Abstract
Purpose
Statins are the mainstay of treatment for patients with familial hypercholesterolaemia (FH). However, their efficacy and safety in children and adolescents with FH has not been well-documented. The purpose of this study was to systematically investigate and meta-analyze the best available evidence from randomized-controlled trials (RCTs) regarding the efficacy and safety of statins in this population.
Methods
A comprehensive search was conducted in PubMed, Scopus and Cochrane, up to 10 January 2020. Data were expressed as mean differences with 95% confidence intervals (CI). The I2 index was employed for heterogeneity.
Results
Ten RCTs were included in the qualitative and quantitative analysis (1191 patients, aged 13.3 ± 2.5 years). Compared with placebo, statins led to a mean relative reduction in total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), triglyceride and apolipoprotein B (apo-B) concentrations by −25.5% (95% CI −30.4%, −20.5%; I2 91%), −33.8% (95% CI −40.1%, −27.4%; I2 90%), −8.4% (95% CI −14.8%, −2.03%; I2 26%) and −28.8% (95% CI −33.9%, −23.6%; I2 83%), respectively. High-density lipoprotein cholesterol (HDL-C) was increased by 3.1% (95% CI 1.1%−5.2%; I2 0%). Statins were well-tolerated, with no significant differences in transaminase and creatine kinase levels or other adverse effects compared with placebo. Statins exerted no effect on growth or sexual development.
Conclusion
Statins are quite effective in reducing TC, LDL-C, TG and apo-B and increasing HDL-C concentrations in children and adolescents with FH. No safety issues were seen with statin use.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Familial hypercholesterolaemia (FH) is the most common monogenic genetic disorder, inherited in an autosomal dominant way and characterized by elevated low-density lipoprotein (LDL) cholesterol (LDL-C) concentrations [1, 2]. Most cases are due to mutations in LDL-receptor (79%), apolipoprotein B (5%) and proprotein convertase subtilisin/kexin type 9 (<1%) genes [1]. FH, in its heterozygous type (HeFH), affects ~1:200–1:300 people worldwide, with most LDL-C concentrations varying between 190 (4.9 mmol/L) and 500 mg/dL (12.9 mmol/L). Homozygous FH (HoFH) is much rarer, affecting 1 in 160,000 to 300,000 individuals, with LDL-C exceeding >500 mg/dL (12.9 mmol/L) [2]. Paediatric patients with LDL-C>190 mg/dL (4.9 mmol/L) or >160 mg/dL (4.1 mmol/L) in combination with family history of premature coronary heart disease and/or high baseline LDL-C concentrations in one parent, provide evidence for the phenotypic diagnosis of FH [1]. The LDL-C threshold for FH diagnosis in cases of confirmed genetic mutation in one parent is 130 mg/dL (3.4 mmol/L) [1, 3, 4].
Prolonged exposure to high LDL-C or other lipids, such as lipoprotein (a) [Lp(a)] concentrations, which are frequently elevated in FH, predispose these patients to an increased risk for cardiovascular (CV) disease (CVD) from early childhood [1]. Among the available lipid-lowering agents, statins constitute the recommended treatment for both children and adults. Atorvastatin, fluvastatin, lovastatin, pravastatin, rosuvastatin and simvastatin, have all been approved in the USA and Europe for children and adolescents with FH, starting from the age of 10 years (pravastatin is approved for use at age ≥8 years) [1]. Starting with the lowest dose, the European Atherosclerosis Society (EAS) recommends a 50% reduction in LDL-C concentrations in patients with FH aged 8–10 years or a target of LDL-C <135 mg/dL (3.5 mmol/L) for those >10 years old [1, 3, 4]. In general, statins seem to be efficacious in children and adolescents with FH [5,6,7,8]. Although there are no long-term data, reports of statin use in children up to 20 years have shown a significant reduction in atherogenic lipids and lipoproteins and, a reduction in non-invasive markers of atherosclerosis and CVD-related events [9]. However, the differential effect of statin type and dose has not been fully elucidated.
The primary aim of this study was to systematically review and meta-analyse the best available evidence from randomized-controlled trials (RCTs) regarding the lipid-lowering efficacy of statins in children and adolescents with FH in comparison with placebo. The secondary aim was to present data concerning adverse effects associated with statin use in this population, as well as comparative data concerning statin type and dose.
Methods
Guidelines followed
This systematic review followed the PRISMA (Preferred Reporting Items for Systematic reviews and Meta-Analyses) guidelines [10]. A completed PRISMA checklist is available as Supplementary Table 1. The present study has already been registered in the Prospective Register of Systematic Reviews (PROSPERO) System (PROSPERO ID: CRD42019121234).
Search strategy
The following PICO (Population, Intervention, Comparison, Outcome) elements were applied as inclusion criteria for the systematic review: (i) Population: children and adolescents with FH; (ii) Intervention: statins (atorvastatin, fluvastatin, lovastatin, pravastatin, rosuvastatin and simvastatin, as well as the newest one, pitavastatin); (iii) Comparator: diet or placebo; (iv) Outcomes: efficacy and safety of statins in children and adolescents with FH. Efficacy was assessed as the absolute and percentage reduction in total cholesterol (TC) and LDL-C concentrations. The percentage of those who achieved the LDL-C target (<135 mg/dL) was also recorded. The increase in transaminase, creatine kinase (CK) and glucose concentrations, the risk myopathy and the impairment of sexual and growth development were the main parameters recorded in terms of safety. Rare side effects potentially related to statin use were also recorded.
