Abstract
Purpose
Early menopause (EM, age at menopause < 45 years) and premature ovarian insufficiency (POI, age at menopause < 40 years) are associated with an increased risk of osteoporosis. However, their association with increased fracture risk has not been established, with studies yielding conflicting results. The primary aim of this systematic review and meta-analysis was to synthesize studies evaluating the association between age at menopause and fracture risk. The secondary aim was to evaluate this effect concerning the site of fractures.
Methods
A comprehensive search was conducted in PubMed, CENTRAL and Scopus, up to 31 January 2018. Data were expressed as odds ratio (OR) with 95% confidence intervals (CI). The I2 index was employed for quantifying heterogeneity.
Results
Eighteen studies were included in the qualitative and quantitative analysis (462,393 postmenopausal women, 12,130 fractures). Compared with women with age at menopause > 45 years, women with EM demonstrated higher fracture risk (OR 1.36, 95% CI 1.11–1.66, p < 0.002, I² 81.5%). Women with POI did not display any difference in fracture risk compared either with women with age at menopause > 40 (OR 1.23, 95% CI 0.72–2.09, p = 0.436, I² 62.5%) or >45 years (OR 0.54, 95% CI 0.22–1.29, p = 0.17, I2 0%). No difference was evident when a separate analysis was performed for vertebral, non-vertebral and hip fractures.
Conclusions
This is the first meta-analysis showing that EM is associated with increased fracture risk compared with normal age at menopause, without any distinct effect on the site of the fracture.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteoporosis is a major global health problem characterized by deterioration in bone microarchitecture, leading to increased fragility and risk of fractures. In the United States, 1.5 million fractures annually are attributed to osteoporosis [1], with an annual medical cost of 282 and 204 million dollars for non-vertebral and hip fractures, respectively [2]. Fractures have been associated with skeletal deformities, significant disability in daily activities and increased mortality (19% within the first year following a hip fracture, with an excess of 1.8% per year) [1, 3].
Menopause is the post-reproductive period in the woman’s life, typically defined as completion of 12 months of amenorrhea or post-bilateral oophorectomy. The mean age is 51 years, although 10% of the female population enters menopause before 45 years. The latter situation is defined as “early” (EM) or “premature” menopause [4]. Notably, about 1% of women enter menopause before the age of 40 (0.1% under the age of 30), defined as “premature ovarian insufficiency” (POI) [4]. Bone loss sharply accelerates during the late peri-menopausal period (1.6% in the spine and 1% in hip) rising to 2 and 1.4%, respectively, during the post-menopausal period [5]. Several studies have shown that EM results in lower BMD at older ages [6, 7]. However, despite the well-documented effect of EM and POI on bone loss, their exact effect on fracture risk is not known, with studies showing either increased or no risk [8,9,10,11,12]. Moreover, their exact effect concerning the site of the skeleton is also not known. To our knowledge, no meta-analysis has been published so far on these topics.
The primary aim of this study was to systematically review and meta-analyze the best evidence regarding the association of age at menopause with the fracture risk. The secondary aim was to evaluate this association concerning the site of fractures (vertebral, non-vertebral, hip).
Materials and methods
Guidelines followed
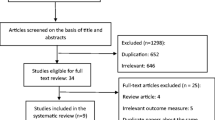
This systematic review followed the MOOSE (Meta-analyses Of Observational Studies in Epidemiology) guidelines [13]. A flow diagram is shown in Fig. 1s completed MOOSE checklist has been submitted as Supplementary Table 1.
Search strategy
The following PICO (Population, Intervention or exposure, Comparison, Outcome) elements were applied as inclusion criteria for the systematic review: (i) Population: post-menopausal women (ii) Intervention: early age at menopause, either EM or POI (iii) Comparison group: women with natural menopause (iv) Outcome: fractures. To identify eligible studies, the main search was conducted in the electronic databases MEDLINE, Scopus and Cochrane (CENTRAL), covering the period from conception until 31 January 2018. More specifically we used using the following search strings for PubMed: (“Menopause, Premature”[Mesh] OR “Primary Ovarian Insufficiency”[Mesh] OR “ovarian insufficiency”[tiab] OR “ovarian failure”[tiab] OR ((menopause[Mesh] OR menopause[tiab] OR menopausal[tiab] OR climacteric[tiab] OR postmenopausal[tiab] OR post-menopausal[tiab]) AND (early[tiab] OR premature[tiab] OR age[tiab] OR years[tiab] OR time[tiab]))) AND (“Osteoporotic Fractures”[Mesh] OR “Fractures, Spontaneous”[Mesh] OR (((“Fractures, Stress”[Mesh] OR “Spinal Fractures”[Mesh] OR “Tibial Fractures”[Mesh] OR “Fractures, Compression”[Mesh] OR “Radius Fractures”[Mesh] OR “Humeral Fractures”[Mesh] OR “Femoral Neck Fractures”[Mesh] OR “Femoral Fractures”[Mesh] “Fractures, Multiple”[Mesh]) OR ((“Fractures, Bone”[Mesh:noexp] OR fractures[tiab] OR fracture[tiab]) AND (hip[tiab] OR femoral[tiab] OR vertebral[tiab] OR “non-vertebral”[tiab] OR “non vertebral”[tiab]))) AND (“Risk Assessment”[Mesh] OR “Risk Factors”[Mesh] OR risk[tiab] OR incident[tiab] OR incidence[tiab] OR prevalence[tiab] OR association[tiab] OR associated[tiab]))) NOT (Animal[mesh] NOT Human[mesh]) NOT (letter[pt] OR comment[pt] OR editorial[pt] OR Review[pt] OR “practice guideline”[ptyp] OR “case reports”[ptyp]). The search strings for Scopus and Cochrane (CENTRAL) are presented in Supplementary Table 2. We also searched for “grey literature” using relevant websites, such as http://www.opengrey.eu, http://greylit.org and https://clinicaltrials.gov. We used the EndNote V8 as our search software.
