Abstract
Atherosclerosis begins in childhood. Fatty streaks, the earliest precursor of atherosclerotic lesions, have been found in the coronary arteries of children of 2 years of age. Hypercholesterolaemia is a risk factor for coronary artery disease. Hypercholesterolaemia can be either primary, when it is characteristic of the main disease, or secondary when it occurs as a result of either a disease process or drug treatment. Given the risk of vascular disease, including myocardial infarction (MI), cerebrovascular accidents (CVA, also known as strokes), peripheral vascular disease (PVD) and ruptured aortic aneurysm, which may follow atherosclerosis, it is important to prevent or slow the early development of atherosclerotic lesions. This prevention necessitates the control of key risk factors such hypercholesterolaemia, dyslipidaemia, hypertension etc. However, at what point this prevention ought to occur, and in what form, is uncertain. Using pharmacological primary prevention for hypercholesterolaemia in the paediatric population is controversial. In an adult patient, hypercholesterolaemia warrants the initiation of a statin. Statins, also known as hydroxymethylglutaryl co-enzyme A inhibitors (or HMG-CoA inhibitors) act by altering cholesterol metabolism. In the paediatric population, the clinical course of vascular disease and the effect of altering this clinical course are less certain. This article reviews the published literature on hypercholesterolaemia in children and the use of statins as a treatment for dyslipidaemia in children. The US National Cholesterol Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents 2012 guidelines (NCEP guidelines) regarding the recognition and treatment of childhood dyslipidaemia are reviewed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Atherosclerosis begins in childhood [1,2,3]. Fatty streaks, the earliest precursor of atherosclerotic lesions, have been found in the coronary arteries of children as young as 2 years [4]. Hypercholesterolaemia is a risk factor for coronary artery disease. Hypercholesterolaemia can be either primary, when it is characteristic of the main disease, or secondary when it occurs as a result of either a disease process or drug treatment.
Given the risk of vascular disease, including myocardial infarction (MI), cerebrovascular accidents (CVA), peripheral vascular disease (PVD) and ruptured aortic aneurysm, any or all of which may follow atherosclerosis, it is important to prevent or slow the early development of atherosclerotic lesions. This prevention necessitates the control of key risk factors such as hypercholesterolaemia, dyslipidaemia, hypertension etc. However, when and how this prevention should occur are uncertain.
Using pharmacological primary prevention for hypercholesterolaemia in the paediatric population is controversial. In an adult patient, the course of action is clear. Hypercholesterolaemia warrants the initiation of a statin [5]. Statins, also known as hydroxymethylglutaryl co-enzyme A inhibitors (or HMG-CoA inhibitors) act by altering cholesterol metabolism. In children, the clinical course of vascular disease and the potential effects of altering this clinical course are less certain.
This article reviews the published literature on hypercholesterolaemia in children and the use of statins as a treatment for dyslipidaemia in children. The US National Cholesterol Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents 2012 guidelines (NCEP guidelines) regarding the recognition and treatment of childhood dyslipidaemia are reviewed.
Materials and methods
In June 2014, a literature review was conducted using Medline and Pubmed. Search terms included ‘hypercholesterolemia’, ‘paediatrics’, ‘statins’, ‘atherosclerosis’, ‘cholesterol reduction’, ‘childhood vascular disease’, ‘paediatric obesity’, ‘dyslipidemia’, ‘hypertriglyceridemia’, and ‘cholesterol’. To increase the sensitivity of the search, the search strategy was not limited to manuscripts about children; but this review paper is. Citation searching and snowball searching were also performed. The research objective was to assess the available literature on hypercholesterolaemia and the use of statins in the paediatric population.
Results
Two hundred articles were retrieved and reviewed for inclusion; 52 were excluded as they did not contain information relevant to the research objective. The 2012 guidelines released by the US Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents regarding the recognition and treatment of childhood dyslipidaemia were also reviewed for this discussion. The remaining articles form the basis of this review.
Discussion
Atherosclerosis in childhood
Atherosclerosis is a complex process in which lipids and inflammatory mediators cause the formation of fatty streaks which progress to plaque formation. The plaque is at risk of rupture and thrombus formation which increases the risk of MI and ischaemic CVA.
Atherosclerosis has its origins in childhood [1,2,3]. This was first asserted by Holman who claimed atherosclerosis was a ‘paediatric problem’ [6]. Several studies since then validate this claim, including the P-Day Study (Pathobiological Determinants of Atherosclerosis in Youth) [2] and the Bogalusa Heart Study [3]. In the P-Day study, post-mortem studies revealed that cardiovascular risk factors such as hypercholesterolaemia and smoking were associated with the size of fatty streaks and plaques in youth [7]. The Bogalusa Heart Study examined the coronary arteries and the aortas of 35 young autopsied subjects, and found a positive association between fatty streaks and low-density lipoprotein cholesterol (LDL-c) and a negative association with high-density lipoprotein cholesterol (HDL-c) [3]. Fatty streaks, the earliest precursor of atherosclerosis, have been found in the coronary arteries of children of 2 years of age [1]. In the Bogalusa study, the prevalence of fatty streaks and fibrous plaques were 50 and 8% respectively during childhood, and 69 and 85% respectively, during young adulthood [1]. These results describe the childhood origins of the first stages of atherosclerosis.