Eligible studies were retrieved via electronic databases of MEDLINE, Scopus and Cochrane (CENTRAL), until the 10th of January 2020. More specifically, the following search string was used for PubMed: (“Hydroxymethylglutaryl-CoA Reductase Inhibitors”[MeSH] OR “atorvastatin”[MeSH] OR “lovastatin”[MeSH] OR “pravastatin”[MeSH] OR “rosuvastatin calcium”[MeSH] OR “simvastatin”[MeSH] OR “statin”[tiab] OR “statins”[tiab] OR “HMG CoA reductase inhibitor”[tiab] OR “HMG CoA reductase inhibitors”[tiab] OR “hydroxymethylglutaryl CoA reductase inhibitor”[tiab] OR “hydroxymethylglutaryl CoA reductase inhibitors”[tiab] “3-hydroxy-3-methyl-glutaryl-CoA reductase inhibitors”[tiab] OR “3-hydroxy-3-methyl-glutaryl-CoA reductase inhibitor”[tiab] OR “atorvastatin”[tiab] OR “fluvastatin”[tiab] OR “lovastatin”[tiab] OR “pitavastatin”[tiab] OR “pravastatin”[tiab] OR “rosuvastatin”[tiab] OR “simvastatin”[tiab]) AND (“child”[tiab] OR “children”[tiab] OR “childhood”[tiab] OR “adolescents”[tiab] OR “adolescence”[tiab] OR “puberty”[tiab] OR “prepuberty”[tiab] OR “youngsters”[tiab] OR “kids” [tiab] OR “paediatric patients”[tiab]) AND (“hyperlipidemias”[MeSH] OR “hypercholesterolaemia”[MeSH] OR “hyperlipoproteinemia type II”[MeSH] OR “hypercholesterolaemia”[tiab] OR “hypercholesterolaemia”[tiab] OR “hyperlipoproteinemia type II”[tiab] OR “hyperlipoproteinaemia type II”[tiab] OR “hypercholesterolemic”[tiab] OR “hypercholesterolaemic”[tiab] OR “dyslipidemias”[tiab] OR “dyslipidaemias”[tiab] OR “hyperlipidemias”[tiab] OR “hyperlipidaemias”[tiab] OR “dyslipidemia”[tiab] OR “dyslipidaemia”[tiab] OR “hyperlipidemia”[tiab] OR “hyperlipidaemia”[tiab] OR “inherited high blood cholesterol”[tiab]) NOT (Animal[MeSH] NOT Human[MeSH]) NOT (letter[pt] OR comment[pt] OR editorial[pt] OR Review[pt] OR “practice guideline”[ptyp] OR “case reports”[ptyp]). Furthermore, “grey literature” was searched for in relevant websites (http://www.opengrey.eu, http://greylit.org and http://clinicaltrials.gov), so that the search was as complete as possible. Endnote V9 was used as search software. The main search was completed independently by two groups of two investigators (KV, PK, CM, KP). In case of discrepancy, this was resolved by either discussion between groups or consultation of an investigator not involved in the initial procedure (PA, DGG).
Trial selection
Specific inclusion criteria were established from the beginning and antecedent to the literature search, as mentioned below: (1) studies conducted in children and adolescents (age range of 8–18 years), diagnosed with FH, according to the Dutch Lipid Clinic Score [1, 3], the US Make Early Diagnosis to Prevent Early Death or the Simon Broome criteria [11,12,13,14] (2) studies with statin monotherapy in the intervention group and placebo or diet in the control group, and, (3) RCTs providing extractable data. Studies were excluded, if they: (1) included patients >18 years of age, (2) assessed different types of lipid-lowering treatment (e.g., ezetimibe) with or without statin, (3) had included patients with a known history of CVD or a CVD risk factor (such as diabetes, hypertension, smoking), (4) were written in a language other than English and/or (5) had no full-text available or were conference abstracts.
Data extraction
Two groups consisting of two researchers each (KV, PK, CM, KP) reviewed all eligible studies. The final data were extracted and recorded as follows: (1) first author, (2) year of publication, (3) country in which the study was conducted, (4) duration of the study, (5) mean age of participants, (6) body mass index (BMI), (7) number of children with HeFH or HoFH, (8) total number of participants and (9) TC, LDL-C, triglyceride (TG), high-density lipoprotein cholesterol (HDL-C), Lp(a), apolipoprotein B (apo-B), glucose, aspartate aminotransferase (AST), alanine aminotransferase (ALT) and CK levels. In case of missing data or ambiguities in study design or trial conduction, the study authors were contacted by e-mail to request additional information. All data were converted into mean ± standard deviation (SD). The following comparisons were made: (1) statins (in general) vs placebo, regarding their effect on the aforementioned lipid and biochemical parameters, (2) mild-to-moderate vs high-intensity statins (direct comparative data from studies or in subgroup analysis), and, (3) statin efficacy according to baseline LDL-C concentrations [>230 mg/dL (>5.9 mmol/L) vs ≤230 mg/dL (≤5.9 mmol/L)].
Statistical analysis
Associations are presented as mean differences ± SD with their 95% confidence intervals (CI). Both absolute and relative differences between statins and placebo regarding lipid profile, liver enzymes, CK and glucose concentrations are reported. In case of median values, these were also transformed in mean values, following the commonly used mathematical models [15]. In cases of missing data on the differences in SD, these were calculated by assuming a correlation coefficient (r) of 0.7 and applying the formula: SD = SQRT ((SD2baseline + SD2after therapy) − (2 × r × SDbaseline × SDafter therapy)) (SQRT: square root). Respective calculations were also made for relative differences by applying the formula: [(lipid parameter after therapy − lipid parameter baseline)/lipid parameter baseline] × 100. A p value of <0.05 was considered significant.
Heterogeneity was tested by the Cochrane chi-square test. The degree of heterogeneity was quantified by the I2 statistics, with values of 30–60% being considered as “moderate” and >60% as a “high degree” of heterogeneity. The random effects model was used for data synthesis, due to the heterogeneity among studies. Publication bias was tested by the Begg-Mazumdar test and the Egger’s test (p > 0.1 indicating the absence of publication bias). All analyses and risk of bias assessment were carried out using Review Manager (RevMan computer program), version 5.3 software (Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014).