In addition, a manual search was conducted through reference lists of reviews and meta-analyses, identified by the above systematic database search. The main search was completed independently by three investigators (PS, NKG, and NK), who checked all the available articles. Any discrepancy was solved by consultation of two investigators, not involved in the initial procedure (PA and DGG).
Trial selection
Specific inclusion criteria were set prior to the literature search, as follows: (i) Studies conducted in post-menopausal women (either hysterectomized or non-hysterectomized) and (ii) Studies providing extractable data. Both cohorts and case-control (cross-sectional) studies were eligible. Studies were excluded if they: (i) Had no control group (without fractures), (ii) Included only pre- or peri-menopausal women, (iii) Were conducted in patients receiving therapy associated with bone loss, such as aromatase inhibitors, (iv) Were written in a language other than English, (v)Included patients with metabolic bone diseases, such as Paget’s disease or rheumatoid arthritis, (vi) Were conducted in animals, (vii) Had no information or proper discrimination on fractures according to the age of menopause.
Data extraction
Three researchers (PS, NKG, and NK) reviewed all eligible studies. The following data were extracted and recorded: (i) First author, (ii) Year of publication, (iii) Country in which the study was conducted, (iv) Study design (case-control or cohort), (v) Duration (available in cohorts), (vi) Total number of participants, (vii) Number of women with EM (age at menopause < 45 years), (viii) Number of women with POI (age at menopause < 40 years), (ix) Number of women with normal age at menopause (>45 years), (x) Number of women with age at menopause > 50 years, (xi) Number of women with age at menopause > 40 years, (xii) Number of cases with fractures and the specific site of fracture in each of these categories. From these data (vii–xii), odds ratios (OR) for fracture risk were calculated. Parameters, such as mean age of the participants at study entry, mean body mass index (BMI), type of menopause (surgical or natural), use of hormone replacement therapy (HRT) or any other anti-osteoporotic medication, method of fracture diagnosis, smoking status, alcohol intake and physical activity, were also recorded, where available. For papers with missing data, we sent e-mails to the authors (two e-mails within a 15-day period), but we did not receive any response. For some older studies (i.e., published before 2000), no communication via e-mail was feasible.
The following comparisons were made: (i) Women with EM compared with those with age at menopause > 45 years, (ii) Women with EM compared with those with age at menopause > 50 years, (iii) Women with POI compared with those with age at menopause > 40 years and (iv) Women with POI compared with those with age at menopause > 45 years. Comparisons according to the site of fractures were performed where available.
Risk of bias and study quality assessment
Newcastle-Ottawa scale (NOS) was used for assessing the quality of each study. NOS evaluates studies based on three criteria: (i) Participant selection, (ii) Comparability of study groups and (iii) Assessment of outcome or exposure. A study can be awarded a maximum of four stars for the selected category, a maximum of two stars for the comparability category and a maximum of three stars for the outcome/exposure category. Finally, each study is characterized as good, fair or poor according to the number of obtained stars [14]. More specifically, the quality of a study is characterized as “good” when it obtains 3 or 4 stars in selection domain and 1 or 2 stars in comparability domain and 2 or 3 stars in outcome/exposure domain. “Fair quality” is a characterization used for 2 stars in selection domain and 1 or 2 stars in comparability domain and 2 or 3 stars in outcome/exposure domain, whereas, a study is considered of “poor quality” in cases of 0–1 stars in selection domain or 0 stars in comparability domain or 0–1 stars in outcome/exposure domain. Data on bias assessment by NOS are presented in Supplementary Table 3.
Statistical analysis
Random effects model was used for data synthesis (Mantel/Haenszel model) in all meta-analyses, as it was anticipated that significant heterogeneity would be present among studies. Associations were reported as OR with 95% confidence intervals (CI). A p-value < 0.05 was considered as statistically significant. Heterogeneity was tested with the Cochrane chi-square test, and the degree of heterogeneity was quantified by the I2 statistics. An I2 30–60% was considered moderate, whereas values > 60% were considered as a high degree of heterogeneity. Publication bias was formally tested with the Begg-Mazumdar test (presented in Funnel plot diagram, with p-values > 0.1 indicating the absence of publication bias) and the Egger’s test (p-values > 0.1 indicating the absence of publication bias). To further explain the heterogeneity among studies, sensitivity analysis, subgroup analysis and meta-regression were planned to be performed. Sensitivity analysis (by the use of random effects model) was used to locate outliers, defined as studies that had large residuals (|z| > 2). Subgroup (stratified) analysis (by the use of random effects model) was performed for categorical variables, such as the continent in which the study was conducted (European versus non-European populations), type of menopause (natural versus surgical), use of HRT or anti-osteoporotic medications and low-energy fractures or fractures in general, as it was anticipated that they could have a significant effect on the main outcome. All subgroup analyses compared women with EM with those of age at menopause > 45 years. Numerical (age at study entry, BMI, BMD at the lumbar or femoral site) and categorical (smoking status, level of physical activity) parameters were planned to be used as predictors of fracture risk (meta-regression by the use of random effects model). All analyses were done with the software Comprehensive MetaAnalysis V2.