Risk factors for atherosclerosis in childhood include obesity, hypertension, dyslipidaemia, family history of cardiovascular disease, smoking and hyperglycaemia [8]. Atherosclerosis in a young person is more likely in the presence of elevated total and LDL cholesterol, elevated BMI and systolic and diastolic hypertension [1, 2]. However, children in whom the atherosclerotic process has already begun are usually asymptomatic [9]. The Muscatine Study demonstrated that increased total cholesterol levels in childhood were associated with increased carotid intimal-medial thickness (CIMT) in adults aged 33–42 years of age [10]. CIMT is a surrogate marker of the atherosclerotic process. However, the severity of atherosclerosis correlates with the duration of hypercholesterolaemia; therefore, early initiation of a statin in a child with familial hypercholesterolaemia (FH) may help to prevent atherosclerosis [11].
The detection of atherosclerosis in children is difficult, as the final stages including MI and CVA rarely manifest in childhood. Also, its detection should ideally be non-invasive. Thus, surrogate markers for measuring atherosclerosis have been developed. These include endothelial dysfunction studies, which measure flow-mediated dilation (FMD), and thickening of the vessel walls, by measuring vessel IMT. Carotid artery IMT predicts MI, CVA and aneurysm [12]. Increased IMT is found in children with FH and in children with a parental history of premature cardiovascular disease [13]. Other non-invasive measures of atherosclerosis include pulse wave velocity (PWV) which estimates arterial stiffness, computed tomography (CT) which quantifies calcium deposits in the coronary arteries, and magnetic resonance imaging (MRI) which measures atheromatous plaques [14]. Of these, CT is not ideal for use in children due to the risk of exposure to ionising radiation. MRI allows examination of the cardiovascular structures and plaques without radiation exposure.
The above surrogate measures of atherosclerosis are not frequently clinically accessible in a typical hospital, usually requiring dedicated expensive equipment and a dedicated laboratory or space and trained personnel. Increasingly, efforts are being made to develop tests that are of more clinical utility. One example is the use of peripheral arterial tonometry (PAT, © Itamar Medical, Israel) to measure endothelial dysfunction, one of the early and potentially reversible steps in the natural history of atherosclerosis.
Primary hyperlipidaemia in children
Hyperlipidaemia may be primary, when it occurs as a main feature of the primary disease causing hyperlipidaemia, or secondary, when it occurs as a consequence of a related disease, disorder or treatment [15]. Primary hyperlipidaemia occurs in children with a genetic predisposition; conditions include heterozygous or homozygous FH (HeFH or HoFH), familial hypertriglyceridaemia, familial combined hyperlipidaemia (FCH) and dysbetalipoproteinaemia. FH and FCH are the most common in paediatric practice [16]. Of these, FH forms the body of research into childhood primary dyslipidaemia with HoFH being less common and presenting with a more severe phenotype and at a younger age than HeFH.
FH is characterised by increased total cholesterol (TC) and increased LDL-c, while triglycerides (TG) and HDL-c are typically normal [16]. FH also has an inflammatory component, in addition to dyslipidaemia [17]. FH comprises HeFH and HoFH. HeFH has a prevalence of 1 in 500 people [18]. HoFH has a prevalence of one per million people [19]. Both HeFH and HoFH are autosomal dominant. Mortality is 100 times higher in FH patients between 20 and 39 years as compared to the normal population [20].
HoFH is caused by mutations to both LDL receptor alleles, and results in elevated plasma LDL-c from birth, cutaneous or tendinous xanthomata, and overt cardiovascular disease early in childhood [20]. HoFH is so severe, that untreated carriers rarely survive past their second decade of life [20]. Total cholesterol concentrations in HoFH range between 18.1 and 31 mmol/L [19]. HoFH is treated with high-dose statins, LDL-c apheresis and liver transplantation.
HeFH is characterised by total cholesterol 6.98–12.93 mmol/L [18] and LDL-c in excess of 5.5 mmol/L [21]. Total cholesterol and LDL-c in HeFH are usually twice the normal level [22, 23]. This dyslipidaemia causes premature cardiovascular disease, with 50% of men and 25% of women developing a cardiovascular event by the age of 50 years [16]. As a result of this risk, those with HeFH are candidates for the early initiation of statin therapy. Unlike HoFH, patients with HeFH do not usually exhibit clinical signs of hypercholesterolaemia in childhood or adolescence. However, children with HeFH have thicker carotid and aortic IMT [24, 25]. Adolescents with HeFH have significant arterial wall thickening and the rate of progression of atherosclerosis is greater than in those without HeFH [9, 26].
Secondary hyperlipidaemia
Secondary hyperlipidaemia, i.e. hyperlipidaemia which occurs as a result of a disease, disorder or treatment, has increased in recent years. Causes of secondary hyperlipidaemia in childhood include obesity, diabetes mellitus, chronic kidney disease, hypothyroidism, liver failure, nephrotic syndrome and connective tissue disease [15, 27]. Drugs which cause a secondary hyperlipidaemia include glucocorticoids, retinoids, beta blockers and anti-retroviral therapies [15].
There has been a dramatic rise in secondary dyslipidaemia related to childhood obesity. In US epidemiological studies, childhood obesity has a prevalence of 20–30% [16, 28]. The dyslipidaemia associated with obesity is characterised by hypertriglyceridaemia and decreasing HDL-c [15, 28, 29]. There is a strong association between childhood obesity and the metabolic syndrome (dyslipidaemia, hypertension and insulin resistance), all of which result in atherosclerosis [28]. Childhood obesity is associated with the risk of cardiovascular disease in adulthood [30].
Obesity-related dyslipidaemia is best treated in the first instance by lifestyle changes such as low-fat diet and exercise [31].