Results
Descriptive data
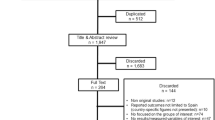
The initial search provided 1052 results, after excluding duplicates, 36 of which were assessed as full-text papers for eligibility (Fig. 1). Ten studies were included in the qualitative and nine in the quantitative analysis [16,17,18,19,20,21,22,23,24,25]. The reasons for study exclusion (n = 26) are presented in Supplementary Table 2. The studies were published between 1996 and 2016, conducted in the following countries: Netherlands (n = 3), USA (n = 1), Japan (n = 1) and Canada (n = 1); three were multi-centre studies. The number of participants ranged from 14 to 214, yielding a total number of 1191 (children or adolescents) with FH, all with HeFH. Mean participants’ age was 13.3 ± 2.5 years (ranging from 10.6 ± 2.9 to 15 ± 2 years), mean BMI was 20.3 ± 3.7 kg/m2 (ranging from 18.7 ± 3.3 to 22.1 ± 3.5 kg/m2; data available for six studies). The study duration ranged from 6 to 96 weeks. With regard to the type and dose of statin, lovastatin (10–40 mg/day) was used in two studies [18, 22], as well as pravastatin (10–40 mg) [16, 21], simvastatin (10–40 mg) [17, 19] and pitavastatin (1–4 mg) [24, 25] whereas atorvastatin (10–20 mg) [20] and rosuvastatin (5–20 mg/day) [23] were used in 1 study each. Of note, the study by Harada-Shiba et al. [25] did not include a placebo-control group and, therefore, was not included in the analysis. The authors compared two different doses of pitavastatin (1 vs 2 mg/day). The study by McCrindle et al. [20] included a mixed population with FH and mixed dyslipidemia, without defining the exact proportion of each type of dyslipidemia. Descriptive characteristics are presented in detail in Table 1. Risk of bias and study quality assessment are presented in Fig. 2.
Efficacy of statins on TC, LDL-C, TG, HDL-C and apo-B
All statins lowered TC and LDL-C levels compared with placebo: −79.3 mg/dL (95% CI −93.9, −64.7; I2 85%) [−2.05 mmol/L (−2.42, 1.67)] and −78.5 mg/dL (95% CI −93.2, −63.8; I2 87%) [−2.03 mmol/L (−2.41, −1.65)], respectively. Mean relative differences in TC and LDL-C with statins compared with placebo were: −25.5% (95% CI −30.4%, −20.5%; I2 91%) and −33.8% (95% CI −40.1%, −27.4%; I2 90%). The respective forest plots are presented in Figs. 3 and 4.
The mean absolute changes in TG [16, 18,19,20,21,22,23,24] and apo-B [17,18,19,20, 22,23,24] by statins vs placebo were: −4.8 mg/dL (95% CI −9.7, 0.1; I2 16%) [−0.05 mmol/L (95% CI −0.10, 0.001)] and −49.6 mg/dL (95% CI −60.4, −38.8; I2 86%) [−0.017 mmol/L (95% CI −0.02, 0.014)]. The respective mean relative differences were significant both for TG and apo-B: −8.4% (95% CI −14.8%, −2.03%; I2 26%) and −28.8% (95% CI −33.9%, −23.6%; I2 83%). Statins raised HDL-C concentrations [16, 18,19,20,21,22,23,24], with mean absolute and relative differences vs placebo: 1.2 mg/dL (95% CI 0.08, 2.29; I2 0%) [0.03 mmol/L (95% CI 0.002, 0.006)] and 3.1% (95% CI 1.1, 5.2; I2 0%), respectively.
Subgroup analysis
Achievement of LDL-C target
Four studies provided data on the percentage of patients achieving the LDL-C target of <135 mg/dL (3.5 mmol/L). This was 60% with atorvastatin 10–20 mg/day [20], 41% with rosuvastatin 10–20 mg/day [for achieving LDL-C <110 mg/dL (<2.85 mmol/L)] [23] and 37.5% for pitavastatin 4 mg/day [24]. The percentage with lower statin doses were 12% for rosuvastatin 5 mg/day [achieving LDL-C <110 mg/dL (<2.85 mmol/L)] [23], 3.8–14.3% for pitavastatin 1 mg/day [24, 25] and 14.3–30.8% for pitavastatin 2 mg/day [24, 25], indicating a dose-dependent effect.
Low- or moderate- vs high-intensity statins
The effect of low-intensity (pravastatin 5–20 mg/day [16, 21]) or moderate-intensity (lovastatin 40 mg/day [18, 22], pitavastatin 4 mg/day [24], pravastatin 40 mg/day [16], simvastatin 20–40 mg/day [17, 19] rosuvastatin 5 mg/day [23] and atorvastatin 20 mg/day [20]) and high-intensity statins (rosuvastatin 20 mg/day) [23] was tested. The respective percentage reduction in TC and LDL-C concentrations with low-intensity statins was −22.3% (95% CI −27.64%, −16.96) and −29.70% (95% CI −36.4%, −22.9%), whereas the respective reductions with moderate-intensity statins was −25.6% (95% CI −30.8%, −20.4%; I2 92%) and −32.78% (95% CI −38.65%, −26.91%; I2 88%). The reduction with high-intensity statins (rosuvastatin 20 mg/day) was −39% (95% CI −43.7%, −34.2%). These effects are illustrated in Supplementary Fig. 1. Two studies provided data on direct comparison according to statin potency, simvastatin 20 vs 40 mg/day [19] and rosuvastatin 5 vs 20 mg/day [23]. The differences in %TC and %LDL-C reduction were significant: −5.42% (95% CI −2.6, −8.2) and −9.4% (95% CI −3.6, −15.23), respectively.
Baseline LDL-C >230 vs ≤230 mg/dL
The absolute and relative LDL-C reduction in patients with baseline LDL-C ≤230 mg/dL (<5.95 mmol/L) by statins was −80.0 mg/dL (95% −88.1, −71.9; I2 17%) [−2.07 mmol/L (95% CI −2.28, −1.86)] and −36.7% (95% CI −41.2%, −32.2%; I2 35%). The absolute and relative LDL-C reduction in patients with baseline LDL-C >230 mg/dL (>5.95 mmol/L) was −79.7 mg/dL (95% −108.4, −51.1; I2 93%) [−2.06 mmol/L (95% CI −2.81, −1.32)] and −32.2% (95% CI −41.6%, −22.8%; I2 35%). These effects are illustrated in Supplementary Fig. 2.