Results
Systematic review
This arch strategy yielded a total of 4894 results after removal of duplicates (7584 results initially), 57 of which were assessed as full-texts for eligibility. Full-text articles were excluded if they: (i) Reported data from a population already used in one of the included studies (n = 1), (ii) Were written in non-English language (n = 8), (iii) Were lacking comparison data between early and normal menopausal groups (n = 11), (iv) Were lacking a control group without fractures (n = 2), (v) Used a threshold of normal age at menopause other than 45 years (i.e., 47–53) (n = 3) and (vi) Had missing or irrelevant data with regard to our study’s endpoint (n = 14). The excluded studies and the reason for this are presented in Supplementary Table 4. Eventually, 18 studies were included in the qualitative and quantitative analysis [8,9,10,11,12, 15,16,17,18,19,20,21,22,23,24,25,26,27].
The characteristics of the studies and their participants are presented in Table 1 and Table 2. Further details about the case-control studies (matching for age or other variables) or the cohort studies (age at baseline), were not available. The included studies were published between 1993 and 2015. Eight of them were case-control, and ten were cohort studies (one of them retrospective). A total number of 462,393 postmenopausal women with 12,130 cases with fractures were included. One study was conducted in Australia, two in Asia, nine in Europe and six in North America (five in the USA). The mean age of the participants was 59.1 ± 5.9 years (data from 17 studies) and the mean BMI 26.4 ± 4.9 kg/m2 (data from 14 studies).
Overall, 14 studies [8,9,10,11,12, 15,16,17,18,19,20, 23, 26, 27] compared women with EM with those of age at menopause > 45 years. Data regarding women with POI were available from six studies [9, 21, 22, 24,25,26]. Four studies [17, 18] included only women with natural menopause, whereas one study [11] only women with surgical menopause. The remainder included both women with natural and surgical menopause or did not specify the type of menopause.
Reported fracture data were based on questionnaires/interviews [17, 21, 24, 25, 27] or were confirmed by medical records [10,11,12, 19, 22], hospital admissions [8, 9, 20], X-rays [15, 16, 22, 23, 26, 27] or by physicians [25]. Most studies [10,11,12, 15, 16, 19, 20, 22, 27], included only low-energy fractures, having excluded those caused by traumatic events or associated with other chronic metabolic bone diseases, such as Paget’s disease or cancer-related fractures. One study [17] acknowledged no application of exclusion criteria. The rest of the studies [8, 9, 20, 21, 23,24,25,26] did not specify the mechanism or the pathology of fractures. Eleven studies [8,9,10, 12, 15, 16, 18,19,20, 23, 26] provided data regarding the site of fracture (vertebral, non-vertebral, hip), comparing EM and normal menopausal groups.
Data on bone mineral density (BMD) or the presence of osteoporosis (as a categorical variable) were available in eight studies. The prevalence of osteoporosis was 8.7 to 38.7% in six studies [15, 17, 22,23,24, 26], in one study the whole population was osteoporotic [21] and in another only BMD values expressed in g/cm2 were available (without T-scores) [27]. One study provided data for each subgroup according to age at menopause, reporting an osteoporosis prevalence of 30% in women with EM and 23% in those with normal age at menopause [17].
Past or current use of HRT was reported in 12 studies [8,9,10,11,12, 15, 17,18,19, 22,23,24, 26], ranging from 1.1 to 79%. However, the exact proportion of those subjects having received HRT was reported only in four studies [9, 15, 17, 19], ranging from 4.5 to 59.2%. Three studies excluded patients who had ever received HRT [16, 20, 25], although bisphosphonate use was reported in one of them (3.1%) [25]. In three studies [21, 26, 27], there was no report on HRT use, although, in one of them [26], 19.2% of patients reported ever use of contraceptives and 41.5% of anti-osteoporotic treatment. Another two studies [21, 25] reported the use of anti-osteoporotic medication, in 3.1 [25] and 61% [21] of the population. The remaining studies either excluded or did not report the use of anti-osteoporotic treatment.
Primary aim: comparison of women with EM with those with normal age at menopause
Thirteen studies [8,9,10,11,12, 15,16,17,18,19,20, 23, 26, 27] provided data on women with EM and those with normal age at menopause. Two studies did not provide numerical data on menopausal women, and OR was used instead for the quantitative analysis [11, 27]. Women with EM demonstrated a higher risk of fracture compared with those of age at menopause > 45 years (OR 1.36, 95% CI 1.11–1.66, p < 0.002, I² 81.5%) (Fig. 2). Increased fracture risk was also evident when the comparison was performed with women of age at menopause > 50 years [8, 9, 16, 18, 20] (OR 1.36, 95% CI 1.09–1.7, p = 0.006, I² 27.8%) (Fig. 3). Furthermore, eight studies [10,11,12, 15, 16, 19, 20, 27] reported exclusively low-energy fractures with a clear definition. When an analysis was conducted only for these studies, women with EM were still at a greater risk of fracture compared with those of age at menopause > 45 years (OR 1.48, 95% CI 1.11–1.97, p = 0.007, I² 88.9%).
Primary aim: comparison of women with POI with those with normal age at menopause
Data analysis from six studies [9, 21, 22, 24,25,26] showed no difference in fracture risk between women with POI and those of age at menopause > 40 years (OR 1.23, 95% CI 0.72–2.09, p = 0.436, I² 62.5%) (Figure 4a). No difference was observed when the former were compared with those of age at menopause > 45 years (OR 0.54, 95% CI 0.22–1.29, p = 0.170, I2 0%) [9, 26] (Figure 4b).