NCEP guidelines 2012
Guidelines relating to childhood hyperlipidaemia and the risk of cardiovascular disease were released by the National Cholesterol Education Program Expert Panel in 2012 (NCEP) [32]. The guidelines stress the early identification of children with dyslipidaemia using universal screening, and the importance of assessing serum lipid profiles. To this end, acceptable, borderline and high values for each of the cholesterol types have been compiled [32].
A positive family history refers to a cardiovascular event, such as myocardial infarction, angina, CVA, coronary artery bypass graft (CABG), stent or angioplasty, in a first degree relative < 55 years if male and < 65 years if female [32]. If LDL-c elevation persists at ≥ 130 mg/dL (3.36 mmol/L), referral to a lipid specialist for addition of second lipid-lowering therapy is required. High-level risk factors include hypertension, current cigarette smoker, BMI ≥ 97th centile and presence of high-risk conditions such as diabetes mellitus, chronic kidney disease and postorthoptic heart transplant. Moderate-level risk factors include hypertension not requiring drug therapy, BMI between 95th and 97th centile, HDL-C < 40 mg/dL (1.03 mmol/L) and the presence of moderate risk conditions such as Kawasaki disease, SLE, HIV and nephrotic syndrome [32].
Initiation of pharmacological therapy should be based on the average of at least two fasting lipid profiles obtained 2 weeks to 3 months apart [32]. The goal of therapy in childhood and adolescence is LDL-c below the 95th centile (≤ 130 mg/dL or 3.36 mmol/L). The guidelines advise that in children < 10 years old, statin therapy should be limited to those with a severe primary hyperlipidaemia, a high-risk condition or evidence of cardiovascular disease and must be initiated by a lipid specialist. For children aged 8–9 years, statin therapy may be considered if the LDL-c remains > 190 mg/dL (4.9 mmol/L) after a trial of lifestyle management, and in the presence of a family history of premature cardiovascular events, a high-level risk factor or at least two moderate-level risk factors [32] (Tables 1 and 2) (Figs. 1).
When to initiate statin therapy based on LDL-C. Adapted from Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents. NHLBI (2012) [32]
Conversion mg/dL to mmol/L: cholesterol is mg/dL × 0.0259 = mmol/L and triglycerides is mg/dL × 0.0113.
Screening for hyperlipidaemia in childhood
The NCEP guidelines recommend universal screening of children aged 9–11 years old and again at 17–21 years old with a non-fasting lipid screen [32]. Previously, targeted screening of those children at increased risk of cardiovascular disease was recommended [33]. However, some studies demonstrated that the AAP targeted screening criteria lacked sensitivity. In one such study, dyslipidaemia requiring pharmacologic treatment was more common among children who did not meet screening criteria than among those who did [34]. In another study of 678 children, the sensitivity of the 2008 AAP screening guidelines was found to be 54–66% [35]. Targeted screening may lack sensitivity due to the factors required to establish increased cardiovascular risk, i.e. family history, and parental serum cholesterol level. Family history may be unknown in some cases, whereas parental cholesterol may not have been checked [14]. There is a concern that universal screening will carry a heavy economic burden, and may cause unnecessary parental anxiety [36].
An ideal screening test is valid, sensitive, specific, simple and safe. It must also be acceptable to the population that it is intended to screen [37]. Screening children for dyslipidaemia, whether targeted or universal, is controversial. Some authors assert that there is no evidence that treatment of dyslipidaemias in childhood will prevent cardiovascular events later [16]. Universal screening will reveal children with dyslipidaemia due to modifiable lifestyle factors, which perhaps should not be treated pharmacologically. However, there are studies which demonstrate that treating hyperlipidaemia early in life delays progression of atherosclerosis [11, 38]. One aspect of screening is to rule out secondary causes of dyslipidaemia, e.g. diabetes mellitus, hypothyroidism and renal failure [14]. Certainly, conditions such as FH confer an increased risk of cardiovascular disease. Since the atherosclerotic process begins early in life, on balance, many authors concur with the expert consensus [32] that early universal screening is justified. However, given the economic implications of universal screening, and its as yet uncertain benefit, it may be more sensible to improve the targeted screening process in the first instance.
A recent survey of family medicine practitioners, paediatricians and general practitioners (n = 548) confirmed that while 74% of respondents believed that lipid screening and treatment would reduce future cardiovascular risk, 34% performed no screening, 50% screened selectively and only 16% performed universal screening [39]. The reasons cited for failing to screen were uneasiness addressing lipid disorders (43%), and unfamiliarity with screening guidelines (31%). Of interest, 57% of respondents were opposed to the use of lipid-lowering medication in children [39]. The reasons behind this opposition were not explored during the study; however, the authors propose that the debate in the medical and lay media surrounding the use of statins in children may have contributed.
Interpreting cholesterol assays in children
Cholesterol levels are age- and maturation-dependent; therefore, it is important to screen for hypercholesterolaemia at the optimal time in childhood [40]. Total cholesterol levels are lower in the prenatal and early postnatal period, and both the total cholesterol and LDL-c begin to rise rapidly from the first week after birth before levelling at the age of 2 years [41]. Levels remain stable until puberty, when LDL-c decreases by approximately 15% [42]. Thus, the NCEP Expert Panel recommends 9–11 years as the optimal period to assess lipids in childhood [32]. This age range (9–11 years) avoids confounding the cholesterol results because of pubertal lipid instability, while also being the best reflection of adult lipid levels [14]. NICE (National Institute for Clinical Excellence) recommends screening before the age of 10 years because after this point LDL-C variation is too great [43].
