Abstract
Non-alcoholic fatty liver disease (NAFLD) constitutes the most common liver disease, one that is still underdiagnosed in pediatric populations (as well as in the general population), this due to the progressive increase in childhood obesity observed both in developed and developing countries during the last few decades. The pathophysiology of the disease has not been thoroughly clarified yet. The condition displays common pathways in adults and children; however, there are age-related differences. Unlike adults, children with NAFLD require extensive laboratory analysis, because underlying pathologies other than obesity may contribute to the evolution of the disease. Despite the presence of several serum markers and imaging techniques that contribute to NAFLD diagnosis, liver biopsy remains the gold standard diagnostic procedure. Early intervention and obesity prevention are mandatory, as NAFLD is reversible at an early stage. If left undiagnosed and untreated, NAFLD can progress to steatohepatitis (NASH) and subsequent liver failure, a potentially lethal complication. Of note, there are no treatment options when advanced liver fibrosis occurs. This review summarizes literature data on NAFLD in childhood indicating that this is an evolving disease and a significant component of the metabolic syndrome. Pediatricians should be aware of this entity, screening children at high risk and providing appropriate early management, in collaboration with pediatric subspecialists.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The dramatic increase in the prevalence of pediatric and adolescent obesity during the last few decades has led to a rise in related comorbidities, such as hypertension, dyslipidemia, insulin resistance, type 2 diabetes mellitus, obstructive sleep apnea, and non-alcoholic fatty liver disease (NAFLD) [1, 2]. The latter comprises a wide spectrum of liver damage, from simple steatosis, which is considered benign and reversible, to more severe forms of the disease, such as non-alcoholic steatohepatitis (NASH), which can result in liver fibrosis, cirrhosis, hepatic failure, and predisposition to hepatocellular carcinoma development later in life [3, 4]. NAFLD is the most common liver disease in pediatric populations in developed and developing countries but still remains an underdiagnosed entity. NAFLD is a hepatic manifestation of the metabolic syndrome and has been associated with both hyperlipidemia and insulin resistance [5, 6]. The current review summarizes data on the pathogenesis, risk factors, and diagnostic work-up as well as the available preventive and treatment options for pediatric NAFLD.
Epidemiology
The rapidly growing pediatric obesity epidemic over the past several decades has led to the increased prevalence of NAFLD in children and adolescents, especially in Western countries [7, 8]. The prevalence of NAFLD in the general population of Western countries is about 20–30%, with 2–3% estimated to have NASH. More specifically in children, the overall prevalence of NAFLD is 3–10%, rising to 40–70% among obese children [9]. As the gold standard for the diagnosis of NAFLD is liver biopsy, the true prevalence of childhood NAFLD and NASH is still unknown. A study based on autopsies of 742 children aged 2 to 19 years who died from trauma estimated that the prevalence of NAFLD, adjusted for age, gender, race, and ethnicity, was overall 9.6%, being lower in children aged 2–4 years (0.7%) and higher in adolescents (17.3%), with the highest prevalence observed in obese children (38%) and in children of Hispanic origin (11.8%) [10]. Another study also based on medico-legal autopsy reports of 265 children revealed that NAFLD was present in 4.2% of children aged 6 months–18 years old, and NASH in 0.3%, while 55.6% of children with NAFLD were obese [11]. Most clinical studies investigating the epidemiology of NAFLD are based on circulating markers, such as serum ALT, or ultrasound findings. These studies mainly estimate the prevalence of the disease only in obese subjects. In a prospective study from Australia, NAFLD was present in 15.2% of adolescents (n = 995) who participated in the Western Australian Pregnancy Cohort (Raine) Study [12]; dietary patterns in early adolescence were associated with an increased risk of NAFLD later in life. In European populations, a study in many obese subjects (n = 16,390) from obesity centers in Germany, Austria, and Switzerland revealed that NAFLD was present in 11% of the sample investigated, predominantly in males [13]. In Asian populations, the prevalence of NAFLD is lower. It was reported to be 7.1% in Iranian, 3% in Indian, and almost 4.5% in Japanese children [14,15,16]. The differences in NAFLD prevalence observed in several ethnicities could be attributed both to genetic factors and to different dietary patterns [17]. Studies in a large cohort of individuals, regardless of obesity, are required in order to accurately determine the prevalence of NAFLD in different ethnicities. Furthermore, replacement of liver biopsy by another non-invasive test of equal validity, either imaging or serologic, is urgently needed. A non-invasive procedure would be of profound importance, not only to diagnose primary NAFLD but most importantly, to recognize exacerbation of this disease under different circumstances, with serial appreciation of the evolving pattern in the same persons.
Pathogenesis, genes, and risk factors for NAFLD
Pathogenesis
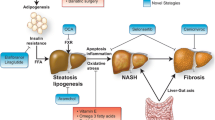
The exact pathophysiological mechanisms leading to fatty liver and progression to NASH are not yet well understood, but it is generally accepted that both genetic and epigenetic factors are involved [18]. The “two-hit” or “multiple-hit” theory was proposed by Day et al. over a decade ago to explain the development and progression of the disease [19]. The “first” hit is the accumulation of fat within the hepatocytes. The most important factor at this stage is obesity and insulin resistance, which are responsible for the increased uptake of free-fatty acids by the hepatocytes [20]. The second “hit” or other multiple additional “hits” are essential for progression of the disease to liver inflammation and subsequent fibrosis, in other words, transition from NAFLD to NASH. Oxidative stress is a major factor associated with liver damage; it is attributed to hypoxia, mitochondrial dysfunction, and lack of antioxidants, leading to the generation of reactive oxygen species (ROS) [20]. ROS are responsible for liver cell damage, either in a direct toxic way (death) or indirectly through apoptosis. Several cytokines are also implicated in progression of the disease to NASH.
There is a direct and statistically significant relationship between obesity, i.e., excessive accumulation of body fat, and NAFLD. Bearing this in mind, together with the fact that, as is today widely accepted, fat, or adipose tissue, is an active endocrine organ secreting several hormones and cytokines, it can be assumed that obesity-related adipose tissue inflammation promotes metabolic dysregulation. Meanwhile, adipose tissue adiponectin is an anti-inflammatory cytokine produced by white adipose tissue: it displays multiple functions, such as improvement of insulin sensitivity, inhibition of free fatty acid uptake, and increased lipid export from hepatic cells [21]. Its expression is decreased in NAFLD patients [22, 23], whereas TNF-a, a proinflammatory cytokine is increased [24]. The abovementioned “two-hit” theory has been applied both in obese children and in adults, although possibly there are differences in disease pathogenesis between adults and children. For instance, unlike in adults [25, 26], the role of central obesity in children with NAFLD has not yet been clearly demonstrated [27,28,29], while it is possible that in childhood, overall obesity is associated with NAFLD development. Furthermore, despite obesity being the most frequent factor for NAFLD and NASH in children, there are several other conditions that could predispose to the disease, especially in children younger than 10 years of age, as presented in Table 1.