Secondary aim: analysis according to the skeletal site of fractures
Comparisons were performed between women with EM and those of normal age at menopause according to the site of fractures. Seven studies [8, 9, 12, 16, 19, 20, 27] provided data on hip fractures, without any difference between the two groups (OR 1.18, 95% CI 0.95–1.46, p = 0.120, I² 73.7%) (Fig. 5a). No difference was demonstrated with respect to non-vertebral (OR 1.66, 95% CI 0.79–3.5, p = 0.177, I² 94.3%) [10, 12, 15] (Fig. 5b) or vertebral fracture risk (OR 1.09, 95% CI 0.87–1.37, p = 0.443, I² 30.0%) [10, 12, 18, 23, 26] (Fig. 5c). Two studies [9, 25] provided data on hip fracture incidence in women with POI, without significant difference when compared with women of age at menopause > 50 years (OR 0.83, 95% CI 0.32-2.17, p = 0.711, I² 63.7%).
Sensitivity analysis
Three studies reported data on the use of anti-osteoporotic medications. When a sensitivity analysis was performed, exclusion of these studies resulted in no changes on the effect of age at menopause (women with POI compared with those of age at menopause > 40 years) on fracture risk (OR 1.21, 95% CI 0.37–3.93, p = 0.742, I² 74.9%).
Subgroup analysis
When women with EM were compared with those of age at menopause > 45 years, a difference was observed only in studies conducted in European (OR 1.75, 95% CI 1.27–2.42, p = 0.001), in contrast to non-European populations (OR 1.049, 95% 0.88–1.24, p = 0.580). The difference was significant between these two subgroups (p = 0.006). Furthermore, the outcomes of the meta-analysis remained unchanged, following a sensitivity analysis that kept only the studies of high quality (low probability of bias) as assesses by the NOS.
Meta-regression
The age of the participants at study entry was associated with the effect of age at menopause on fracture risk (Q 6.98, df 1, p = 0.008) (Fig. 6). No such association was found for BMI. Meta-regression for the other pre-defined parameters (BMD, smoking status, physical activity) was not evaluable due to the small number of studies (<10) or a different way of definition.
Discussion
To the best of our knowledge, this systematic review and meta-analysis that included >462,000 postmenopausal women and >12,000 fracture cases, is the first reporting on the association between the age of menopause and risk of fractures. EM was associated with a higher risk of fractures compared with women with age at menopause > 45 or >50 years. No such association was found between POI and fracture risk, probably due to the relatively small number of fractures in this population (176 versus 1356 in EM). Another explanation could be that women with POI may be more likely to be treated with estrogens compared with women with early menopause. Moreover, no association was found according to the site of the fracture.
Estrogens are essential for obtaining peak bone mass in both genders. EM leads to early estrogen depletion providing the main pathogenetic mechanism for accelerated bone loss and the development of osteoporosis in later life [6, 7]. Estrogen decreases osteoclast formation and activity and increases osteoblast formation, differentiation, proliferation and function, through its receptor, mainly type α (ERα)] [28]. The beneficial effect of HRT on fracture risk reduction has been well-established. In the Women’s Health Initiative (WHI), conjugated equine estrogen (CEE) 0.625 mg/d combined with medroxyprogesterone acetate (MPA) 2.5 mg/d was associated with a significant reduction in the risk of total [hazard ratio (HR) 0.76, 95% CI 0.69–0.85] and hip fractures (HR 0.66, 95% CI 0.45–0.98) [29]. Similar reductions were observed in the estrogen-alone arm of the WHI [30]. The absolute risk reduction was estimated at 12 fractures per 1000 women after 5 years on CEE plus MPA and 8 fractures with CEE alone. The effect of estrogen on bone mass seems to be dose-dependent [31]. Of note, after discontinuation of estrogen therapy, BMD declines to a rate that is comparable to the one occurring within the first 2 years of menopause in untreated women [32]. However, recent data show that bone microarchitecture changes [33] and anti-fracture efficacy may persist five years after discontinuation of HRT [34]. Beyond estrogen depletion, another plausible explanation for the association of age at menopause with fracture risk could be the co-existence of risk factors for both entities, such as smoking [35, 36], physical activity [35, 36], BMI [35,36,37] and socioeconomic and educational status [36, 38].
The impact of genetic variants on the age at menopause may account for ~50% of its variation [39]. Genome-wide association studies (GWAS) have identified several polymorphisms in genes implicated in DNA repair (XO1, HELQ, UIMC1, FAM175A, FANCI, TLK1, POLG, PRIM1) and immune function (IL11, NLRP11, BAT2) that are associated with the earlier timing of menopause [40]. Other genes implicated in the determination of the age at menopause are the anti-Mullerian hormone (AMH) and its receptor 2 (AMHR2) gene [41], as well as, the follicular stimulating hormone receptor (FHSR) genes [42]. Other GWAS have identified gene polymorphisms related to increased risk of osteoporosis and fractures [RANKL, OPG, ERα, major histocompatibility complex [43] and vitamin D receptor (VDR)] [44]. Whether these polymorphisms also predispose to fracture risk is unknown. Notably, some studies have demonstrated an association of ER [45] and VDR gene variants (BsmI and TaqI) [46] with age at menopause, providing a potential genetic linkage. More specifically, women homozygous for the PP genotype of the ER, present an earlier onset of menopause by 1.1 years or 2.4 times higher risk of surgical menopause compared with those carrying the pp genotype [45]. Similarly, homozygotes of the minor allele of BsmI, AA, are under a two-fold higher risk of surgical menopause compared with homozygotes of the major allele, GG. This is also the case for CC allele of the TaqI polymorphism, compared with the TT haplotype [46]. In general, the age at menopause seems to be the integration of both genetic and environmental factors [35, 36].