When interpreting cholesterol assays gender and ethnicity should also be considered. Females have higher total cholesterol, LDL-c and HDL-c than males [40]. Regarding ethnicity, black children have higher HDL-c, lower triglycerides and higher total cholesterol than white and Hispanic children [44, 45].
Treatment options for paediatric dyslipidaemia: diet
Dietary modification and the introduction of exercise must be the cornerstone of paediatric dyslipidaemia management, whether or not pharmacological therapy must also be instituted. Dietary changes instituted in childhood are more successful if the entire family adheres to the new diet [15]. Particularly in paediatric medicine, family-based therapy is essential to effect dietary and lifestyle changes. The Cardiovascular Health Integrated Lifestyle Diet (CHILD 1) is the first stage in dietary change for children with dyslipidaemia, excess weight, obesity and risk factor clustering [32]. CHILD 1 is also the recommended diet for children with a positive family history of premature cardiovascular disease, dyslipidaemia, obesity, primary hypertension, diabetes or exposure to smoking in the home [32]. The diet recommends that in children with hypercholesterolaemia, intake of total fat can be safely limited to 30% of total calories, saturated fat intake limited to 7–10% of calories and dietary cholesterol limited to 300 mg/day (7.75 mmol/L) [32]. In children < 1 year old, dietary fat ought not to be restricted as fats are important in infancy for brain and cognitive development [32].
A healthy diet ought to be recommended for all children with dyslipidaemia regardless of the aetiology of their condition. However, children with secondary hyperlipidaemia can be expected to benefit more from a healthy diet than can children with primary hyperlipidaemia. In particular, children with HeFH and HoFH will inevitably require statin therapy in addition to lifestyle changes. Notwithstanding, dietary and lifestyle changes in a family-based setting are the cornerstone of treatment of paediatric dyslipidaemias.
Treatment options for paediatric dyslipidaemia: statins
Statins: method of action
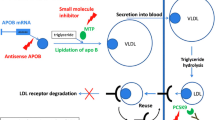
Statins, or 3-hydroxy-3-methyl co-enzyme A (HMG-CoA) reductase inhibitors, inhibit HMG-CoA, the rate-limiting step in endogenous cholesterol synthesis. This results in decreased intracellular cholesterol levels, and in the up-regulation of LDL-c receptors, resulting in increased LDL-c clearance from the circulation.
Statins: efficacy
Statins are efficient at reducing LDL-c in children with FH. The LDL-c reduction achieved by statins in children ranges from 21 to 39% [46], which varies by dose and agent used. A Cochrane review conducted in 2010, of 897 children with HeFH, concluded that statins reduce LDL-c by an average of 30% [47].
In addition to lowering LDL-c, statin therapy has been shown to improve the surrogate markers of atherosclerosis, flow-mediated dilation and intimal-medial thickness. In one study, after 28 weeks treatment with simvastatin, FMD improved by 4% in the children with HeFH, compared with placebo-treated healthy controls [48]. Statins also improve carotid intimal-medial thickness. Wiegman et al., in a study of 214 children with HeFH, demonstrated that after 2 years of therapy with pravastatin, CIMT reduced by 2%, compared with a 1% reduction in the untreated control group [9, 26]. A follow-up study demonstrated that early initiation of statin therapy resulted in smaller increases in CIMT, thus the authors concluded that early initiation of statin treatment delays the progression of CIMT in adolescents and young adults [11].
Statins side effect profile
In adults, the most common side effects associated with statins are increased hepatic transaminase levels, elevations in creatine kinase, rhabdomyolysis and potential teratogenicity [4]. These side effects may be seen in the paediatric population also [14]. For example, elevations in alanine aminotransferase and/or aspartate aminotransferase have been reported in 1–5% of children who were treated with simvastatin or atorvastatin [48, 49]. However, adverse effects such as increased hepatic transaminase levels and myopathy are rare in the paediatric population [14]. In most studies on the use of statins in children with FH, good tolerance of statins is reported in the statin-treated groups [18, 40].
Statins: safety
There are concerns about the safety of statins in the paediatric population. Concerns include the lack of long-term studies to evaluate the life-time effects of initiating statins during childhood; the longest controlled trial is only 2 years long [26]. Also at issue is whether inhibiting cholesterol synthesis at critical periods during childhood development may have adverse consequences. The steroid hormones (adrenal and gonadal) depend on cholesterol for synthesis. Therefore, there is a concern that statin therapy during childhood and adolescence may affect cholesterol-dependent production of steroid hormones in the gonads and adrenal glands [4]. Puberty may be affected by statin therapy, theoretically at least, because cholesterol is required for synthesis of all the sex steroid hormones [15]. However, Wiegman et al. found no evidence of puberty being affected in their randomised control trial of children with FH [26]. De Ferranti et al. point out that starting statin therapy at 8 years of age, when the brain and other organ systems are in a dynamic stage of development, may affect the central nervous system, immune function, hormones, energy metabolism or other systems in unanticipated ways [30]. An observational study of 185 Pravastatin treated children with FH demonstrated no signs of early maturation or delayed puberty [50]. There are additional concerns regarding the use of statins in adolescent girls, with potential effects on pituitary hormones (luteinising hormone, LH, and follicle stimulating hormone, FSH), menstrual cycle length and physical development [51]. Cholesterol is a precursor of the adrenal (DHEAS and cortisol) and gonadal (estradiol) hormones. Statin treatment may result in decreased production of these hormones by inhibiting the rate-limiting enzyme involved in cholesterol production. However, in a 24-week study during which adolescent girls with FH were treated with Lovastatin, there were no adverse effects recorded [51].