Genes
The role of genes in the pathogenesis of NAFLD is supported by studies demonstrating that different ethnicities have different prevalence of the disease, by the fact that not all patients with NAFLD progress to NASH and, finally, by the fact that NAFLD displays some hereditability, as 18% of patients with NASH have a first degree relative suffering from NAFLD [29, 30]. This latter observation should, at least in part, be interpreted with caution, as close relatives tend to live in relatively similar conditions, notably with regard to dietary patterns, and to be exposed to approximately the same environmental influences. Several genes have been implicated in NAFLD pathogenesis at several stages. For instance, a single-nucleotide polymorphism (SNP) of the patatin-like phospholipase-3 (PNPLA-3) gene which encodes for adiponutrin expression, namely the G allele, has been associated with liver fat accumulation and liver enzyme increase [31], although in children, no association with liver histological severity has to date been confirmed [32]. This SNP displays the highest prevalence in Hispanic populations and the lowest in African-Americans [33, 34]. A study performed in 200 children and adolescents with NAFLD (58% obese and 32% overweight) showed that the rs738409 I148M PNPLA3 polymorphism was the main determinant of steatosis severity, also linked with dietary components, such as sweetened beverage consumption [35]. Moreover, SNPs resulting in enhanced IL-6 and TNF-a gene expression have also been associated with insulin resistance and NASH [23]. Lipins, proteins that promote fatty acid oxidation by acting as co-regulators of gene expression by DNA-bound transcription factors, have also been associated with liver steatosis [36]. Lipin1 rs13412852 SNP is associated with severity of liver damage and progression of fibrosis in pediatric patients with histological NAFLD [37]. The transmembrane 6 superfamily member 2 (TM6SF2) gene, predominantly expressed in the liver and intestine, is associated with plasma triglyceride (TG) concentrations [38]. Carriers of the TM6SF2 E167K variant have fatty liver due to reduced secretion of very low-density lipoproteins. As a result, they have lower circulating lipids and reduced risk of myocardial infarction, while being more prone to progressive NASH [39]. On the other hand, SNPs of the UGT1A1 gene that lead to increased levels of bilirubin (Gilbert’s syndrome) are associated with decreased incidence of NAFLD [40, 41]. These genes involved in NAFLD pathogenesis are summarized in Table 2.
Risk factors
Several risk factors have been associated with NAFLD development in children [40, 42]. The role of BMI and obesity has been previously discussed. Age per se is a risk factor, as NAFLD is far more prevalent in adolescents, rare in children < 10 years old, while probably absent in those under 3 years of age, in whom the presence of NAFLD is debated and does not exist as a clinicopathological entity [10, 40]. Sex hormones and the frequently unhealthy dietary patterns of adolescents could explain the higher prevalence of NAFLD in this age group [12]. Boys are more often affected by the disease than girls at a ratio of 2:1, regardless of age and BMI [9, 43, 44], but females have a different metabolic profile to that of males; the latter have especially elevated cholesterol and serum LDL concentrations, which are associated with a greater NAFLD risk than in girls [45]. On the other hand, the impact of gender on the development of NASH has yet to be elucidated. Indicatively, in adults, the prevalence of advanced fibrosis is higher in post-menopausal women than in men of similar age, a finding that additionally highlights the role of the loss of estrogens which occurs in NASH progression [46]. Ethnicity is an important factor, since, as already mentioned, large epidemiological studies showed that NAFLD is more common in Hispanic individuals compared to Caucasians and African-Americans [47,47,49]. Dyslipidemia is another risk factor, and a large percentage of children (20–80% according to different studies) may present with hypercholesteremia and/or hypertriglyceridemia [50, 51]. Individuals with NAFLD and elevated triglycerides are more likely to develop NASH. There is a strong association between fatty liver and metabolic syndrome and insulin resistance and presence of type 2 diabetes mellitus [52]. Children with metabolic syndrome have a five-fold increased risk for fatty liver compared to normal individuals. Celiac disease is an independent risk factor for NAFLD regardless of metabolic syndrome, but clinicians should also be aware of the co-existence of atypical celiac disease in obese children with liver dysfunction [53].
Dietary habits, including consumption of sugar-sweetened beverages, which has increased fivefold since the 1950s, have been associated with metabolic syndrome and fatty liver [54]. The fact that approximately 75% of all foods and beverages contain sugar in several forms constitutes a major public health problem. Concerns exist regarding the role of fructose in the predisposition for fatty liver. Two reviews published in 2014 suggested that the overall strength of evidence from observational studies is insufficient to prove an association between a hypercaloric fructose diet and fatty liver in healthy adults [55, 56]. Studies on this topic generally display inconsistent findings and are usually small, of short duration (less than 4 weeks), or of poor quality. Despite the lack of strong evidence relating high fructose consumption to NAFLD induction in healthy individuals, in patients with NAFLD, daily fructose ingestion is associated with increased hepatic fibrosis [57]. In obese adolescents, the risk of NAFLD is lower in those with lower fructose consumption [58], and, inversely, children with NAFLD are more prone to fructose beverage consumption than healthy children [59]. Endotoxins released by gut bacteria after fructose consumption seem to play an important role in NAFLD. Serum endotoxin levels are higher in children with NAFLD than in healthy individuals, and their levels are associated with insulin resistance and circulating concentrations of inflammatory cytokines [60]. Regarding low-calorie sweeteners, such as aspartame, stevia, and tryptophan, and their association in the pathogenesis of NAFLD, the only studies that presently exist are in experimental models (rats) [61,61,63]. Aspartame, when used long term, has been associated with hyperglycemia and serum triglyceride elevation, while upregulation of leptin and downregulation of adiponectin have been observed [61]. On the other hand, stevia-derived compounds and tryptophan supplementation in mice diet seem to reduce hepatic steatosis [63]. The abovementioned research findings should also be further investigated in humans.
Regarding other contributing factors, breastfeeding is protective against the disease and progression to NASH, as it protects from later development of obesity [64]. Some studies demonstrate that low birth weight neonates and children who showed rapid catch-up-growth also have an increased risk for subsequent obesity and NAFLD [65]; however, it is possible that accelerated infant weight gain during the first 3 months of life is a stronger risk factor for NAFLD than low birth weight [66]. Finally, obstructive sleep apnea is another risk factor for fatty liver disease, regardless of the presence of metabolic syndrome manifestations [67].
Clinical presentation and differential diagnosis of NAFLD
Most children with NAFLD are obese adolescents who are usually asymptomatic. Symptoms are inconsistent and may include vague pain at the right upper quadrant (indicative of NASH), malaise, and fatigue. On clinical examination, findings can be hepatomegaly (almost 50% of patients) and acanthosis nigricans, indicative of NASH and insulin resistance, respectively [40, 68]. Waist circumference is a valuable anthropometric parameter for NAFLD and NASH evaluation in children [40, 69] and was proposed to be used among other parameters (besides total bilirubin and total cholesterol) for the construction of a non-invasive prediction model for the progression from NAFLD to NASH [68]. Therefore, it is essential to construct charts for waist circumference according to ethnicity and age to be used in clinical practice [69].