In the current study, the chronological age of the participants affected the association of age at menopause with fracture risk. Indeed, in some studies, chronological age was a stronger predictor of fractures than age at menopause [25]. Advanced chronological age is associated with an increased risk of fractures, particularly after the age of 60 years [26]. In a recent study, the 25-year risk of hip fracture was significantly higher in women ≥ 80 years compared with those aged < 70 years (22.6% versus 13.9%) [47]. In women aged 70–74 years, the incidence of hip fractures was 7-fold higher compared with those of 50–54 years [20]. It should be noted that chronological age for each subgroup, according to the age of menopause, was available only from three studies (two studies with EM and one with POI) [17, 20, 25]. Thus, more detailed conclusions could not be drawn on this concept. Similarly, time since menopause, which is the period from the final menstrual period since the study entry, which might have also affected fracture risk, was reported only in two studies [17, 25], according to age at menopause.
The present study has certain limitations. First, both cohorts and case-control studies were included which might have contributed to the heterogeneity of data. However, after performing a subgroup analysis, no difference was found among them. Second, different definitions for the fractures were applied in the original studies, including both clinical and morphological fractures. Third, past or current use of HRT was reported in most studies to a variable extent (with limited data on the duration and the exact regimen). HRT may have different effects on idiopathic POI or EM compared with bilateral oophorectomy at the same age. This reporting bias might have contributed to the heterogeneity of data. This was also the case with the use of anti-osteoporotic medications (data from three studies were available). However, when a sensitivity analysis was performed after exclusion of these studies, no changes were observed. Fourth, the number of studies providing data on risk factors for fracture (other than age and BMI) was not sufficient to perform meta-regression analysis and, therefore, their contribution to the heterogeneity could not be estimated. Fifth, in most women the age of natural (non-surgical) menopause was self-reported, which was subject to recall bias. Sixth, no clear discrimination according to the type of menopause could be performed. From the studies included in the meta-analysis, it was practically impossible to discriminate between natural and iatrogenic (specifically surgical menopause). Only one study [11] included a homogenous sample of women with surgical menopause, defined as “bilateral oophorectomy”. Four studies [17, 18] included only women with natural menopause (defined as ≥12 months of amenorrhea), whereas the remainder did not discriminate between natural and surgical menopause (which could be either bilateral oophorectomy or hysterectomy or both). Nevertheless, the fracture risk does not seem to be affected in direct comparisons between surgical and natural menopause, as reported in one study [20]. Seventh, another limitation is that no data were available regarding the exact time of fracture occurrence after menopause. Eighth, we must also acknowledge a selection bias by excluding non-English articles. Ninth, the difference in fracture reporting according to the site of the skeleton is also another source of information bias.
Conclusions
In conclusion, this systematic review and meta-analysis in post-menopausal women provided evidence that women entering menopause at an earlier age (<45 years) have an increased risk for fractures compared with those with age at menopause > 45 or >50 years. Thus, EM is not only associated with increased risk of osteoporosis, but also with an increased risk of fractures. However, this was not evident for women with POI, possibly due to the small number of studies and low fracture rates. No difference in risk could be detected with respect to the site of fractures. A potential clinical implication of these findings is a closer follow-up for women with EM, regarding their risk of fracture than those with normal age at menopause, regardless of the existence of other factors. Future well-designed, prospective cohorts and interventional studies in women with POI, that include reports on different types of fractures, would further clarify the association between age at menopause and bone health.
References
D.M. Black, C.J. Rosen, Clinical practice. Postmenopausal osteoporosis. N. Engl. J. Med. 374(3), 254–262 (2016)
C. Pike, H.G. Birnbaum, M. Schiller, H. Sharma, R. Burge, E.T. Edgell, Direct and indirect costs of non-vertebral fracture patients with osteoporosis in the US. Pharmacoeconomics 28(5), 395–409 (2010)
P. Vestergaard, L. Rejnmark, L. Mosekilde, Increased mortality in patients with a hip fracture-effect of pre-morbid conditions and post-fracture complications. Osteoporos. Int. 18(12), 1583–1593 (2007)
S.R. Davis, I. Lambrinoudaki, M. Lumsden, G.D. Mishra, L. Pal, M. Rees, N. Santoro, T. Simoncini, Menopause. Nat. Rev. Dis. Prim. 1, 15004 (2015)
J.S. Finkelstein, S.E. Brockwell, V. Mehta, G.A. Greendale, M.R. Sowers, B. Ettinger, J.C. Lo, J.M. Johnston, J.A. Cauley, M.E. Danielson, R.M. Neer, Bone mineral density changes during the menopause transition in a multiethnic cohort of women. J. Clin. Endocrinol. Metab. 93(3), 861–868 (2008). https://doi.org/10.1210/jc.2007-1876.