Several studies have demonstrated the safety of statins for use in childhood in the short term, up to 2 years [11, 26, 48, 50,51,52]. A meta-analysis of six studies (n = 798; 12 to 104 weeks of treatment) evaluating the safety and effectiveness of statins in children with FH found no statistically significant increases in adverse events compared with placebo [46]. There were no growth issues, side effects, muscle effects or liver toxicity [46]. In a study which assessed the use of statins in 214 children with FH, there was no effect on growth, development, adrenal or gonadal hormones [26]. This study was subject to follow-up giving a total treatment length of 4.5 years; no serious laboratory adverse events were reported, and statin treatment had no untoward effects on sexual maturation [11]. A Cochrane review summarised that statins are not associated with clinically significant changes in growth, maturation, liver enzymes, serum creatine kinase or myopathy [47]. However, the Cochrane review did not speak to the long-term safety of statins when initiated in childhood, as the studies thus far have had a short duration, with all lasting less than 2 years.
Statins: use in FH
The NCEP Expert Panel advise statins as first-line therapy for HeFH and HoFH, after initial measures such as diet and exercise [32]. However, the NCEP also acknowledges that dietary measures alone are unlikely to achieve sufficient LDL-c reduction in primary hyperlipidaemia [32]. Statin treatment should be started at > 8 years old but, in HoFH, treatment may be initiated at earlier ages [14]. The treatment goal of statin therapy in paediatric FH patients is a ≥ 50% reduction in LDL cholesterol or LDL cholesterol < 130 mg/dL (3.36 mmol/L) [14]. More aggressive LDL-c targets should be considered for those with additional CVD risk factors. The initiation of statin therapy in children should be carried out in consultation with a lipid specialist [32]. Although statins may be effective in HoFH patients, most will require the addition of LDL apheresis, or liver transplantation [14].
The use of statins in pre-pubertal children is recommended only in the most severe cases of primary hyperlipidaemia [53]. The American Heart Association recommends commencing statins after the age of 10 years and Tanner II puberty in males, or after 10 years and the onset of menses in female patients [54]. Although several studies of statin treatment to date have not demonstrated any effect on puberty [26, 50], it may be appropriate to delay initiating statin treatment until the onset of puberty if possible. This demonstrates that normal puberty can occur in that individual patient.
Statin use in children must be monitored for muscle and liver toxicity through assessment of hepatic transaminases and creatine kinase [32]. In addition, female adolescents receiving statin therapy must be counselled about the possible teratogenicity of the drug and receive contraception advice if indicated. Although, O’Gorman et al. advise caution when prescribing the oral contraceptive pill (OCP), as it is contraindicated in hypertriglyceridaemia and may be pro-thrombotic in FH [15].
Statins: use in secondary hyperlipidaemia
Obese children with the dyslipidaemic phenotype of elevated triglycerides and low HDL-c comprise the majority of children with lipid abnormalities [4]. However, the majority of studies on the use of statins in the paediatric population have focused on children with HeFH. The data taken from those studies on children with primary hyperlipidaemia may not be representative of the children with secondary hyperlipidaemia, which has a different aetiology. Thus, some authors advise caution in extrapolating information from these studies in order to treat secondary dyslipidaemia [4, 15]. For example, O’Gorman et al. state that dietary changes may be of more benefit to children with obesity-related dyslipidaemia than to those with primary hyperlipidaemia, and that lower doses of statins may be required as a result [15]. A balanced caloric intake with a diet rich in wholegrains, fish, fruit, vegetables and low-fat dairy and low in added-sugar and salt may improve the cholesterol profile in a child with obesity-related hyperlipidaemia, and ought to be the first treatment attempted. Only when dietary and lifestyle changes have failed should treatment with statins be considered in this cohort [15]. Children with primary hyperlipidaemia may see less improvement in their cholesterol profile following the adoption of a healthy diet because primary hyperlipidaemia has a distinct aetiology in which the LDL-c receptor alleles are mutated and this leads to elevated plamsa LDL-c.
The NCEP Expert Panel guidelines focus primarily on children with primary hyperlipidaemia. Kennedy et al. assert that because the majority of childhood dyslipidaemias are now due to secondary hyperlipidaemia, the guidelines no longer apply to the majority of at-risk children [4]. One author takes issue with using statins to treat childhood dyslipidaemia caused by modifiable lifestyle factors, and poses the scenario of ever stronger adult drugs being used to treat childhood obesity and its complications, e.g. beta blockers, diuretics, aspirin and insulin [30]. Certainly, unless children with obesity-related secondary hyperlipidaemia incorporate a healthy diet and exercise into their lives, they will remain at risk of cardiovascular disease in adulthood regardless of statin therapy begun in childhood.
Treatment options for paediatric dyslipidaemia: other pharmacological agents
Bile acid-binding sequestrants
Bile acid-binding sequestrants work by binding the cholesterol in bile acids in the intestinal lumen, which prevents its reuptake into the blood. Bile acid-binding sequestrants were originally the first-line agent for hypercholesterolaemia but they were supplanted by statins. The NCEP Expert Panel guidelines now advise that bile acid-binding sequestrants be used in combination with a statin for patients who fail to meet LDL-c target levels with either medication alone [32]. There is no increase in adverse effects when both are used together, while the efficacy of both agents in combination appears to be additive [32]. Bile acid-binding sequestrants are not ideal for use in the paediatric population due to their side effect profile which includes gastrointestinal effects, namely abdominal pain nausea and constipation [4, 14, 15, 40].