Suspicion of NAFLD is raised when obese children present with elevated serum liver enzymes (mainly ALT and γ-GT) and/or increased hepatic echogenicity on liver ultrasound, and diagnosis is established when all other causes of fatty liver have been excluded. According to an ESPGHAN position paper [40], in all infants and children < 10 years of age, a detailed diagnostic laboratory evaluation is essential to exclude all other causes of fatty liver. In older children (> 10 years), NAFLD is most likely attributable to obesity; however, disorders with hepatic manifestations should always be suspected in children. The differential diagnosis of NAFLD in children is shown in Table 1. The suggested detailed laboratory evaluation of these children is displayed in Table 3. It should be stated with emphasis that pediatricians need to be aware of the metabolic disorders causing liver dysfunction in both children and adolescents, and that obesity with elevated serum transaminase levels should not be the only factor to justify making a diagnosis of NAFLD [40].
A problem in clinical practice is the evaluation of liver transaminases in children since the cutoff of serum ALT in pediatric populations has not yet been established. There is no consensus on defining the upper normal limits of serum ALT, which display gender differences [70]. Some investigators define as pathological an ALT value > 40 U/L [28, 71, 72], but the SAFETY study revealed that the sensitivity achieved by using as 95th percentile ALT levels of 25.8 U/L in boys and 22.1 U/L in girls is too low to detect liver diseases, such as NAFLD [70]. Pediatricians should be aware that (a) children with normal or mildly elevated ALT serum levels can present with fibrosis in liver biopsy and that ALT is not a reliable marker for NAFLD staging [73], (b) high serum levels of γGT are associated with advanced liver fibrosis in NAFLD patients [74], and (c) the AST:ALT ratio is generally > 1 and increases as hepatic fibrosis progresses [75]. Clinicians should be aware of cases of diverse etiology with hypertransaminasemia and/or hyperenzymemias or pseudotransaminasemias (macrotransaminasemia).
Liver biopsy
Nowadays, liver biopsy is considered the most reliable tool not only for the diagnosis of NAFLD, differentiating this entity from NASH, but also for exploring the degree of inflammation and fibrosis and ruling out co-existence of underlying diseases, such as autoimmune hepatitis. The main drawback is the invasiveness of the technique, not easily applied in pediatric populations, which might lead to severe complications, and always carrying the risk of inadequate sampling [40, 76].
Regarding histological findings from the liver biopsy, NAFLD is established when at least 5% of the hepatocytes present with micro- or macrovesicular steatosis without severe inflammation or fibrosis, and this criterion is identical for children and adults [77, 78]. This stage is the most benign one and is reversible when treated early. The histological pattern of NASH in children, which may differ from that in adults, is of two types: type 1 (adult type) and type 2 (pediatric type) [79]. Type 1 steatosis is characterized by ballooning degeneration and lobular inflammation, with or without perisinusoidal inflammation. Type 2 steatosis displays portal inflammation, with or without portal fibrosis, but ballooning degeneration and perisinusoidal inflammation are absent. However, there is an overlap, and both histological types can be observed in some pediatric patients [77, 78]. NASH type 2 ranges from 29 to 51% in children with fatty liver, being more frequent in younger children, especially boys of Asian and Hispanic descent and in those with more severe obesity, than individuals with NASH type 1 and is associated with advanced fibrosis [80, 81]. It seems that these two histological types have different etiology, pathogenesis, and response to treatment.
Several grading systems have been proposed for the assessment of the severity of NASH according to the histological findings from liver biopsy, such as the Brunt score and the NASH Clinical Research Network scoring system [77, 82]. According to these scores, there are four stages of fibrosis. Since the NASH score does not include portal inflammation, a histological feature characteristic of pediatric NASH, a new score, namely, the PNHS (Pediatric NAFLD Histological Score), has been proposed. A strong correlation between the PNHS score and the presence of NASH has been demonstrated [83].
A major clinical question is the ideal timing for liver biopsy in children with NAFLD. According to an ESPGHAN position paper (2012), criteria used for the timing to perform liver biopsy in pediatric patients are those proposed by Roberts et al. [40, 83] (Table 4), who stress that all laboratory investigations should be conducted before a liver biopsy is performed [40, 84].
The limitations and risks of liver biopsy have led to an effort to identify non-invasive biomarkers, which will reliably assess liver fibrosis and will replace liver biopsy as the gold standard method for NASH diagnosis and staging. These markers are related to apoptosis, oxidative stress, hepatic inflammation, and hepatic fibrosis. An example of a serum apoptosis marker is the intracellular intermediate filament protein cytokeratin-18 (CK-18), which is highly expressed in NAFLD patients, both adults and children, especially in those with NASH [85,85,86,87,89]. Nevertheless, there are conflicting results regarding the utility of this biomarker, as it is also over-expressed in other liver diseases.
Oxidative stress markers are molecules involved in oxidation pathways in the mitochondria, which are involved in the hepatic damage observed in NAFLD. Studies in pediatric populations showed correlation of some of these proteins (e.g., hepatic malondialdehyde, carbonyls, and bilirubin,) with hepatic fibrosis [90]. A study in 109 obese children and adolescents could not demonstrate a relationship between obesity with or without liver steatosis and oxidative stress [91]. In contrast, variants of the UGT1A1 gene expressing as high levels of bilirubin contribute to reduced risk of disease onset [92]. Hepatic inflammation serum markers are cytokines: low levels of adiponectin and high levels of TNF-alpha and leptin have been observed in pediatric patients with NASH [93, 94], but their utility in predicting hepatic fibrosis in obese subjects is in doubt [95].
As far as liver fibrosis markers are concerned, these are classified as non-specific and specific. Non-specific liver fibrosis markers in children are serum level AST/ALT > 1 (with low sensitivity), high γ-GT levels, and waist circumference [20, 68, 75]. Specific serum liver fibrosis markers panels have been suggested, such as FibroTest and the FIB4 index, but these panels have been tested mainly in adults [96, 97]. In children, proposed panels are (a) the pediatric NAFLD fibrosis index, which is based on age, waist circumference, and serum triglyceride concentrations, although longitudinal assessment is required for its implementation in clinical practice [83], and (b) the European Liver Fibrosis (ELF) panel that comprises hyaluronic acid, amino-terminal propeptide of type III collagen, and tissue inhibitor of metalloproteinase I, having high specificity and sensitivity compared with liver biopsy findings; however, this should be confirmed by further studies [98].
Imaging techniques
Several imaging techniques have been investigated, mainly in adults, for NAFLD screening, which have advantages and disadvantages regarding diagnostic accuracy, specificity, and sensitivity, cost, and availability, although none of them can reliably distinguish NAFLD from NASH.