S.S. Faubion, C.L. Kuhle, L.T. Shuster, W.A. Rocca, Long-term health consequences of premature or early menopause and considerations for management. Climacteric 18(4), 483–491 (2015)
L.T. Shuster, D.J. Rhodes, B.S. Gostout, B.R. Grossardt, W.A. Rocca, Premature menopause or early menopause: long-term health consequences. Maturitas 65(2), 161–166 (2010)
R.G. Cumming, R.J. Klineberg, Breastfeeding and other reproductive factors and the risk of hip fractures in elderly women. Int. J. Epidemiol. 22(4), 684–691 (1993)
F. Parazzini, A. Tavani, E. Ricci, C. La Vecchia, Menstrual and reproductive factors and hip fractures in post menopausal women. Maturitas 24(3), 191–196 (1996)
A. Papaioannou, L. Joseph, G. Ioannidis, C. Berger, T. Anastassiades, J.P. Brown, D.A. Hanley, W. Hopman, R.G. Josse, S. Kirkland, T.M. Murray, W.P. Olszynski, L. Pickard, J.C. Prior, K. Siminoski, J.D. Adachi, Risk factors associated with incident clinical vertebral and nonvertebral fractures in postmenopausal women: the Canadian Multicentre Osteoporosis Study (CaMos). Osteoporos. Int. 16(5), 568–578 (2005)
M. Tuppurainen, H. Kroger, R. Honkanen, E. Puntila, J. Huopio, S. Saarikoski, E. Alhava, Risks of perimenopausal fractures—a prospective population-based study. Acta Obstet. Gynecol. Scand. 74(8), 624–628 (1995)
A. Paganini-Hill, K.A. Atchison, J.A. Gornbein, A. Nattiv, S.K. Service, S.C. White, Menstrual and reproductive factors and fracture risk: the Leisure World Cohort Study. J. Womens. Health. 14(9), 808–819 (2005)
D.F. Stroup, J.A. Berlin, S.C. Morton, I. Olkin, G.D. Williamson, D. Rennie, D. Moher, B.J. Becker, T.A. Sipe, S.B. Thacker, Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 283(15), 2008–2012 (2000)
G.A. Wells, B. Shea, D. O’Connell, J. Peterson, V. Welch, P. Tugwell, The Newcastle-Ottawa Scale (NOS) for assessing the quality of non-randomised studies in meta-analyses. (2009). http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp [cited 2009 Oct 19]
M. Varenna, L. Binelli, F. Zucchi, P. Beltrametti, M. Gallazzi, L. Sinigaglia, Is the metatarsal fracture in postmenopausal women an osteoporotic fracture? A cross-sectional study on 113 cases. Osteoporos. Int. 7(6), 558–563 (1997)
D. Huo, D.S. Lauderdale, L. Li, Influence of reproductive factors on hip fracture risk in Chinese women. Osteoporos. Int. 14(8), 694–700 (2003)
D.J. van Der Voort, P.H. van Der Weijer, R. Barentsen, Early menopause: increased fracture risk at older age. Osteoporos. Int. 14(6), 525–530 (2003)
M. van der Klift, C.E. de Laet, E.V. McCloskey, O. Johnell, J.A. Kanis, A. Hofman, H.A. Pols, Risk factors for incident vertebral fractures in men and women: the Rotterdam Study. J. Bone Miner. Res. 19(7), 1172–1180 (2004)
M.R. Jenkins, A.V. Denison, Smoking status as a predictor of hip fracture risk in postmenopausal women of northwest Texas. Prev. Chronic Dis. 5(1), A09 (2008)
E. Banks, G.K. Reeves, V. Beral, A. Balkwill, B. Liu, A. Roddam, Million Women Study, C.: Hip fracture incidence in relation to age, menopausal status, and age at menopause: prospective analysis. PLoS. Med. 6(11), e1000181 (2009)
E. Lespessailles, F.E. Cotte, C. Roux, P. Fardellone, F. Mercier, A.F. Gaudin, Prevalence and features of osteoporosis in the French general population: the Instant study. Jt. Bone Spine 76(4), 394–400 (2009)
F.A. Tremollieres, J.M. Pouilles, N. Drewniak, J. Laparra, C.A. Ribot, P. Dargent-Molina, Fracture risk prediction using BMD and clinical risk factors in early postmenopausal women: sensitivity of the WHO FRAX tool. J. Bone Miner. Res. 25(5), 1002–1009 (2010)
C.S. Shin, M.J. Kim, S.M. Shim, J.T. Kim, S.H. Yu, B.K. Koo, H.Y. Cho, H.J. Choi, S.W. Cho, S.W. Kim, S.Y. Kim, S.O. Yang, N.H. Cho, The prevalence and risk factors of vertebral fractures in Korea. J. Bone Miner. Metab. 30(2), 183–192 (2012)
A.K. Pfister, C.A. Welch, M.K. Emmett, N.W. Sheets, Risk factors predicting fractures in early postmenopausal women. W. V. Med. J. 109(3), 14–15 (2013). 8-12
S.D. Sullivan, A. Lehman, F. Thomas, K.C. Johnson, R. Jackson, J. Wactawski-Wende, M. Ko, Z. Chen, J.D. Curb, B.V. Howard, Effects of self-reported age at nonsurgical menopause on time to first fracture and bone mineral density in the Women’s Health Initiative Observational Study. Menopause 22(10), 1035–1044 (2015)
I. Lambrinoudaki, M. Flokatoula, E. Armeni, P. Pliatsika, A. Augoulea, A. Antoniou, A. Alexandrou, M. Creatsa, C. Panoulis, S. Dendrinos, X. Papacharalambous, Vertebral fracture prevalence among Greek healthy middle-aged postmenopausal women: association with demographics, anthropometric parameters, and bone mineral density. Spine J. 15(1), 86–94 (2015)