Adverse events (AEs)
All statins were generally well-tolerated. There was no difference in AST, ALT and CK concentrations between statin therapy and placebo. Only one study reported data on fasting plasma glucose (FPG) concentrations, showing a mild increase with the statin (lovastatin 40 mg/day) compared with placebo (2.6 ± 7.0 vs −0.9 ± 14.0 mg/dL, respectively; p < 0.05) [18]. Available data on changes in these parameters are presented in Table 2.
The incidence of AEs was similar in both treatment and placebo groups, ranging from 0 to 73.8% (<20% in most studies) and were mostly unrelated to statin therapy. Almost all AEs resolved during the administration period. The vast majority of AEs were of mild intensity [16, 18,19,20,21,22,23,24,25]. Treatment-related AE rates ranged from 0–15% and were similar across all treatment groups [19, 20, 22, 24, 25]. Serious AEs, although not related to statin treatment, were very rare. Briefly, these included: bruising and purpura (1/67 receiving lovastatin 10–40 mg/day, vs 1/65 skin rash and 1/65 myalgia in the placebo group) [18], infectious mononucleosis (1/106 receiving simvastatin 10 mg/day) [19], depression (1/140 receiving atorvastatin 10–20 mg/day) [20] vesicular rash progressing to cellulitis (1/73 receiving rosuvastatin 20 mg/day vs 1/46 blurred vision in the placebo group) [23], operation to revise previous scar (1/14 receiving pitavastatin 1 mg/day) [25]. There were no deaths or life-threatening AEs, such as rhabdomyolysis, leading to disability or requiring hospitalization (Table 3).
The rates of permanent discontinuation due to AEs were very low, ranging from 0% [16, 22, 23, 25] to 0.7–1.9% [18, 20, 24]. The rates of transaminase elevation of >3-fold the upper limit of normal (ULN) were very low, ranging from 0% (lovastatin 10–40 mg/day [18, 22]), pitavastatin 1–4 mg/day [24, 25], pravastatin 5–40 mg/day [16, 21] to 1% (atorvastatin 10–20 mg/day [20]), 1.8% (simvastatin 10–40 mg/day) [19] and 2% (rosuvastatin 10–20 mg/day [23]). The respective rates of CK elevation of >10-fold of the ULN ranged from 0% (atorvastatin 10–20 mg/day [20]), lovastatin 10–40 mg/day [18, 22], pitavastatin 1–4 mg/day [24], pravastatin 5–40 mg/day [16, 21] and rosuvastatin 5 mg/day [23] to 0.9% (simvastatin 10–40 mg/day [19]), 4.4% (rosuvastatin 10–20 mg/day [23]) and 7% (pitavastatin 1–2 mg/day [25]). Myalgia was reported only in 2 studies: 4/149 (2.7%) patients receiving rosuvastatin 5–20 mg/day [23] and 1/24 (4.1%) receiving pitavastatin 4 mg/day [24]. All cases were attributed to exercise and resolved during treatment continuation.
No change in gonadal steroid concentrations were observed [19, 21, 22, 24, 25]. Furthermore, no clinical signs of growth or sexual impairment assessed by Tanner staging were noticed during statin treatment [19, 20, 22, 23, 25].
Discussion
This study provided an updated overview of the efficacy and safety of statin treatment in children and adolescents with FH. Despite heterogeneity, statins, as a whole, significantly lowered TC, LDL-C, apo-B and, to a lesser extent, TG concentrations compared with diet and placebo. The increase in HDL-C was also significant. However, no direct comparative data with regard to the type of statin are available. The effect on TC, LDL-C and apo-B seems to be potency-dependent. More than half of patients may achieve the LDL-C target with high-intensity statin dose (i.e. rosuvastatin 20 mg/day), whereas this seems to be lower with low-to-moderate intensity statins (4–14%). Baseline LDL-C concentrations do not seem to predict the lipid-lowering effect of statins in children.
All statins were generally well-tolerated. There was no difference in transaminase or CK elevation compared with placebo. Treatment-related AEs were rare (0–15%). Permanent withdrawal due to AEs was rare (0–2%). Serious AEs were not related to statin use. Transaminase elevation >3-fold the ULN, CK >10-fold the ULN and myalgia was rare (0–2%, 0–7% and 0–4%, mostly seen with pitavastatin and rosuvastatin). Despite the paucity of data on glucose metabolism (only one study reported a slight increase in FPG compared with placebo), there were no reports of new-onset diabetes mellitus (NODM) in FH children or adolescents treated with statins. Data from statin trials in adults indicate a very low risk of transaminase elevation >3-fold the ULN [odds ratio (OR) 1.26 (95% CI 0.99, 1.62)], depending on the dose and potency of statin [26]. Moreover, recent trials in patients with abnormal liver tests and at high CVD risk have shown efficacy in reducing CV morbidity, as well as improvement in liver histology [27, 28]. Non-alcoholic fatty liver disease remains underdiagnosed in paediatric populations, sharing common pathways with adults, with an overall prevalence of 3–10%, rising to 40–70% in obese children. Except for weight loss strategies, the evidence for pharmacological intervention (including insulin sensitizers, anti-oxidants, probiotics and prebiotics) is weak. No data exist for the use of statins in this setting [29].
The risk of statin-induced myositis or rhabdomyolysis is low, confined to high-dose statins and cases of drug interactions [26]. No difference with placebo seems to exist in terms of myalgia risk [26]. Concerning the risk of NODM, statin trials in adults have shown that it is generally low [OR 1.09 (95% CI 1.02–1.17)] [30] depending on the dose and potency of statin and mostly involving patients already at high risk of type 2 diabetes mellitus, such as those with obesity and acanthosis nigricans [26]. However, controversy still exists regarding the exact mechanisms underlying this risk, since a differential effect on insulin resistance has been reported [31]. Of note, evidence supports that long duration of high-intensity statin therapy is not associated with a higher risk of NODM in patients with FH [32, 33].