Cholesterol absorption inhibitors
Ezetimibe, a cholesterol absorption inhibitor, is currently approved for the treatment of FH in children ≥ 10 years of age [4]. Ezetimibe selectively inhibits cholesterol at the intestinal brush border, but does not affect absorption of triglycerides or the fat-soluble vitamins [15]. Although Ezetimibe has been shown to decrease LDL-c by up to 20% [55], there is no evidence that it decreases cardiovascular disease [4]. The ENHANCE study, which trialled Ezetimibe and Simvastatin in combination versus Simvastatin alone in adults with FH, demonstrated that Ezetimibe failed to improve CIMT over a 2-year period, despite achieving reductions in LDL-c and C-reactive protein (CRP) [55]. CIMT was used as a surrogate marker to assess the progression of atherosclerosis. The ENHANCE study demonstrated that Ezetimibe does lower LDL-c, whether it prevents progression of atherosclerosis is now unclear. The NCEP Expert Panel guidelines state that cholesterol absorption inhibitors must be instituted only in consultation with a lipid specialist [32]. Given the uncertainty about whether the use of Ezetimibe confers any cardiovascular benefit in a cohort with familial hypercholesterolaemia, it may be sensible to await a randomised trial which explores its efficacy in children with familial hypercholesterolaemia.
Niacin and fibrates
Niacin, a hydrophilic B-complex vitamin, works by decreasing hepatic production of very low-density lipoprotein (VLDL). Due to its serious side effects, poor tolerance and lack of safety data in paediatric patients, niacin therapy is usually reserved for children with HoFH or elevated lipoprotein-A levels [4]. Side effects of niacin include flushing, impaired glucose tolerance, liver failure, hyperuricaemia and myopathy [15, 40]. The NCEP Expert Panel guidelines state that niacin must be instituted only in consultation with a lipid specialist [32].
Fibrates work by inhibiting the synthesis and increasing the clearance of the VLDL apolipoprotein B, which then leads to a decrease in VLDL production. They also inhibit peripheral lipolysis and decrease hepatic extraction of free fatty acids, which reduces hepatic triglyceride production [40]. Fibrates are useful for reducing triglycerides and increasing HDL-c. Their effect on LDL-c is variable [4]. Their primary use is in treating primary hypertriglyceridaemia. The fibrates have a similar side effect profile to statins, with rhabdomyolysis and myopathy increasing in incidence when statins and fibrates are used in combination [40]. The NCEP Expert Panel guidelines state that fibrates must be instituted only in consultation with a lipid specialist [32].
Conclusion
There is clear evidence that atherosclerosis begins in childhood, with the deposition of cholesterol and the formation of fatty streaks in the carotid and coronary arteries. Hypercholesterolaemia is a key factor in the atherosclerotic process. Atherosclerosis may result in thrombotic plaque rupture causing MI, CVA, peripheral vascular disease or aortic aneurysm rupture. Given the risk of these cardiovascular events occurring, it is of vital importance to control risk factors such as hyperlipidaemia.
In order to diagnose and treat childhood hyperlipidaemia, the NCEP Expert Panel guidelines have recommended universal cholesterol screening for all children between the ages of 9 and 11 years. Once diagnosed, control of dyslipidaemia in childhood must begin with lifestyle intervention including a healthy low-fat diet and exercise. This may be followed by the initiation of pharmacological therapy, of which statins are first-line. However, the control of hyperlipidaemia using statin therapy has been dogged by controversy. Most authors accept the necessity of the use of statins in treating children with primary hyperlipidaemia, namely HeFH and HoFH. However, this consensus is absent on the issue of treating secondary hypercholesterolaemia caused by modifiable lifestyle factors such as obesity.
There are concerns that statin therapy may interfere with normal growth, development and puberty. There have been several short-term studies that demonstrate both the safety and efficacy of statins in the paediatric population. Long-term follow-up is lacking in the paediatric population. With the recommendation for universal screening in childhood, and the possibility that more children with dyslipidaemia will come to light as a result, it is time to conduct a randomised control trial on the safety and efficacy of statins to discover if there are any long-term effects to initiating statin treatment in childhood.
Ongoing studies are required in order to fully understand the indications, risks and benefits to the use of statins in childhood dyslipidaemia.
