310–23.
Wijdicks EFM, Hijdra a, Young GB, Bassetti CL, Wiebe S. Practice parameter: prediction of outcome in comatose survivors after cardiopulmonary resuscitation (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2006;67(2):203–10.
Fugate JE, Wijdicks EFM, Mandrekar J, et al. Predictors of neurologic outcome in hypothermia after cardiac arrest. Ann Neurol. 2010;68(6):907–14.
Engel H, Ben Hamouda N, Portmann K, et al. Serum procalcitonin as a marker of post-cardiac arrest syndrome and long-term neurological recovery, but not of early-onset infections, in comatose post-anoxic patients treated with therapeutic hypothermia. Resuscitation. 2013;84(6):776–81.
The Hypothermia after Cardiac Arrest Study Group. Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med. 2002;346(8):549–56.
Bernard S, Gray T, Buist M, et al. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med. 2002;346(8):557–63.
Oddo M, Schaller M-D, Feihl F, Ribordy V, Liaudet L. From evidence to clinical practice: Effective implementation of therapeutic hypothermia to improve patient outcome after cardiac arrest. Crit Care Med. 2006;34(7):1865–73.
Nielsen N, Wetterslev J, Cronberg T, et al. Targeted temperature management at 33 °C versus 36 °C after cardiac arrest. N Engl J Med. 2013;369(23):2197–206.
Horn J, Cronberg T, Taccone FS. Prognostication after cardiac arrest. Curr Opin Crit Care. 2014;20(3):280–6.
Stub D, Bernard S, Duffy SJ, Kaye DM. Post cardiac arrest syndrome: a review of therapeutic strategies. Circulation. 2011;123(13):1428–35.
Greer DM, Rosenthal ES, Wu O. Neuroprognostication of hypoxic-ischaemic coma in the therapeutic hypothermia era. Nat Rev Neurol. 2014;10(4):190–203.
Cronberg T, Brizzi M, Liedholm L, et al. Neurological prognostication after cardiac arrest—recommendations from the Swedish Resuscitation Council. Resuscitation. 2013;84:867–72.
Oddo M, Rossetti AO. Early multimodal outcome prediction after cardiac arrest in patients treated with hypothermia. Crit Care Med. 2014;42(6):1340–7.
Alvarez V, Sierra-Marcos A, Oddo M, Rossetti AO. Yield of intermittent versus continuous EEG in comatose survivors of cardiac arrest treated with hypothermia. Crit Care. 2013;17(5):R190.
Thenayan EL, Savard M, Sharpe MD, Norton L, Young B. Electroencephalogram for prognosis after cardiac arrest. J Crit Care. 2010;25(2):300–4.
Tsetsou S, Oddo M, Rossetti AO. Clinical outcome after a reactive hypothermic EEG following cardiac arrest. Neurocrit Care. 2013;19(3):283–6.
Hirsch LJ, LaRoche SM, Gaspard N, et al. American Clinical Neurophysiology Society’s Standardized Critical Care EEG Terminology: 2012 version. J Clin Neurophysiol. 2013;30(1):1–27.
Rossetti AO, Oddo M, Logroscino G, Kaplan PW. Prognostication after cardiac arrest and hypothermia: a prospective study. Ann Neurol. 2010;67(3):301–7.
Booth CM, Boone RH, Tomlinson G, Detsky AS. Is this patient dead, vegetative, or severely neurologically impaired? Assessing outcome for comatose survivors of cardiac arrest. J Am Med Assoc. 2004;291(7):870–9.
Posner J, Sapper C, Schiff N, Plum F. Plum and Posner’s diagnosis of stupor and coma. 4th ed. New York: Oxford University Press; 2007.
Jennett B, Teasdale G. Aspects of coma after severe head injury. Lancet. 1977;1(8017):878–81.
Van Putten MJAM. The N20 in post-anoxic coma: are you listening? Clin Neurophysiol. 2012;123(7):1460–4.
Rossetti AO, Carrera E, Oddo M. Early EEG correlates of neuronal injury after brain anoxia. Neurology. 2012;78(11):796–802.
Cronberg T, Rundgren M, Westhall E, et al. Neuron-specific enolase correlates with other prognostic markers after cardiac arrest. Neurology. 2011;77(7):623–30.
Noirhomme Q, Lehembre R, LugoZdel R, et al. Automated analysis of background EEG and reactivity during therapeutic hypothermia in comatose patients after cardiac arrest. Clin EEG Neurosci. 2014;45:6–13.
Crepeau AZ, Fugate JE, Mandrekar J, et al. Value analysis of continuous EEG in patients during therapeutic hypothermia after cardiac arrest. Resuscitation. 2014;85(6):785–9.
Crepeau AZ, Rabinstein A, Fugate JE, et al. Continuous EEG in therapeutic hypothermia after cardiac arrest: prognostic and clinical value. Neurology. 2013;80(4):339–44.
Acknowledgments
The authors thank Christine Staehli (RN), the EEG fellows and technologists, and the ICU fellows and nurses for their help in data collection. The Swiss National Science Foundation provides financial support to AOR and EJ (CR3213_143780) and MO (320030_138191).
Disclosures
The authors declare that they have no other conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Juan, E., Novy, J., Suys, T. et al. Clinical Evolution After a Non-reactive Hypothermic EEG Following Cardiac Arrest. Neurocrit Care 22, 403–408 (2015). https://doi.org/10.1007/s12028-014-0095-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-014-0095-4