Concerns exist as to whether statins could interfere with steroid hormone production and, thus, affect growth and gonadal development. None of the studies included in this systematic review raised any concern regarding this issue either at a clinical or at a laboratory level. However, only a few studies have reported long-term efficacy and safety data on statin in children and adolescents. An older study with atorvastatin (10–40 mg/day) in 16 males, aged 10–17 (median 13) years, showed sustained efficacy and achievement of LDL-C target (<130 mg/dL) in all patients for 3 years. No AE, growth or mental impairment were observed during follow-up [34]. A 10-year safety data have also been reported in patients treated with pravastatin 20–40 mg/day (follow-up of patients included in the study by Wiegman et al., published in 2004) [9]. Compared with non-FH siblings, no significant difference was observed in gonadal steroid and gonadotropin concentrations in both male and female patients with FH [35]. A decrease in dehydroepiandrosterone sulphate (DHEAS) concentrations only in males treated with pravastatin was noticed after 10 years of treatment, without impairment of mental maturation and growth [35, 36]. Concerning DHEAS, either increase (lovastatin) [18], decrease (simvastatin) [19] on null-effect has (pravastatin) [21] been reported, without any impact on sexual development.
The atherosclerotic process in patients with FH seems to initiate during early childhood (7 years), as shown by greater carotid intima-media thickness (cIMT) compared with their non-FH controls [37, 38]. Few data exist as to whether intervention with statins at this stage can attenuate this process. In an open-label study, rosuvastatin 5–20 mg/day mitigated the progression of carotid atherosclerosis in FH children to the level of their unaffected controls, achieving a similar cIMT after 2 years of treatment [39]. Furthermore, a 20-year follow-up study of the initial Wiegman’s [21] cohort slowed that mean progression of cIMT in patients with FH, treated with pravastatin since childhood, to the level of their unaffected siblings over time. Moreover, the cumulative incidence of CVD events and death at 39 years was lower among patients with FH on statin treatment than among their affected parents (1 vs 26% and 0 vs 7%, respectively) [9].
Efficacy and safety of statins in children and adolescents have also been evaluated in other meta-analyses. Older ones included both RCTs and prospective cohort studies, yielding comparable results [5] also reporting data on surrogate CVD indices, such as cIMT. A recent meta-analysis, published in 2018 (10 RCTs), only focused on changes in lipid profile [7]. There were only brief reports on statin safety. The authors also included the studies by van der Graaf et al. [40] and Rodenburg et al. [41], which we excluded because in the former the patients were treated with simvastatin plus ezetimibe. The latter study reported follow-up data of patients already incorporated in another study [21]. Notably, the authors did not include the study by Couture et al. [17]. A very recent Cochrane systematic review (n = 9 RCTs) [8] reported alterations in lipid profile in alignment with the present study. Regarding safety profile, AEs related to statin use appeared comparable among participants, at least in the short-term. However, it must be pointed out that our meta-analysis further reported comparative data according to different statin intensity, baseline LDL-C concentrations and achievement of LDL-C target.
Our study has certain limitations. First, a direct comparison between different statin types was not available. Second, there were missing data on absolute and relative mean differences in lipid and biochemical parameters, concerning SD, which were imputed using the mathematical models described in the methods section. However, these could not have any significant impact on our results, after performing subgroup analysis. Third, direct comparison between equivalent statin doses (i.e., rosuvastatin 5 mg vs atorvastatin 10 mg or simvastatin 20 mg) was not feasible. Fourth, no fixed statin dose was administered in all studies (dose titration to achieve the LDL-C target or different dose according to patients’ age, were reported). Fifth, the combination of statins with other lipid-lowering medications (i.e., ezetimibe) in children cannot be assessed based on currently available data.
Conclusions
Statins are quite effective in reducing TC, LDL-C, apo-B and, to a lesser extent, TG concentrations compared with diet and placebo. Use of statins may also lead to a modest increase in HDL-C levels. The lipid-lowering effect in children appears to be dependent on statin potency. All statins were generally well-tolerated, with no difference in transaminase or CK elevation rates compared with placebo. There was no detrimental effect on sexual or growth development. The key issue is to establish evidence in RCTs of longer duration in children, directly comparing different doses and types of statins. The effect of such treatment on CVD risk should also be assessed.
References