The most common imaging technique used for NAFLD diagnosis is ultrasound due to its wide availability, low cost, safety (lack of radiation), and high sensitivity and specificity for detection of steatosis in liver parenchyma [99]. Portal hypertension is also easily assessed. Steatosis appears as a bright or hyperechoic lesion of the liver, but in order to avoid false-positive interpretations, liver echogenicity should exceed that of the renal cortex and spleen, resembling that of the pancreas. Also, an attenuation of the ultrasound wave should be noted, while loss of definition of the diaphragm and poor delineation of the intrahepatic architecture should also be observed [100]. A study performed in 208 pediatric patients with biopsy-proven NAFLD demonstrated that ultrasound displays excellent accuracy in NAFLD diagnosis but cannot determine fibrosis stage [101]. Another limitation is that it is operator dependent, and therefore, the operator’s experience is an important factor for diagnostic reliability.
Magnetic resonance imaging (MRI) is very often used for fatty liver assessment in adults. The MRI type widely applied in such cases is chemical shift gradient-echo (GRE) imaging with in-phase and opposed-phase acquisitions, and the quantification of hepatic fat in hepatocytes is achieved by assessing the degree of signal intensity loss [98]. The reported specificity and sensitivity for GRE is 100% and 81%, respectively [102]. A common disadvantage of ultrasound, MRI, and computed axial tomography is the fact that a low amount of liver fat, i.e., < 30% wet weight, is usually not detected [103]. MRI is very appealing for application in children with NAFLD, since it is non-invasive and not associated with X-ray radiation, though more studies comparing MRI findings with liver biopsy histopathological findings are necessary. Proton MR spectroscopy (MRS) is the most accurate magnetic resonance method for fatty liver assessment since it can detect steatosis grade 1 (5–30% of fat) [104]. On the other hand, this technique is very expensive, requires operator expertise, is not available in all imaging units, and does not generate anatomic images.
Another imaging method for liver fibrosis assessment is elastography, which can use either ultrasound or FibroScan, and whose principle is based on mechanical excitation and generation of images from the tissue response to the localized excitation [105]. It has, however, several limitations. In obese subjects (BMI > 28), quantification of liver fibrosis is difficult, and steatosis may be confused with fibrosis [40]. Furthermore, this imaging technique can detect only advanced fibrosis, but cannot differentiate fibrosis of intermediate stages [106]. MR elastography is a new and promising technique that could complement MRS for the estimation of the degree of liver steatosis, but further studies in both adults and children are needed to prove its superiority over other established methods [107].
Treatment of NAFLD
The optimal management of pediatric NAFLD is obesity prevention, as other treatment options in children are quite limited. Lifestyle interventions, such as a healthy diet and moderate exercise, are the only recommendations for the pediatric population so far [108, 109].
Weight reduction in children with NAFLD is mandatory because the reduction of free fatty acids load from food results in better peripheral glucose utilization [110]. Clinical studies in obese children revealed that even a minimal reduction in weight can lead to an improvement of serum transaminase levels and ultrasonographic findings [111, 112] as well as to the reduction of ROS generation and inflammation [113]. It is very important for pediatricians to be aware that early stages of NAFLD (steatosis only) are reversible and intervention at this stage is crucial. Reduction of dietary intake of saturated fat and fructose (contained in many soft drinks) and an increased intake of fibers and omega-3 fatty acids are recommended. Weight loss should be achieved gradually (500 g/week), as excessive and rapid weight loss can result in more severe liver damage. In younger children, whose growth is particularly important, weight loss is not always needed, and no further weight gain is recommended.
Physical activity is also important, and both aerobic and resistance exercise are required to reduce hepatic fat content [40]. The combination of a healthy diet intervention and an increase in physical activity can improve even the histological findings [114]. Lifestyle interventions should be followed, if possible, by all family members to achieve improved patient compliance, and psychological support may also be required. Pediatricians have a leading role in patient management along with other pediatric subspecialties (gastroenterologists and endocrinologists).
The use of pharmacologic agents in pediatric populations is still controversial [108, 109]. Drugs such as insulin sensitizers (metformin), cytoprotective agents (ursodeoxycholic acid), and hypolipidemic drugs have minimal effects, while the latter are not allowed in NASH [115,115,117]. Antioxidant agents, like vitamin E, are promising, but they do not display better efficacy than weight loss alone in NAFLD [115]. A study in 44 obese children and adolescents showed that antioxidant supplements used daily for 4 months had a significant impact on serum transaminase levels but did not reduce γGT and serum markers of inflammation [118].
Probiotics and prebiotics positively modulate gut microbiota and could be potential treatment modalities in NAFLD patients, although the data in pediatric populations are as yet sparse [109]. Very promising in pediatric patients are omega-3 long chain polyunsaturated fatty acids, present in several natural foods. A 6-month administration of omega-3 fatty acids in children resulted in an increase in insulin sensitivity and improvement of liver echo findings [119], while these agents were even shown to improve liver histology in pediatric NAFLD [120]. Recent findings indicate that a combination of pharmacologic agents, like docosahexaenoic acid, choline, and vitamin E, could provide better efficacy in children with steatohepatitis [121].
Finally, in adults, bariatric surgery for severe cases of NAFLD yielded good results as regards hepatic histology, this probably attributable to the significant weight loss. Studies in children are necessary to replicate these results, although bariatric surgery is generally not recommended in growing children and adolescents [122, 123].
Conclusions
NAFLD occurs in children and adolescents mainly because of childhood obesity and is associated with increased morbidity and mortality in adulthood. As there are no effective drugs to modify the natural course of NAFLD, which, if left untreated, may lead to irreversible liver damage and even hepatocellular carcinoma, the only effective management is prevention of childhood obesity from the very beginning. Efforts should be made to sensitize health care professionals and parents to avoid the devastating effects of childhood obesity through effective prevention and promotion of a healthy lifestyle, including a healthy diet and moderate physical activity.