References
Berenson GS, Srinivasan SR, Bao W, Newman WP, Tracy RE, Wattigney WA (1998) Association between multiple cardiovascular risk factors and atherosclerosis in children and young adults. The Bogalusa Heart Study. N Engl J Med 338:1650–1656
HC MG Jr, McMahan CA, Zieske AW et al (2000) Associations of coronary heart disease risk factors with the intermediate lesion of atherosclerosis in youth: the Pathobiological Determinants of Atherosclerosis in Youth (PDAY) Research Group. Arterioscler Thromb Vasc Biol 20(8):1998–2004
Newman WP 3rd, Freedman DS, Voors AW et al (1986) Relation of serum lipoprotein levels and systolic blood pressure to early atherosclerosis. The Bogalusa Heart Study. N Engl J Med 314(3):138–144
Kennedy MJ, Jellerson KD, Snow MZ, Zacchetti ML (2013) Challenges in the pharmacologic management of obesity and secondary dyslipidemia in children and adolescents. Pediatr Drugs 15:335–342
Shepherd J, Cobbe SM, Ford I et al (1995) Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. West of Scotland Coronary Prevention Study Group. N Engl J Med 333(20):1301–1307
Holman RL (1961) Atherosclerosis—a pediatric nutrition problem? Am J Clin Nutr 9:565–569
Relationship of atherosclerosis in young men to serum lipoprotein cholesterol concentrations and smoking (1990) A preliminary report from the Pathobiological Determinants of Atherosclerosis in Youth (PDAY) Research Group. JAMA 264(23):3018–3024
Hong YM (2010) Atherosclerotic CVD beginning in childhood. Korean Circ J 40(1):1–9
Wiegman A, de Groot E, Hutten BA et al (2004) Arterial intima-media thickness in children heterozygous for familial hypercholesterolaemia. Lancet 363:369–370
Davis PH, Dawson JD, Riley WA, Lauer RM (2001) Carotid intimal-medial thickness is related to cardiovascular risk factors measured from childhood through middle age: the Muscatine Study. Circulation 104(23):2815–2819
Rodenburg J, Vissers MN, Wiegman A, van Trotsenburg ASP, van der Graaf A, de Groot E, Wijburg FA, Kastelein JJP, Hutten BA (2007) Statin treatment in children with familial hypercholesterolemia: the younger, the better. Circulation 116:664–668
Lorenz MW, Markus HS, Bots ML, Rosvall M, Sitzer M (2007) Prediction of clinical cardiovascular events with carotid intima-media thickness—a systematic review and meta-analysis. Circulation 115:459–467
Ford ES, Mokdad AH, Ajani UA (2004) Trends in risk factors for cardiovascular disease among children and adolescents in the United States. Pediatrics 114(6):1534–1544
Daniels SR, Gidding SS, de Ferranti SD (2011) Pediatric aspects of familial hypercholesterolemias: recommendations from the National Lipid Association Expert Panel on Familial Hypercholesterolemia. J Clin Lipidol 5:S30–S37
O’Gorman CS, O’Neill MB, Conwell LS (2010) Considering statins for cholesterol-reduction in children if lifestyle and diet changes do not improve their health: a review of the risks and benefits. Vasc Health Risk Manag 7:1–14
Peterson AL, McBride PE (2012) A review of guidelines for dyslipidemia in children and adolescents. WMJ 111(6):271–281
Holven KB, Narverud I, Lindvig HW et al Subjects with FH are characterized by an inflammatory phenotype despite long-term intensive cholesterol lowering treatment. Atherosclerosis 233(2):561–567
Belay B, Belamarich PF, Tom-Revzon C (2007) The use of statins in pediatrics: knowledge base, limitations, and future directions. Pediatrics 119(2):370–380
WHO Human Genetics Programme (1999) Familial hypercholesterolaemia (FH): report of a second WHO consultation, Paris, 3 October 1997. Geneva: World Health Organization.
Raal FJ, Pilcher GJ, Panz VR, van Deventer HE, Brice BC, Blom DJ, Marais AD (2011) Reduction in mortality in subjects with homozygous familial hypercholesterolemia associated with advances in lipid-lowering therapy. Circulation 124(20):2202–2207
Descamps OS, Tenoutasse S, Stephenne X, Gies I, Beauloye V, Lebrethon MC, de Beaufort C, de Waele K, Scheen A, Rietzschel E, Mangano A, Panier JP, Ducobu J, Langlois M, Balligand JL, Legat P, Blaton V, Muls E, van Gaal L, Sokal E, Rooman R, Carpentier Y, de Backer G, Heller FR (2011) Management of familial hypercholesterolemia in children and young adults: consensus paper developed by a panel of lipidologists, cardiologists, paediatricians, nutritionists, gastroenterologists, general practitioners and a patient organization. Atherosclerosis 218(2):272–280
SoutaR AK, Naoumova RP (2007) Mechanisms of disease: genetic causes of familial hypercholesterolemia. Nat Clin Pract Cardiovasc Med 4(4):214–225
Goldstein JL, Brown MS (2009) The LDL receptor. Arterioscler Thromb Vasc Biol 29(4):431–438
Noto N, Okada T, Abe Y, Miyashita M, Kanamaru H, Karasawa K, Ayusawa M, Sumitomo N, Mugishima H (2011) Changes in the textural characteristics of intima-media complex in young patients with familial hypercholesterolemia: implication for visual inspection on B-mode ultrasound. J Am Soc Echocardiogr 24(4):438–443
Di Salvo G, D’Aiello AF, Castaldi B et al (2012) Early left ventricular abnormalities in children with heterozygous familial hypercholesterolemia. J Am Soc Echocardiogr 25(10):1075–1082
Wiegman A, Hutten BA, de Groot E (2004) Efficacy and safety of statin therapy in children with familial hypercholesterolemia: a randomized controlled trial. JAMA 292:331–337
EspinheiraMdo C, Vasconcelos C, Medeiros AM (2013) Hypercholesterolemia—a disease with expression since childhood. Rev Port Cardiol 32(5):379–386
Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Soliman EZ, Sorlie PD, Sotoodehnia N, Turan TN, Virani SS, Wong ND, Woo D, Turner MB, American Heart Association Statistics Committee and Stroke Statistics Subcommittee (2012) Executive summary: heart disease and stroke statistics-2012 update: a report from the American Heart Association. Circulation 125(1):188–197