S.R. Cummings, M.C. Nevitt, W.S. Browner, K. Stone, K.M. Fox, K.E. Ensrud, J. Cauley, D. Black, T.M. Vogt, Risk factors for hip fracture in white women. Study of Osteoporotic Fractures Research Group. N. Engl. J. Med. 332(12), 767–773 (1995)
B.L. Riggs, S. Khosla, L.J. Melton 3rd, : Sex steroids and the construction and conservation of the adult skeleton. Endocr. Rev. 23(3), 279–302 (2002)
J.E. Rossouw, G.L. Anderson, R.L. Prentice, A.Z. LaCroix, C. Kooperberg, M.L. Stefanick, R.D. Jackson, S.A. Beresford, B.V. Howard, K.C. Johnson, J.M. Kotchen, J. Ockene, Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the women’s health initiative randomized controlled trial. JAMA 288(3), 321–333 (2002)
G.L. Anderson, M. Limacher, A.R. Assaf, T. Bassford, S.A. Beresford, H. Black, D. Bonds, R. Brunner, R. Brzyski, B. Caan, R. Chlebowski, D. Curb, M. Gass, J. Hays, G. Heiss, S. Hendrix, B.V. Howard, J. Hsia, A. Hubbell, R. Jackson, K.C. Johnson, H. Judd, J.M. Kotchen, L. Kuller, A.Z. LaCroix, D. Lane, R.D. Langer, N. Lasser, C.E. Lewis, J. Manson, K. Margolis, J. Ockene, M.J. O’Sullivan, L. Phillips, R.L. Prentice, C. Ritenbaugh, J. Robbins, J.E. Rossouw, G. Sarto, M.L. Stefanick, L. Van Horn, J. Wactawski-Wende, R. Wallace, S. Wassertheil-Smoller, Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. JAMA 291(14), 1701–1712 (2004)
H. Mizunuma, Y. Taketani, H. Ohta, H. Honjo, I. Gorai, A. Itabashi, M. Shiraki, Dose effects of oral estradiol on bone mineral density in Japanese women with osteoporosis. Climacteric 13(1), 72–83 (2010)
F.A. Tremollieres, J.M. Pouilles, C. Ribot, Withdrawal of hormone replacement therapy is associated with significant vertebral bone loss in postmenopausal women. Osteoporos. Int. 12(5), 385–390 (2001)
G. Papadakis, D. Hans, E. Gonzalez-Rodriguez, P. Vollenweider, G. Waeber, P.M. Marques-Vidal, O. Lamy, The benefit of menopausal hormone therapy on bone density and microarchitecture persists after its withdrawal. J. Clin. Endocrinol. Metab. 101(12), 5004–5011 (2016)
N.B. Watts, J.A. Cauley, R.D. Jackson, A.Z. LaCroix, C.E. Lewis, J.E. Manson, J.M. Neuner, L.S. Phillips, M.L. Stefanick, J. Wactawski-Wende, C. Crandall, No increase in fractures after stopping hormone therapy: results from the women’s health initiative. J. Clin. Endocrinol. Metab. 102(1), 302–308 (2017)
E.B. Gold, S.L. Crawford, N.E. Avis, C.J. Crandall, K.A. Matthews, L.E. Waetjen, J.S. Lee, R. Thurston, M. Vuga, S.D. Harlow, Factors related to age at natural menopause: longitudinal analyses from SWAN. Am. J. Epidemiol. 178(1), 70–83 (2013)
U. Stepaniak, K. Szafraniec, R. Kubinova, S. Malyutina, A. Peasey, H. Pikhart, A. Pajak, M. Bobak, Age at natural menopause in three central and eastern European urban populations: the HAPIEE study. Maturitas 75(1), 87–93 (2013)
V. Mpalaris, P. Anagnostis, D.G. Goulis, I. Iakovou, Complex association between body weight and fracture risk in postmenopausal women. Obes. Rev. 16(3), 225–233 (2015)
M.C. Navarro, M. Sosa, P. Saavedra, P. Lainez, M. Marrero, M. Torres, C.D. Medina, Poverty is a risk factor for osteoporotic fractures. Osteoporos. Int. 20(3), 393–398 (2009)
J.S. Laven, Genetics of early and normal menopause. Semin. Reprod. Med. 33(6), 377–383 (2015)
L. Stolk, J.R. Perry, D.I. Chasman, C. He, M. Mangino, P. Sulem, M. Barbalic, L. Broer, E.M. Byrne, F. Ernst, T. Esko, N. Franceschini, D.F. Gudbjartsson, J.J. Hottenga, P. Kraft, P.F. McArdle, E. Porcu, S.Y. Shin, A.V. Smith, S. van Wingerden, G. Zhai, W.V. Zhuang, E. Albrecht, B.Z. Alizadeh, T. Aspelund, S. Bandinelli, L.B. Lauc, J.S. Beckmann, M. Boban, E. Boerwinkle, F.J. Broekmans, A. Burri, H. Campbell, S.J. Chanock, C. Chen, M.C. Cornelis, T. Corre, A.D. Coviello, P. d’Adamo, G. Davies, U. de Faire, E.J. de Geus, I.J. Deary, G.V. Dedoussis, P. Deloukas, S. Ebrahim, G. Eiriksdottir, V. Emilsson, J.G. Eriksson, B.C. Fauser, L. Ferreli, L. Ferrucci, K. Fischer, A.R. Folsom, M.E. Garcia, P. Gasparini, C. Gieger, N. Glazer, D.E. Grobbee, P. Hall, T. Haller, S.E. Hankinson, M. Hass, C. Hayward, A.C. Heath, A. Hofman, E. Ingelsson, A.C. Janssens, A.D. Johnson, D. Karasik, S.L. Kardia, J. Keyzer, D.P. Kiel, I. Kolcic, Z. Kutalik, J. Lahti, S. Lai, T. Laisk, J.S. Laven, D.A. Lawlor, J. Liu, L.M. Lopez, Y.V. Louwers, P.K. Magnusson, M. Marongiu, N.G. Martin, I.M. Klaric, C. Masciullo, B. McKnight, S.E. Medland, D. Melzer, V. Mooser, P. Navarro, A.B. Newman, D.R. Nyholt, N.C. Onland-Moret, A. Palotie, G. Pare, A.N. Parker, N.L. Pedersen, P.H. Peeters, G. Pistis, A.S. Plump, O. Polasek, V.J. Pop, B.M. Psaty, K. Raikkonen, E. Rehnberg, J.I. Rotter, I. Rudan, C. Sala, A. Salumets, A. Scuteri, A. Singleton, J.A. Smith, H. Snieder, N. Soranzo, S.N. Stacey, J.M. Starr, M.G. Stathopoulou, K. Stirrups, R.P. Stolk, U. Styrkarsdottir, Y.V. Sun, A. Tenesa, B. Thorand, D. Toniolo, L. Tryggvadottir, K. Tsui, S. Ulivi, R.M. van