References
Ogden CL, Carroll MD, Curtin LR, Lamb MM, Flegal KM (2010) Prevalence of high body mass index in US children and adolescents, 2007-2008. JAMA 3:242–249
Pan L, Freedman DS, Gillespie C, Park S, Sherry B (2011) Incidences of obesity and extreme obesity among US adults: findings from the 2009 Behavioral Risk Factor Surveillance System. Popul Health Metrics 9(1):56
Brunt EM (2010) Pathology of nonalcoholic fatty liver disease. Nat Rev Gastroenterol Hepatol 7:195–203
Mencin AA, Lavine JE (2011) Nonalcoholic fatty liver disease in children. Curr Opin Clin Nutr Metab Care 14(2):151–157
Schwimmer JB, Pardee PE, Lavine JE, Blumkin AK, Cook S (2008) Cardiovascular risk factors and the metabolic syndrome in pediatric nonalcoholic fatty liver disease. Circulation 3:277–283
Della Corte C, Alisi A, Saccari A, De Vito R, Vania A, Nobili V (2012) Nonalcoholic fatty liver in children and adolescents: an overview. J Adolesc Health 51:305–312
Barshop NJ, Sirlin CB, Schwimmer JB, Lavine JE (2008) Review article: epidemiology, pathogenesis and potential treatments of paediatric non-alcoholic fatty liver disease. Aliment Pharmacol Ther 28:13–24
Moya M (2008) An update in prevention and treatment of pediatric obesity. World J Pediatr 4:173–185
Bellentani S, Scaglioni F, Marino M, Bedogni G (2010) Epidemiology of non-alcoholic fatty liver disease. Dig Dis 28:155–161
Schwimmer JB, Deutsch R, Kahen T, Lavine JE, Stanley C, Behling C (2006) Prevalence of fatty liver in children and adolescents. Pediatrics 118:1388–1393
Rorat M, Jurek T, Kuchar E, Szenborn L, Golema W, Halon A (2013) Liver steatosis in polish children assessed by medicolegal autopsies. World J Pediatr 9:68–72
Oddy WH, Herbison CE, Jacoby P, Ambrosini GL, O'Sullivan TA, Ayonrinde OT et al (2013) The Western dietary pattern is prospectively associated with nonalcoholic fatty liver disease in adolescence. Am J Gastroenterol 108:778–785
Wiegand S, Keller KM, Röbl M, L’Allemand D, Reinehr T, Widhalm K, APV-Study Group and the German Competence Network Adipositas et al (2010) Obese boys at increased risk for nonalcoholic liver disease: evaluation of 16,390 overweight or obese children and adolescents. Int J Obes 34:1468–1474
Tominaga K, Fujimoto E, Suzuki K, Hayashi M, Ichikawa M, Inaba Y (2009) Prevalence of non-alcoholic fatty liver disease in children and relationship to metabolic syndrome, insulin resistance, and waist circumference. Environ Health Prev Med 14:142–149
Alavian SM, Mohammad-Alizadeh AH, Esna-Ashari F, Ardalan G, Hajarizadeh B (2009) Non-alcoholic fatty liver disease prevalence among school-aged children and adolescents in Iran and its association with biochemical and anthropometric measures. Liver Int 29:159–163
Chaturvedi K, Vohra P (2012) Non-alcoholic fatty liver disease in children. Indian Pediatr 49:757–758
Alisi A, Feldstein AE, Villani A, Raponi M, Nobili V (2012) Pediatric nonalcoholic fatty liver disease: a multidisciplinary approach. Nat Rev Gastroenterol Hepatol 9:152–161
Day CP (2010) Genetic and environmental susceptibility to non-alcoholic fatty liver disease. Dig Dis 28:255–260
Janczyk W, Socha P (2012) Non-alcoholic fatty liver disease in children. Clin Res Hepatol Gastroenterol 36:297–300
Giorgio V, Prono F, Graziano F, Nobili V (2013) Pediatric non-alcoholic fatty liver disease: old and new concepts on development, progression, metabolic insight and potential treatment targets. BMC Pediatr 25:13–40
Cambuli VM, Musiu MC, Incani M, Paderi M, Serpe R, Marras V et al (2008) Assessment of adiponectin and leptin as biomarkers of positive metabolic outcomes after lifestyle intervention in overweight and obese children. J Clin Endocrinol Metab 93:3051–3057
Burgert TS, Taksali SE, Dziura J, Goodman TR, Yeckel CW, Papademetris X et al (2006) Alanine aminotransferase levels and fatty liver in childhood obesity: associations with insulin resistance, adiponectin, and visceral fat. J Clin Endocrinol Metab 91:4287–4294
Giby VG, Ajith TA (2014) Role of adipokines and peroxisome proliferator-activated receptors in nonalcoholic fatty liver disease. World J Hepatol 6:570–579
Monteiro PA, Antunes Bde M, Silveira LS, Christofaro DG, Fernandes RA, Freitas Junior IF (2014) Body composition variables as predictors of NAFLD by ultrasound in obese children and adolescents. BMC Pediatr 14:25
Paniagua JA, Escandell-Morales JM, Gil-Contreras D, Berral de la Rosa FJ, Romero-Jimenez M, Gómez-Urbano A et al (2014) Central obesity and altered peripheral adipose tissue gene expression characterize the NAFLD patient with insulin resistance: role of nutrition and insulin challenge. Nutrition 30:177–185
do S Alve de Carvalho M, Coelho Cabral P, Kruze Grande de Arruda I, Goretti Pessoa Araújo de Burgos M, da Silva Diniz A, Barros Pernambuco JR et al (2012) Risk factors associated with hepatic steatosis: a study in patients in the Northeast Brazil. Nutr Hosp 27:1344–1350
Martins C, Freitas I Jr, Pizarro A, Aires L, Silva G, Santos MP et al (2013) Cardiorespiratory fitness, but not central obesity or C-reactive protein, is related to liver function in obese children. Pediatr Exerc Sci 25:3–11
Kelishadi R, Abtahi SH, Qorbani M, Heshmat R, Esmaeil Motlagh M, Taslimi M et al (2012) First National Report on aminotransaminases' percentiles in children of the Middle East and North Africa (MENA): the CASPIAN-III study. Hepat Mon 12:e7711
Schwimmer JB, Celedon MA, Lavine JE, Salem R, Campbell N, Schork NJ et al (2009) Heritability of nonalcoholic fatty liver disease. Gastroenterology 136:1585–1592
Willner IR, Waters B, Patil SR, Reuben A, Morelli J, Riely CA (2001) Ninety patients with nonalcoholic steatohepatitis: insulin resistance, familial tendency, and severity of disease. Am J Gastroenterol 96:2957–2961
Lin YC, Chang PF, Chang MH, Ni YH (2014) Genetic variants in GCKR and PNPLA3 confer susceptibility to nonalcoholic fatty liver disease in obese individuals. Am J Clin Nutr 99:869–874
Rotman Y, Koh C, Zmuda JM, Kleiner DE, Liang TJ, NASH CRN (2010) The association of genetic variability in patatin-like phospholipase domain-containing protein 3 (PNPLA3) with histological severity of nonalcoholic fatty liver disease. Hepatology 52:894–903
Walker RW, Sinatra F, Hartiala J, Weigensberg M, Spruijt-Metz D, Alderete TL et al (2013) Genetic and clinical markers of elevated liver fat content in overweight and obese Hispanic children. Obesity (Silver Spring) 21:E790–E797