A. Wiegman, S.S. Gidding, G.F. Watts, M.J. Chapman, H.N. Ginsberg, M. Cuchel, L. Ose, M. Averna, C. Boileau, J. Boren, E. Bruckert, A.L. Catapano, J.C. Defesche, O.S. Descamps, R.A. Hegele, G.K. Hovingh, S.E. Humphries, P.T. Kovanen, J.A. Kuivenhoven, L. Masana, B.G. Nordestgaard, P. Pajukanta, K.G. Parhofer, F.J. Raal, K.K. Ray, R.D. Santos, A.F. Stalenhoef, E. Steinhagen-Thiessen, E.S. Stroes, M.R. Taskinen, A. Tybjaerg-Hansen, O. Wiklund, Familial hypercholesterolaemia in children and adolescents: gaining decades of life by optimizing detection and treatment. Eur. Heart J. 36(36), 2425–2437 (2015). https://doi.org/10.1093/eurheartj/ehv157
P. Anagnostis, P. Siolos, D. Krikidis, D.G. Goulis, J.C. Stevenson, Should we consider lipoprotein (a) in cardiovascular disease Risk assessment in patients with familial hypercholesterolaemia? Cur. Pharm. Des. 24(31), 3665–3671 (2018). https://doi.org/10.2174/1381612824666181010150958
B.G. Nordestgaard, M.J. Chapman, S.E. Humphries, H.N. Ginsberg, L. Masana, O.S. Descamps, O. Wiklund, R.A. Hegele, F.J. Raal, J.C. Defesche, A. Wiegman, R.D. Santos, G.F. Watts, K.G. Parhofer, G.K. Hovingh, P.T. Kovanen, C. Boileau, M. Averna, J. Boren, E. Bruckert, A.L. Catapano, J.A. Kuivenhoven, P. Pajukanta, K. Ray, A.F. Stalenhoef, E. Stroes, M.R. Taskinen, A. Tybjaerg-Hansen, Familial hypercholesterolaemia is underdiagnosed and undertreated in the general population: guidance for clinicians to prevent coronary heart disease: consensus statement of the European Atherosclerosis Society. Eur. Heart J. 34(45), 3478–3490a (2013). https://doi.org/10.1093/eurheartj/eht273
F. Mach, C. Baigent, A.L. Catapano, K.C. Koskinas, M. Casula, L. Badimon, M.J. Chapman, G.G. De Backer, V. Delgado, B.A. Ference, I.M. Graham, A. Halliday, U. Landmesser, B. Mihaylova, T.R. Pedersen, G. Riccardi, D.J. Richter, M.S. Sabatine, M.R. Taskinen, L. Tokgozoglu, O. Wiklund; Group, E.S.C.S.D., 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur. Heart J. 41(1), 111–188 (2020). https://doi.org/10.1093/eurheartj/ehz455
C. Arambepola, A.J. Farmer, R. Perera, H.A. Neil, Statin treatment for children and adolescents with heterozygous familial hypercholesterolaemia: a systematic review and meta-analysis. Atherosclerosis 195(2), 339–347 (2007). https://doi.org/10.1016/j.atherosclerosis.2006.09.030
C.S. O’Gorman, M.F. Higgins, M.B. O’Neill, Systematic review and metaanalysis of statins for heterozygous familial hypercholesterolemia in children: evaluation of cholesterol changes and side effects. Pediatr. Cardiol. 30(4), 482–489 (2009). https://doi.org/10.1007/s00246-008-9364-3
G. Radaelli, G. Sausen, C.C. Cesa, F.S. Santos, V.L. Portal, J.L. Neyeloff, L.C. Pellanda, Statin treatments and dosages in children with familial hypercholesterolemia: meta-analysis. Arq. Bras. Cardiol. 111(6), 810–821 (2018). https://doi.org/10.5935/abc.20180180
Vuorio, A., Kuoppala, J., Kovanen, P.T., Humphries, S.E., Tonstad, S., Wiegman, A., Drogari, E., Ramaswami, U.: Statins for children with familial hypercholesterolemia. Cochrane Database Syst. Rev. 2019(11) (2019). https://doi.org/10.1002/14651858.CD006401.pub5
I.K. Luirink, A. Wiegman, D.M. Kusters, M.H. Hof, J.W. Groothoff, E. de Groot, J.J.P. Kastelein, B.A. Hutten, 20-Year Follow-up of statins in children with familial hypercholesterolemia. N. Engl. J. Med. 381(16), 1547–1556 (2019). https://doi.org/10.1056/NEJMoa1816454
A. Liberati, D.G. Altman, J. Tetzlaff, C. Mulrow, P.C. Gotzsche, J.P. Ioannidis, M. Clarke, P.J. Devereaux, J. Kleijnen, D. Moher, The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 339, b2700 (2009). https://doi.org/10.1136/bmj.b2700
Programme, W.H.O.H.G.: Familial hypercholesterolaemia (FH): report of a second WHO consultation, Geneva, 4 September 1998. In., vol. WHO/HGN/FH/CONS/99.2. World Health Organization, Geneva, (1999)
Scientific Steering Committee on behalf of the Simon Broome Register Group, Risk of fatal coronary heart disease in familial hypercholesterolaemia. BMJ 303(6807), 893–896 (1991). https://doi.org/10.1136/bmj.303.6807.893
R.R. Williams, S.C. Hunt, M.C. Schumacher, R.A. Hegele, M.F. Leppert, E.H. Ludwig, P.N. Hopkins, Diagnosing heterozygous familial hypercholesterolemia using new practical criteria validated by molecular genetics. Am. J. Cardiol. 72(2), 171–176 (1993). https://doi.org/10.1016/0002-9149(93)90155-6
Representatives of the Global Familial Hypercholesterolemia, C. Wilemon, K.A. Patel, J. Aguilar-Salinas, C. Ahmed, C.D. Alkhnifsawi, M. Almahmeed, W. Alonso, R. Al-Rasadi, K. Badimon, L. Bernal, L.M. Bogsrud, M.P. Braun, L.T. Brunham, L. Catapano, A.L. Cillíková, K. Corral, P. Cuevas, R. Defesche, J.C. Descamps, O.S. de Ferranti, S. Eiselé, J.-L. Elikir, G. Folco, E. Freiberger, T. Fuggetta, F. Gaspar, I.M. Gesztes, Á.G. Grošelj, U. Hamilton-Craig, I. Hanauer-Mader, G. Harada-Shiba, M. Hastings, G. Hovingh, G.K. Izar, M.C. Jamison, A. Karlsson, G.N. Kayikçioglu, M. Koob, S. Koseki, M. Lane, S. Lima-Martinez, M.M. López, G. Martinez, T.L. Marais, D. Marion, L. Mata, P. Maurina, I. Maxwell, D. Mehta, R. Mensah, G.A. Miserez, A.R. Neely, D. Nicholls, S.J. Nohara, A. Nordestgaard, B.G. Ose, L. Pallidis, A. Pang, J. Payne, J. Peterson, A.L. Popescu, M.P. Puri, R. Ray, K.K. Reda, A. Sampietro, T. Santos, R.D. Schalkers, I. Schreier, L. Shapiro, M.D. Sijbrands, E. Soffer, D. Stefanutti, C. Stoll, M. Sy, R.G. Tamayo, M.L. Tilney, M.K. Tokgözoglu, L. Tomlinson, B. Vallejo-Vaz, A.J. Vazquez-Cárdenas, A. de Luca, P.V. Wald, D.S. Watts, G.F. Wenger, N.K. Wolf, M. Wood, D. Zegerius, A. Gaziano, T.A. Gidding, S.S.: Reducing the clinical and public health burden of familial hypercholesterolemia: a global call to action. JAMA Cardiol. https://doi.org/10.1001/jamacardio.2019.5173 (2020)