Manlhiot C, Larsson P, Gurofsky RC, Smith RW, Fillingham C, Clarizia NA, Chahal N, Clarke JT, McCrindle BW (2009) Spectrum and management of hypertriglyceridemia among children in clinical practice. Pediatrics 123(2):458–465
De Ferranti S, Ludwig DS (2008) Storm over statins—the controversy surrounding pharmacologic treatment of children. N Engl J Med 359(13):1309–1312
Ho M, Garnett SP, Baur LA, Burrows T, Stewart L, Neve M, Collins C (2013) Impact of dietary and exercise interventions on weight change and metabolic outcomes in obese children and adolescents: a systematic review and meta-analysis of randomized trials. JAMA Pediatr 167(8):759–768
(2011) Expert panel on integrated guidelines for cardiovascular health and risk reduction in children and adolescent. NHLBI. Pediatrics 128(Suppl 5):S213–S256. https://doi.org/10.1542/peds.2009-2107C
Haney EM, Huffman LH, Bougatsos C et al (2007) Screening and treatment for lipid disorders in children and adolescents: systematic evidence review for the US Preventive Services Task Force. Pediatrics 120(1):189–214
Ritchie SK, Murphy EC, Ice C et al (2010) Universal versus targeted blood cholesterol screening among youth: the CARDIAC project. Pediatrics 126(2):260–265
Eissa MA, Wen E, Mihalopoulos NL, Grunbaum JA, Labarthe DR (2009) Evaluation of AAP guidelines for cholesterol screening in youth: Project Heart Beat! Am J Prev Med 37(1):S71–S77
De Ferranti SD, Daniels SR, Gillman (2012) NHLBI integrated guidelines on cardiovascular disease risk reduction: can we clarify the controversy about cholesterol screening and treatment in childhood? Clin Chem 58(12):1626–1630
Wilson JMG, Jungner G (1968) Principles and practice of screening for disease. World Health Organization, Geneva
HC MG Jr, McMahan CA, Gidding SS (2008) Preventing heart disease in the 21st century: implications of the Pathobiological Determinants of Atherosclerosis in Youth (PDAY) study. Circulation 117(9):1216–1227
Dixon DB, Kornblum AP, Steffen L (2014) Implementation of lipid screening guidelines in children by primary pediatric providers. J Pediatr 164(3):572–576
Daniels SR, Greer FR (2008) Lipid screening and cardiovascular health in childhood. Pediatrics 122(1):198–208
Myśliwiec M, Walczak M, Małecka-Tendera E, Dobrzańska A, Cybulska B, Filipiak K, Mazur A, Jarosz-Chobot P, Szadkowska A, Rynkiewicz A, Chybicka A, Socha P, Brandt A, Bautembach-Minkowska J, Zdrojewski T, Limon J, Gidding SS, Banach M (2014) Management of familial hypercholesterolemia in children and adolescents. Position paper of the Polish Lipid Expert Forum. J Clin Lipidol 8(2):173–180
Jolliffe CJ, Janssen I (2006) Distribution of lipoproteins by age and gender in adolescents. Circulation 114:1056–1062
Wierzbicki AS, Humphries SE, Minhas R (2008) Familial hypercholesterolemia: summary of NICE guideline. BMJ 337:a1095
Labarthe DR, Dai S, Fulton J (2003) Cholesterol screening in children: insights from Project Heart Beat! and NHANES III. Prog Pediatr Cardiol 17(2):169–178
Bradley CB, Harrell JS, McMurray RG (1997) Prevalence of high cholesterol, high blood pressure, and smoking among elementary school children in North Carolina. N C Med J 58(5):362–367
Avis HJ, Vissers MN, Stein EA (2007) A systematic review and meta-analysis of statin therapy in children with familial hypercholesterolemia. Arterioscler Thromb Vasc Biol 27:1803–1810
Vuorio A, Kuoppala J, Kovanen PT (2014) Statins for children with familial hypercholesterolemia. Cochrane Database Syst Rev 7:CD006401. https://doi.org/10.1002/14651858
De Jongh S, Lilien MR, op’tRoodt J et al (2002) Early statin therapy restores endothelial function in children with familial hypercholesterolemia. J Am Coll Cardiol 40:2117–2121
McCrindle BW, Ose L, Marais AD (2003) Efficacy and safety of atorvastatin in children and adolescents with familial hypercholesterolemia or severe hyperlipidemia: a multicenter, randomized, placebo-controlled trial. J Pediatr 142:74–80
Carreau V, Girardet JP, Bruckert E (2011) Long-term follow-up of statin treatment in a cohort of children with familial hypercholesterolemia. Efficacy and tolerability. Pediatr Drugs 13:267–275
Clauss SB, Holmes KW, Hopkins P (2005) Efficacy and safety of lovastatin therapy in adolescent girls with heterozygous familial hypercholesterolemia. Pediatricsm 116:682–688
Knipscheer HC, Boelen CC, Kastelein JJ et al (1996) Short-term efficacy and safety of pravastatin in 72 children with familial hypercholesterolemia. Pediatr Res 39:867–871
Gandelman K, Glue P, Laskey R, Jones J, LaBadie R, Ose L (2011) An eight-week trial investigating the efficacy and tolerability of atorvastatin for children and adolescents with heterozygous familial hypercholesterolemia. Pediatr Cardiol 32(4):433–441
McCrindle BW (2007) Summary of the American Heart Association’s scientific statement on drug therapy of high-risk lipid abnormalities in children and adolescents. Arterioscler Thromb Vasc Biol 27(5):982–985
Kastelein JJ, Akdim F, Stroes ES, Zwinderman AH, Bots ML, Stalenhoef AF, Visseren FL, Sijbrands EJ, Trip MD, Stein EA, Gaudet D, Duivenvoorden R, Veltri EP, Marais AD, de Groot E, ENHANCE Investigators (2008) Simvastatin with or without ezetimibe in familial hypercholesterolemia. N Engl J Med 358(14):1431–1443
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This study does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
King, K., Macken, A., Blake, O. et al. Cholesterol screening and statin use in children: a literature review. Ir J Med Sci 188, 179–188 (2019). https://doi.org/10.1007/s11845-018-1835-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11845-018-1835-9