Dam, Y.T. van der Schouw, C.H. van Gils, P. van Nierop, J.M. Vink, P.M. Visscher, M. Voorhuis, G. Waeber, H. Wallaschofski, H.E. Wichmann, E. Widen, C.J. Wijnands-van Gent, G. Willemsen, J.F. Wilson, B.H. Wolffenbuttel, A.F. Wright, L.M. Yerges-Armstrong, T. Zemunik, L. Zgaga, M.C. Zillikens, M. Zygmunt, A.M. Arnold, D.I. Boomsma, J.E. Buring, L. Crisponi, E.W. Demerath, V. Gudnason, T.B. Harris, F.B. Hu, D.J. Hunter, L.J. Launer, A. Metspalu, G.W. Montgomery, B.A. Oostra, P.M. Ridker, S. Sanna, D. Schlessinger, T.D. Spector, K. Stefansson, E.A. Streeten, U. Thorsteinsdottir, M. Uda, A.G. Uitterlinden, C.M. van Duijn, H. Volzke, A. Murray, J.M. Murabito, J.A. Visser, K.L. Lunetta, Meta-analyses identify 13 loci associated with age at menopause and highlight DNA repair and immune pathways. Nat. Genet. 44(3), 260–268 (2012)
M.G. Braem, M. Voorhuis, Y.T. van der Schouw, P.H. Peeters, L.J. Schouten, M.J. Eijkemans, F.J. Broekmans, N.C. Onland-Moret, Interactions between genetic variants in AMH and AMHR2 may modify age at natural menopause. PLoS. One. 8(3), e59819 (2013)
A. La Marca, G. Sighinolfi, C. Argento, V. Grisendi, L. Casarini, A. Volpe, M. Simoni, Polymorphisms in gonadotropin and gonadotropin receptor genes as markers of ovarian reserve and response in in vitro fertilization. Fertil. Steril. 99(4), 970–978.e971 (2013)
U. Styrkarsdottir, B.V. Halldorsson, S. Gretarsdottir, D.F. Gudbjartsson, G.B. Walters, T. Ingvarsson, T. Jonsdottir, J. Saemundsdottir, J.R. Center, T.V. Nguyen, Y. Bagger, J.R. Gulcher, J.A. Eisman, C. Christiansen, G. Sigurdsson, A. Kong, U. Thorsteinsdottir, K. Stefansson, Multiple genetic loci for bone mineral density and fractures. N. Engl. J. Med. 358(22), 2355–2365 (2008)
G.R. Ji, M. Yao, C.Y. Sun, Z.H. Li, Z. Han, BsmI, TaqI, ApaI, and FokI polymorphisms in the vitamin D receptor (VDR) gene and risk of fracture in Caucasians: a meta-analysis. Bone 47(3), 681–686 (2010)
A.E. Weel, A.G. Uitterlinden, I.C. Westendorp, H. Burger, S.C. Schuit, A. Hofman, T.J. Helmerhorst, J.P. van Leeuwen, H.A. Pols, Estrogen receptor polymorphism predicts the onset of natural and surgical menopause. J. Clin. Endocrinol. Metab. 84(9), 3146–3150 (1999)
V. Dvornyk, J.R. Long, P.Y. Liu, H. Shen, R.R. Recker, H.W. Deng, Polymorphisms of the vitamin D receptor gene predict the onset of surgical menopause in Caucasian females. Gynecol. Endocrinol. 22(10), 552–556 (2006)
D.M. Black, J.A. Cauley, R. Wagman, K. Ensrud, H.A. Fink, T.A. Hillier, L.Y. Lui, S.R. Cummings, J.T. Schousboe, N. Napoli, The ability of a single BMD and fracture history assessment to predict fracture over 25 years in postmenopausal women: the study of osteoporotic fractures. J. Bone Miner. Res. 33(3), 389–395 (2018)
Author contributions
P.A. designed the research, extracted and analysed the data and wrote the first draft of the paper. P.S., N.K.G., N.K., A.M.A., and K.C. searched the literature, extracted and analysed the data. P.S. was responsible for the statistical analysis and reviewed the manuscript. S.A.P., M.P., E.T., E.K., I.L., and J.C.S. reviewed the manuscript and provided critical scientific input. D.G.G. resolved discrepancies regarding the quality of the studies included in the meta-analysis, provided critical scientific input and had the primary responsibility for the paper’s final content.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
J.C.S. has received grants/research support from Abbott, Mylan and Pfizer; consulting fees from Abbott, Mylan and Pfizer; and speaker’s honoraria from Abbott, Bayer, Gedeon Richter, Menarini, Mylan, and Pfizer. The remaining authors declare that they have no conflict of interest.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Anagnostis, P., Siolos, P., Gkekas, N.K. et al. Association between age at menopause and fracture risk: a systematic review and meta-analysis. Endocrine 63, 213–224 (2019). https://doi.org/10.1007/s12020-018-1746-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-018-1746-6