X. Wan, W. Wang, J. Liu, T. Tong, Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med. Res. Methodol. 14, 135 (2014). https://doi.org/10.1186/1471-2288-14-135
H.C. Knipscheer, C.C. Boelen, J.J. Kastelein, D.E. van Diermen, B.E. Groenemeijer, A. van den Ende, H.R. Buller, H.D. Bakker, Short-term efficacy and safety of pravastatin in 72 children with familial hypercholesterolemia. Pediatr. Res. 39(5), 867–871 (1996). https://doi.org/10.1203/00006450-199605000-00021
P. Couture, L.D. Brun, F. Szots, M. Lelievre, D. Gaudet, J.P. Despres, J. Simard, P.J. Lupien, C. Gagne, Association of specific LDL receptor gene mutations with differential plasma lipoprotein response to simvastatin in young French Canadians with heterozygous familial hypercholesterolemia. Arterioscler. Thromb. Vasc. Biol. 18(6), 1007–1012 (1998)
E.A. Stein, D.R. Illingworth, P.O. Kwiterovich Jr., C.A. Liacouras, M.A. Siimes, M.S. Jacobson, T.G. Brewster, P. Hopkins, M. Davidson, K. Graham, F. Arensman, R.H. Knopp, C. DuJovne, C.L. Williams, J.L. Isaacsohn, C.A. Jacobsen, P.M. Laskarzewski, S. Ames, G.J. Gormley, Efficacy and safety of lovastatin in adolescent males with heterozygous familial hypercholesterolemia: a randomized controlled trial. JAMA 281(2), 137–144 (1999). https://doi.org/10.1001/jama.281.2.137
S. de Jongh, L. Ose, T. Szamosi, C. Gagne, M. Lambert, R. Scott, P. Perron, D. Dobbelaere, M. Saborio, M.B. Tuohy, M. Stepanavage, A. Sapre, B. Gumbiner, M. Mercuri, A.S. van Trotsenburg, H.D. Bakker, J.J. Kastelein, Efficacy and safety of statin therapy in children with familial hypercholesterolemia: a randomized, double-blind, placebo-controlled trial with simvastatin. Circulation 106(17), 2231–2237 (2002). https://doi.org/10.1161/01.cir.0000035247.42888.82
B.W. McCrindle, L. Ose, A.D. Marais, Efficacy and safety of atorvastatin in children and adolescents with familial hypercholesterolemia or severe hyperlipidemia: a multicenter, randomized, placebo-controlled trial. J. Pediatr. 143(1), 74–80 (2003). https://doi.org/10.1016/s0022-3476(03)00186-0
A. Wiegman, B.A. Hutten, E. de Groot, J. Rodenburg, H.D. Bakker, H.R. Buller, E.J. Sijbrands, J.J. Kastelein, Efficacy and safety of statin therapy in children with familial hypercholesterolemia: a randomized controlled trial. JAMA 292(3), 331–337 (2004). https://doi.org/10.1001/jama.292.3.331
S.B. Clauss, K.W. Holmes, P. Hopkins, E. Stein, M. Cho, A. Tate, A.O. Johnson-Levonas, P.O. Kwiterovich, Efficacy and safety of lovastatin therapy in adolescent girls with heterozygous familial hypercholesterolemia. Pediatrics 116(3), 682–688 (2005). https://doi.org/10.1542/peds.2004-2090
H.J. Avis, B.A. Hutten, C. Gagne, G. Langslet, B.W. McCrindle, A. Wiegman, J. Hsia, J.J. Kastelein, E.A. Stein, Efficacy and safety of rosuvastatin therapy for children with familial hypercholesterolemia. J. Am. Coll. Cardiol. 55(11), 1121–1126 (2010). https://doi.org/10.1016/j.jacc.2009.10.042
M.J. Braamskamp, C. Stefanutti, G. Langslet, E. Drogari, A. Wiegman, N. Hounslow, J.J. Kastelein, Efficacy and safety of pitavastatin in children and adolescents at high future cardiovascular risk. J. Pediatr. 167(2), 338–343.e335 (2015). https://doi.org/10.1016/j.jpeds.2015.05.006
M. Harada-Shiba, O. Arisaka, A. Ohtake, T. Okada, H. Suganami, Efficacy and safety of pitavastatin in Japanese male children with familial hypercholesterolemia. J. Atheroscler. Thromb. 23(1), 48–55 (2016). https://doi.org/10.5551/jat.28753. Epub 20 Apr 2015
C.S. Desai, S.S. Martin, R.S. Blumenthal, Non-cardiovascular effects associated with statins. BMJ 349, g3743 (2014). https://doi.org/10.1136/bmj.g3743
V.G. Athyros, K. Tziomalos, T.D. Gossios, T. Griva, P. Anagnostis, K. Kargiotis, E.D. Pagourelias, E. Theocharidou, A. Karagiannis, D.P. Mikhailidis, Safety and efficacy of long-term statin treatment for cardiovascular events in patients with coronary heart disease and abnormal liver tests in the Greek Atorvastatin and Coronary Heart Disease Evaluation (GREACE) Study: a post-hoc analysis. Lancet 376(9756), 1916–1922 (2010). https://doi.org/10.1016/s0140-6736(10)61272-x
K. Kargiotis, V.G. Athyros, O. Giouleme, N. Katsiki, E. Katsiki, P. Anagnostis, C. Boutari, M. Doumas, A. Karagiannis, D.P. Mikhailidis, Resolution of non-alcoholic steatohepatitis by rosuvastatin monotherapy in patients with metabolic syndrome. World J. Gastroenterol. 21(25), 7860–7868 (2015). https://doi.org/10.3748/wjg.v21.i25.7860
F. Tzifi, A. Fretzayas, G. Chrousos, C. Kanaka-Gantenbein, Non-alcoholic fatty liver infiltration in children: an underdiagnosed evolving disease. Hormones 18(3), 255–265 (2019). https://doi.org/10.1007/s42000-019-00107-7