Santoro N, Kursawe R, D'Adamo E, Dykas DJ, Zhang CK, Bale AE et al (2010) A common variant in the patatin-like phospholipase 3 gene (PNPLA3) is associated with fatty liver disease in obese children and adolescents. Hepatology 52:1281–1290
Nobili V, Liccardo D, Bedogni G, Salvatori G, Gnani D, Bersani I et al (2014) Influence of dietary pattern, physical activity, and I148M PNPLA3 on steatosis severity in at-risk adolescents. Genes Nutr 9:392
Csaki LS, Dwyer JR, Fong LG, Tontonoz P, Young SG, Reue K (2013) Lipins, lipinopathies, and the modulation of cellular lipid storage and signaling. Prog Lipid Res 52:305–316
Valenti L, Motta BM, Alisi A, Sartorelli R, Buonaiuto G, Dongiovanni P et al (2012) LPIN1 rs13412852 polymorphism in pediatric nonalcoholic fatty liver disease. J Pediatr Gastroenterol Nutr 54:588–593
Mahdessian H, Taxiarchis A, Popov S, Silveira A, Franco-Cereceda A, Hamsten A et al (2014) TM6SF2 is a regulator of liver fat metabolism influencing triglyceride secretion and hepatic lipid droplet content. Proc Natl Acad Sci U S A 111:8913–8918
Dongiovanni P, Petta S, Maglio C, Fracanzani AL, Pipitone R, Mozzi E et al (2014) TM6SF2 gene variant disentangles nonalcoholic steatohepatitis from cardiovascular disease. Hepatology 61:506–514
Vajro P, Lenta S, Socha P, Dhawan A, McKiernan P, Baumann U et al (2012) Diagnosis of nonalcoholic fatty liver disease in children and adolescents: position paper of the ESPGHAN hepatology committee. J Pediatr Gastroenterol Nutr 54:700–713
Lin YC, Chang PF, Hu FC, Chang MH, Ni YH (2009) Variants in the UGT1A1 gene and the risk of pediatric nonalcoholic fatty liver disease. Pediatrics 124(6):e1221–e1227
Welsh JA, Karpen S, Vos MB (2013) Increasing prevalence of nonalcoholic fatty liver disease among United States adolescents, 1988-1994 to 2007-2010. J Pediatr 162:496–500
Gupta R, Bhangoo A, Matthews NA, Anhalt H, Matta Y, Lamichhane B et al (2011) The prevalence of non-alcoholic fatty liver disease and metabolic syndrome in obese children. J Pediatr Endocrinol Metab 24:907–911
Imhof A, Kratzer W, Boehm B, Meitinger K, Trischler G, Steinbach G et al (2007) Prevalence of non-alcoholic fatty liver and characteristics in overweight adolescents in the general population. Eur J Epidemiol 22:889–897
Fernandes MT, Ferraro AA, de Azevedo RA, Fagundes Neto U (2010) Metabolic differences between male and female adolescents with non-alcoholic fatty liver disease, as detected by ultrasound. Acta Paediatr 99:1218–1223
Suzuki A, Abdelmalek MF, Schwimmer JB, Lavine JE, Scheimann AO, Unalp-Arida A et al (2012) Nonalcoholic steatohepatitis clinical research network. Association between puberty and features of nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol 10:786–794
Wolfgram PM, Connor EL, Rehm JL, Eickhoff JC, Reeder SB, Allen DB (2014) Ethnic differences in the effects of hepatic fat deposition on insulin resistance in nonobese middle school girls. Obesity (Silver Spring) 22:243–248
Deboer MD, Wiener RC, Barnes BH, Gurka MJ (2013) Ethnic differences in the link between insulin resistance and elevated ALT. Pediatrics 132:e718–e726
Hudson OD, Nunez M, Shaibi GQ (2012) Ethnicity and elevated liver transaminases among newly diagnosed children with type 2 diabetes. BMC Pediatr 12:174
Papandreou D, Karabouta Z, Rousso I (2012) Are dietary cholesterol intake and serum cholesterol levels related to nonalcoholic Fatty liver disease in obese children? Cholesterol 2012:572820
Nakamuta M, Fujino T, Yada R, Yada M, Yasutake K, Yoshimoto T et al (2009) Impact of cholesterol metabolism and the LXRalpha-SREBP-1c pathway on nonalcoholic fatty liver disease. Int J Mol Med 23:603–608
Sundaram SS, Zeitler P, Nadeau K (2009) The metabolic syndrome and nonalcoholic fatty liver disease in children. Curr Opin Pediatr 21:529–535
Ludvigsson JF, Elfström P, Broomé U, Ekbom A, Montgomery SM (2007) Celiac disease and risk of liver disease: a general population-based study. Clin Gastroenterol Hepatol 5:63–69
Bray GA, Popkin BM (2014) Dietary sugar and body weight: have we reached a crisis in the epidemic of obesity and diabetes?: health be damned! Pour on the sugar. Diabetes Care 37:950–956
Chung M, Ma J, Patel K, Berger S, Lau J, Lichtenstein AH (2014) Fructose, high-fructose corn syrup, sucrose, and nonalcoholic fatty liver disease or indexes of liver health: a systematic review and meta-analysis. Am J Clin Nutr 100:833–849
Chiu S, Sievenpiper JL, de Souza RJ, Cozma AI, Mirrahimi A, Carleton AJ et al (2014) Effect of fructose on markers of non-alcoholic fatty liver disease (NAFLD): a systematic review and meta-analysis of controlled feeding trials. Eur J Clin Nutr 68:416–423
Abdelmalek MF, Suzuki A, Guy C, Unalp-Arida A, Colvin R, Johnson RJ et al (2010) Nonalcoholic steatohepatitis clinical research network. Increased fructose consumption is associated with fibrosis severity in patients with nonalcoholic fatty liver disease. Hepatology 51:1961–1971
O’Sullivan TA, Oddy WH, Bremner AP, Sherriff JL, Ayonrinde OT, Olynyk JK et al (2014) Lower fructose intake may help protect against development of nonalcoholic fatty liver in adolescents with obesity. Pediatr Gastroenterol Nutr 58:624–631
Jin R, Le NA, Liu S, Farkas Epperson M, Ziegler TR, Welsh JA et al (2012) Children with NAFLD are more sensitive to the adverse metabolic effects of fructose beverages than children without NAFLD. J Clin Endocrinol Metab 97:E1088–E1098
Jin R, Willment A, Patel SS, Sun X, Song M, Mannery YO et al (2014) Fructose induced endotoxemia in pediatric nonalcoholic fatty liver disease. Int J Hepatol 2014:560620
Lebda MA, Tohamy HG, El-Sayed YS (2017) Long-term soft drink and aspartame intake induces hepatic damage via dysregulation of adipocytokines and alteration of the lipid profile and antioxidant status. Nutr Res 41:47–55
Holvoet P, Rull A, García-Heredia A, López-Sanromà S, Geeraert B, Joven J et al (2015) Stevia-derived compounds attenuate the toxic effects of ectopic lipid accumulation in the liver of obese mice: a transcriptomic and metabolomic study. Food Chem Toxicol 77:22–33
Ritze Y, Bárdos G, Hubert A, Böhle M, Bischoff SC (2014) Effect of tryptophan supplementation on diet-induced non-alcoholic fatty liver disease in mice. Br J Nutr 112:1–7
Nobili V, Bedogni G, Alisi A, Pietrobattista A, Alterio A, Tiribelli C et al (2009) A protective effect of breastfeeding on the progression of non-alcoholic fatty liver disease. Arch Dis Child 94:801–805
Nobili V, Alisi A, Panera N, Agostoni C (2008) Low birth weight and catch-up-growth associated with metabolic syndrome: a ten year systematic review. Pediatr Endocrinol Rev 6:241–247
Breij LM, Kerkhof GF, Hokken-Koelega AC (2014) Accelerated infant weight gain and risk for nonalcoholic fatty liver disease in early adulthood. J Clin Endocrinol Metab 99:1189–1195
Nobili V, Cutrera R, Liccardo D, Pavone M, Devito R, Giorgio V et al (2014) Obstructive sleep apnea syndrome affects liver histology and inflammatory cell activation in pediatric nonalcoholic fatty liver disease, regardless of obesity/insulin resistance. Am J Respir Crit Care Med 189:66–76
Eng K, Lopez R, Liccardo D, Nobili V, Alkhouri N (2014) A non-invasive prediction model for non-alcoholic steatohepatitis in paediatric patients with non-alcoholic fatty liver disease. Dig Liver Dis 46:1008–1013
Bacopoulou F, Efthymiou V, Landis G, Rentoumis A, Chrousos GP (2015) Waist circumference, waist-to-hip ratio and waist-to-height ratio reference percentiles for abdominal obesity among Greek adolescents. BMC Pediatr 4:15–50
Schwimmer JB, Dunn W, Norman GJ, Pardee PE, Middleton MS, Kerkar N et al (2010) SAFETY study: alanine aminotransferase cutoff values are set too high for reliable detection of pediatric chronic liver disease. Gastroenterology 138:1357–1364
Elizondo-Montemayor L, Ugalde-Casas PA, Lam-Franco L, Bustamante-Careaga H, Serrano-González M, Gutiérrez NG et al (2014) Association of ALT and the metabolic syndrome among Mexican children. Obes Res Clin Pract 8:e79–e87
Wei C, Ford A, Hunt L, Crowne EC, Shield JP (2011) Abnormal liver function in children with metabolic syndrome from a UK-based obesity clinic. Arch Dis Child 96:1003–1007
Poustchi H, George J, Esmaili S, Esna-Ashari F, Ardalan G, Sepanlou SG et al (2011) Gender differences in healthy ranges for serum alanine aminotransferase levels in adolescence. PLoS One 6:e21178
Molleston JP, Schwimmer JB, Yates KP, Murray KF, Cummings OW, Lavine JE et al (2014) NASH clinical research network. Histological abnormalities in children with nonalcoholic fatty liver disease and normal or mildly elevated alanine aminotransferase levels. J Pediatr 164:707–713
Patton HM, Lavine JE, Van Natta ML, Schwimmer JB, Kleiner D, Molleston J (2008) Nonalcoholic steatohepatitis clinical research network. Clinical correlates of histopathology in pediatric nonalcoholic steatohepatitis. Gastroenterology 135:1961–1971
Wieckowska A, Feldstein AE (2008) Diagnosis of nonalcoholic fatty liver disease: invasive versus noninvasive. Semin Liver Dis 28:386–395
Brunt EM, Janney CG, Di Bisceglie AM, Neuschwander-Tetri BA, Bacon BR (1999) Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. Am J Gastroenterol 94:2467–2474
Carter-Kent C, Yerian LM, Brunt EM, Angulo P, Kohli R, Ling SC et al (2009) Nonalcoholic steatohepatitis in children: a multicenter clinicopathological study. Hepatology 50:1113–1120
Schwimmer JB, Behling C, Newbury R, Deutsch R, Nievergelt C, Schork NJ et al (2005) Histopathology of pediatric nonalcoholic fatty liver disease. Hepatology 42:641–649
Nobili V, Marcellini M, Devito R, Ciampalini P, Piemonte F, Comparcola D et al (2006) NAFLD in children: a prospective clinical-pathological study and effect of lifestyle advice. Hepatology 44:458–465
Takahashi Y, Fukusato T (2010) Pediatric nonalcoholic fatty liver disease: overview with emphasis on histology. World J Gastroenterol 16:5280–5285
Juluri R, Vuppalanchi R, Olson J, Unalp A, Van Natta ML, Cummings OW et al (2011) Generalizability of the nonalcoholic steatohepatitis clinical research network histologic scoring system for nonalcoholic fatty liver disease. J Clin Gastroenterol 45:55–58
Alkhouri N, De Vito R, Alisi A, Yerian L, Lopez R, Feldstein AE et al (2012) Development and validation of a new histological score for pediatric non-alcoholic fatty liver disease. J Hepatol 57:1312–1318
Roberts EA (2007) Pediatric nonalcoholic fatty liver disease (NAFLD): a "growing" problem? J Hepatol 46:1133–1142
Feldstein AE, Wieckowska A, Lopez AR, Liu YC, Zein NN, McCullough AJ (2009) Cytokeratin-18 fragment levels as noninvasive biomarkers for nonalcoholic steatohepatitis: a multicenter validation study. Hepatology 50:1072–1078
Chan WK, Sthaneshwar P, Nik Mustapha NR, Mahadeva S (2014) Limited utility of plasma M30 in discriminating non-alcoholic steatohepatitis from steatosis--a comparison with routine biochemical markers. PLoS One 9:e105903
Vuppalanchi R, Jain AK, Deppe R, Yates K, Comerford M, Masuoka HC et al (2014) Relationship between changes in serum levels of keratin 18 and changes in liver histology in children and adults with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol 12:2121–2130
Feldstein AE, Alkhouri N, De Vito R, Alisi A, Lopez R, Nobili V (2013) Serum cytokeratin-18 fragment levels are useful biomarkers for nonalcoholic steatohepatitis in children. Am J Gastroenterol 108:1526–1531
Fitzpatrick E, Mitry RR, Quaglia A, Hussain MJ, DeBruyne R, Dhawan A (2010) Serum levels of CK18 M30 and leptin are useful predictors of steatohepatitis and fibrosis in paediatric NAFLD. J Pediatr Gastroenterol Nutr 51:500–506
Nobili V, Parola M, Alisi A, Marra F, Piemonte F, Mombello C et al (2010) Oxidative stress parameters in paediatric non-alcoholic fatty liver disease. Int J Mol Med 26:471–476
Torun E, Gökçe S, Ozgen İT, Aydın S, Cesur Y (2014) Serum paraoxonase activity and oxidative stress and their relationship with obesity-related metabolic syndrome and non-alcoholic fatty liver disease in obese children and adolescents. J Pediatr Endocrinol Metab 27:667–675
Puri K, Nobili V, Melville K, Corte CD, Sartorelli MR, Lopez R et al (2013) Serum bilirubin level is inversely associated with nonalcoholic steatohepatitis in children. J Pediatr Gastroenterol Nutr 57:114–118
Lebensztejn DM, Wojtkowska M, Skiba E, Werpachowska I, Tobolczyk J, Kaczmarski M (2009) Serum concentration of adiponectin, leptin and resistin in obese children with non-alcoholic fatty liver disease. Adv Med Sci 54:177–182
Lebensztejn DM, Kowalczuk D, Tarasów E, Skiba E, Kaczmarski M (2010) Tumor necrosis factor alpha and its soluble receptors in obese children with NAFLD. Adv Med Sci 55:74–79