N. Sattar, D. Preiss, H.M. Murray, P. Welsh, B.M. Buckley, A.J. de Craen, S.R. Seshasai, J.J. McMurray, D.J. Freeman, J.W. Jukema, P.W. Macfarlane, C.J. Packard, D.J. Stott, R.G. Westendorp, J. Shepherd, B.R. Davis, S.L. Pressel, R. Marchioli, R.M. Marfisi, A.P. Maggioni, L. Tavazzi, G. Tognoni, J. Kjekshus, T.R. Pedersen, T.J. Cook, A.M. Gotto, M.B. Clearfield, J.R. Downs, H. Nakamura, Y. Ohashi, K. Mizuno, K.K. Ray, I. Ford, Statins and risk of incident diabetes: a collaborative meta-analysis of randomised statin trials. Lancet 375(9716), 735–742 (2010). https://doi.org/10.1016/S0140-6736(09)61965-6
P. Anagnostis, D. Selalmatzidou, S.A. Polyzos, A. Panagiotou, A. Slavakis, A. Panagiotidou, V.G. Athyros, A. Karagiannis, D.P. Mikhailidis, M. Kita, Comparative effects of rosuvastatin and atorvastatin on glucose metabolism and adipokine levels in non-diabetic patients with dyslipidaemia: a prospective randomised open-label study. Int. J. Clin. Pract. 65(6), 679–683 (2011). https://doi.org/10.1111/j.1742-1241.2011.02655.x
V. Panz, A. Immelman, J. Paiker, G. Pilcher, F. Raal, High-dose statin therapy does not induce insulin resistance in patients with familial hypercholesterolemia. Metab. Syndr. Relat. Disord. 10(5), 351–357 (2012). https://doi.org/10.1089/met.2012.0063
J. Skoumas, C. Liontou, C. Chrysohoou, C. Masoura, K. Aznaouridis, C. Pitsavos, C. Stefanadis, Statin therapy and risk of diabetes in patients with heterozygous familial hypercholesterolemia or familial combined hyperlipidemia. Atherosclerosis 237(1), 140–145 (2014). https://doi.org/10.1016/j.atherosclerosis.2014.08.047
V.G. Athyros, A.A. Papageorgiou, A.G. Kontopoulos, Long-term treatment with atorvastatin in adolescent males with heterozygous familial hypercholesterolemia. Atherosclerosis 163(1), 205–206 (2002). https://doi.org/10.1016/s0021-9150(02)00005-9
M.J. Braamskamp, D.M. Kusters, A. Wiegman, H.J. Avis, F.A. Wijburg, J.J. Kastelein, A.S. van Trotsenburg, B.A. Hutten, Gonadal steroids, gonadotropins and DHEAS in young adults with familial hypercholesterolemia who had initiated statin therapy in childhood. Atherosclerosis 241(2), 427–432 (2015). https://doi.org/10.1016/j.atherosclerosis.2015.05.034
D.M. Kusters, H.J. Avis, E. de Groot, F.A. Wijburg, J.J. Kastelein, A. Wiegman, B.A. Hutten, Ten-year follow-up after initiation of statin therapy in children with familial hypercholesterolemia. JAMA 312(10), 1055–1057 (2014). https://doi.org/10.1001/jama.2014.8892
A. Wiegman, E. de Groot, B.A. Hutten, J. Rodenburg, J. Gort, H.D. Bakker, E.J. Sijbrands, J.J. Kastelein, Arterial intima-media thickness in children heterozygous for familial hypercholesterolaemia. Lancet 363(9406), 369–370 (2004). https://doi.org/10.1016/s0140-6736(04)15467-6
D.M. Kusters, A. Wiegman, J.J. Kastelein, B.A. Hutten, Carotid intima-media thickness in children with familial hypercholesterolemia. Circ. Res. 114(2), 307–310 (2014). https://doi.org/10.1161/circresaha.114.301430
M. Braamskamp, G. Langslet, B.W. McCrindle, D. Cassiman, G.A. Francis, C. Gagne, D. Gaudet, K.M. Morrison, A. Wiegman, T. Turner, E. Miller, D.M. Kusters, J.S. Raichlen, P.D. Martin, E.A. Stein, J.J.P. Kastelein, B.A. Hutten, Effect of rosuvastatin on carotid intima-media thickness in children with heterozygous familial hypercholesterolemia: the CHARON study (hypercholesterolemia in children and adolescents taking rosuvastatin open label). Circulation 136(4), 359–366 (2017). https://doi.org/10.1161/CIRCULATIONAHA.116.025158
A. van der Graaf, C. Cuffie-Jackson, M.N. Vissers, M.D. Trip, C. Gagne, G. Shi, E. Veltri, H.J. Avis, J.J. Kastelein, Efficacy and safety of coadministration of ezetimibe and simvastatin in adolescents with heterozygous familial hypercholesterolemia. J. Am. Coll. Cardiol. 52(17), 1421–1429 (2008). https://doi.org/10.1016/j.jacc.2008.09.002
J. Rodenburg, M.N. Vissers, A. Wiegman, E.R. Miller, P.M. Ridker, J.L. Witztum, J.J. Kastelein, S. Tsimikas, Oxidized low-density lipoprotein in children with familial hypercholesterolemia and unaffected siblings: effect of pravastatin. J. Am. Coll. Cardiol. 47(9), 1803–1810 (2006). https://doi.org/10.1016/j.jacc.2005.12.047
Author contributions
PA designed the study, analysed the data and wrote the first draft of the paper. K.V., P.K., C.M. and K.P. searched the literature and extracted the data. V.G.A. and D.P.M. reviewed the manuscript and provided critical scientific input. D.G.G. resolved discrepancies regarding the quality of the studies included in the meta-analysis, provided critical scientific input and had the primary responsibility for the paper’s final content. All authors approved the final version of the text.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
D.P.M. has given talks and attended conferences sponsored by Amgen, AstraZeneca and Libytec. The other authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary material
Rights and permissions
About this article
Cite this article
Anagnostis, P., Vaitsi, K., Kleitsioti, P. et al. Efficacy and safety of statin use in children and adolescents with familial hypercholesterolaemia: a systematic review and meta-analysis of randomized-controlled trials. Endocrine 69, 249–261 (2020). https://doi.org/10.1007/s12020-020-02302-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-020-02302-8