Koot BG, van der Baan-Slootweg OH, Bohte AE, Nederveen AJ, van Werven JR, Tamminga-Smeulders CL et al (2013) Accuracy of prediction scores and novel biomarkers for predicting nonalcoholic fatty liver disease in obese children. Obesity (Silver Spring) 21:583–590
Ratziu V, Massard J, Charlotte F, Messous D, Imbert-Bismut F, Bonyhay L et al (2006) LIDO study group; CYTOL study group. Diagnostic value of biochemical markers (FibroTest-FibroSURE) for the prediction of liver fibrosis in patients with non-alcoholic fatty liver disease. BMC Gastroenterol 6:6
Shah AG, Lydecker A, Murray K, Tetri BN, Contos MJ, Sanyal AJ (2009) Nash clinical research network. Comparison of noninvasive markers of fibrosis in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol 7:1104–1112
Nobili V, Parkes J, Bottazzo G, Marcellini M, Cross R, Newman D et al (2009) Performance of ELF serum markers in predicting fibrosis stage in pediatric non-alcoholic fatty liver disease. Gastroenterology 136:160–167
Mazhar SM, Shiehmorteza M, Sirlin CB (2009) Noninvasive assessment of hepatic steatosis. Clin Gastroenterol Hepatol 7:135–140
Hamer OW, Aguirre DA, Casola G, Lavine JE, Woenckhaus M, Sirlin CB (2006) Fatty liver: imaging patterns and pitfalls. Radiographics 26:1637–1653
Shannon A, Alkhouri N, Carter-Kent C, Monti L, Devito R, Lopez R et al (2011) Ultrasonographic quantitative estimation of hepatic steatosis in children with NAFLD. J Pediatr Gastroenterol Nutr 53:190–195
Kim SH, Lee JM, Han JK, Lee JY, Lee KH, Han CJ et al (2006) Hepatic macrosteatosis: predicting appropriateness of liver donation by using MR imaging--correlation with histopathologic findings. Radiology 240:116–129
Limanond P, Raman SS, Lassman C, Sayre J, Ghobrial RM, Busuttil RW et al (2004) Macrovesicular hepatic steatosis in living related liver donors: correlation between CT and histologic findings. Radiology 230:276–280
Bohte AE, van Werven JR, Bipat S, Stoker J (2011) The diagnostic accuracy of US, CT, MRI and 1H-MRS for the evaluation of hepatic steatosis compared with liver biopsy: a meta-analysis. Eur Radiol 21:87–97
Boursier J, Isselin G, Fouchard-Hubert I, Oberti F, Dib N, Lebigot J et al (2010) Acoustic radiation force impulse: a new ultrasonographic technology for the widespread noninvasive diagnosis of liver fibrosis. Eur J Gastroenterol Hepatol 22:1074–1084
Marginean CO, Marginean C (2012) Elastographic assessment of liver fibrosis in children: a prospective single center experience. Eur J Radiol 81:e870–e874
Loomba R, Wolfson T, Ang B, Booker J, Behling C, Peterson M et al (2014) Magnetic resonance elastography predicts advanced fibrosis in patients with nonalcoholic fatty liver disease: a prospective study. Hepatology 60:1920–1928
Vajro P, Lenta S, Pignata C, Salerno M, D'Aniello R, De Micco I et al (2012) Therapeutic options in pediatric non-alcoholic fatty liver disease: current status and future directions. Ital J Pediatr 38:55
Alisi A, Nobili V (2012) Non-alcoholic fatty liver disease in children now: lifestyle changes and pharmacologic treatments. Nutrition 28:722–726
Ryan MC, Itsiopoulos C, Thodis T, Ward G, Trost N, Hofferberth S et al (2013) The Mediterranean diet improves hepatic steatosis and insulin sensitivity in individuals with non-alcoholic fatty liver disease. J Hepatol 59:138–143
Reinehr T, Schmidt C, Toschke AM, Andler W (2009) Lifestyle intervention in obese children with non-alcoholic fatty liver disease: 2-year follow-up study. Arch Dis Child 94:437–442
Verduci E, Pozzato C, Banderali G, Radaelli G, Arrizza C, Rovere A et al (2013) Changes of liver fat content and transaminases in obese children after 12-mo nutritional intervention. World J Hepatol 5:505–512
Shah K, Stufflebam A, Hilton TN, Sinacore DR, Klein S, Villareal DT (2009) Diet and exercise interventions reduce intrahepatic fat content and improve insulin sensitivity in obese older adults. Obesity (Silver Spring) 17:2162–2168
Nobili V, Manco M, Devito R, Di Ciommo V, Comparcola D, Sartorelli MR et al (2008) Lifestyle intervention and antioxidant therapy in children with nonalcoholic fatty liver disease: a randomized, controlled trial. Hepatology 48:119–128
Lavine JE, Schwimmer JB, Van Natta ML, Molleston JP, Murray KF, Rosenthal P et al (2011) Effect of vitamin E or metformin for treatment of nonalcoholic fatty liver disease in children and adolescents: the TONIC randomized controlled trial. JAMA 305:1659–1668
Dohil R, Schmeltzer S, Cabrera BL, Wang T, Durelle J, Duke KB et al (2011) Enteric-coated cysteamine for the treatment of paediatric non-alcoholic fatty liver disease. Aliment Pharmacol Ther 33:1036–1044
Vajro P, Franzese A, Valerio G, Iannucci MP, Aragione N (2000) Lack of efficacy of ursodeoxycholic acid for the treatment of liver abnormalities in obese children. J Pediatr 136:739–743
Murer SB, Aeberli I, Braegger CP, Gittermann M, Hersberger M, Leonard SW et al (2014) Antioxidant supplements reduced oxidative stress and stabilized liver function tests but did not reduce inflammation in a randomized controlled trial in obese children and adolescents. J Nutr 144:193–201
Nobili V, Bedogni G, Alisi A, Pietrobattista A, Risé P, Galli C et al (2011) Docosahexaenoic acid supplementation decreases liver fat content in children with non-alcoholic fatty liver disease: double-blind randomised controlled clinical trial. Arch Dis Child 96:350–353
Nobili V, Carpino G, Alisi A, De Vito R, Franchitto A, Alpini G et al (2014) Role of docosahexaenoic acid treatment in improving liver histology in pediatric nonalcoholic fatty liver disease. PLoS One 9(2):e88005
Zöhrer E, Alisi A, Jahnel J, Mosca A, Della Corte C, Crudele A et al (2017) Efficacy of docosahexaenoic acid-choline-vitamin E in paediatric NASH: a randomized controlled clinical trial. Appl Physiol Nutr Metab 42:948–954
Pardee PE, Lavine JE, Schwimmer JB (2009) Diagnosis and treatment of pediatric nonalcoholic steatohepatitis and the implications for bariatric surgery. Semin Pediatr Surg 18:144–151
Weiner RA (2010) Surgical treatment of non-alcoholic steatohepatitis and non-alcoholic fatty liver disease. Dig Dis 28:2742–2279
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tzifi, F., Fretzayas, A., Chrousos, G. et al. Non-alcoholic fatty liver infiltration in children: an underdiagnosed evolving disease. Hormones 18, 255–265 (2019). https://doi.org/10.1007/s42000-019-00107-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42000-019-00107-7