Abstract
Bazedoxifene (BZA) is a selective estrogen receptor modulator that reduces the risk of fracture and improves bone mineral density in post-menopausal women with osteoporosis. The aim of the present systematic review and meta-analysis was to investigate effects of BZA on bone mineral density (BMD) and fracture in post-menopausal osteoporotic women. We searched PubMed, Cochrane Central Register of Controlled Trials, Web of Sciences, Embase, and Scopus from until November 30, 2016. All randomized controlled trials that compared the effects of BZA on BMD and the incidence of vertebral and non-vertebral fractures in post-menopausal osteoporotic women compared with a control group were eligible for inclusion. Meta-analyses were conducted to calculate relative risk (RR) with 95% confidence interval (CI) for the association of BZA and vertebral and non-vertebral fractures compared with placebo. Nine randomized clinical trials met our inclusion criteria. Studies results showed that BZA significantly improves BMD, although we were not able to pool the results. Meta-analysis showed that the pooled effect of BZD on vertebral fracture was protective and significant (RR = 0.63; 95% CI 0.48, 0.83; P = 0.001). But pooled results did not show any association between taking BZD and the incidence of non-vertebral fracture (RR = 0.97; 95% CI 0.83, 1.13; P = 0.683). Evidence suggests that bazedoxifene is generally effective and safe in preventing bone loss and vertebral fracture in post-menopausal women with osteoporosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteoporosis is a silent disease but its impacts are serious in public health [1,2,3]. There are approximately 200 million people with this disease in the world [3]. In the USA, an estimated two million osteoporotic fractures occur annually, resulting in more than half a million hospitalizations [1] and in the EU, it was estimated that in 2010, 6.6% of men and 22.1% of women aged over 50 years had osteoporosis, and that 3.5 million fractures occurred [4].
In post-menopausal women (PM), the level of endogenous estrogen decreases and leads to osteoporosis [5]. The characteristics of post-menopausal osteoporosis are low BMD and deterioration of bone structure, which is associated with an increased risk of hip, vertebral and non-vertebral fractures [6].
Bisphosphonates, hormone therapy, parathyroid hormone, denosumab, strontium ranelate (outside the USA), and selective estrogen receptor modulators (SERMs) are therapies currently used to treat and prevent osteoporosis [7, 8]. Each of these treatments has its unique benefits and complications and may not be appropriate for all women [8].
SERMs are a class of compounds that interact with estrogen receptors (ERs) and exert agonist or antagonist effects on ERs in a tissue-specific manner. Tamoxifen, a first-generation SERM, is able to maintain BMD in post-menopausal women. Raloxifene, a second-generation SERM, was used to prevent post-menopausal osteoporosis [9, 10]. BZD is a third-generation SERM that has been approved for the prevention and treatment of osteoporosis in post-menopausal women at increased risk for fractures [8, 11, 12].
There are several multicenter, national, and international randomized clinical trials (RCTs) that were conducted to show BZA effects post-menopausal osteoporosis in women. Our purpose was to systematically review the literature regarding the effect of BZA on BMD and the incidence of vertebral and non-vertebral fractures in post-menopausal osteoporotic women.
Methods
Databases and Search Strategy
We performed a literature search in PubMed, Cochrane Central Register of Controlled Trials, Web of Sciences, Embase, and Scopus from the 6th to 30th of November 2016. The search terms used are listed in Table 1.
Eligibility Criteria
Inclusion criteria included a focus on RCTs comparing the effects of BZA on BMD and the incidence of vertebral and non-vertebral fractures in post-menopausal osteoporotic women compared with a control group. We excluded narrative review articles, animal studies, and human cell and tissue culture studies. Also, irrelevant outcome measures, duplicate papers about the same study, and secondary analysis of previous trials were our exclusion criteria.
Study Selection
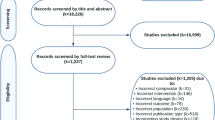
Two authors (MK and HM) reviewed the search results separately to find potentially eligible studies. The publications were sorted by titles and abstracts, and eligible studies were selected for full-text review. During this stage, all the irrelevant studies and duplicates were excluded (Fig. 1). Then, they independently assessed each of the selected articles for inclusion in the study using the inclusion and exclusion criteria previously mentioned.
Data Extraction
Data were extracted independently by two authors (MK and HM) and any discrepancies that arose were solved by a third author (NK). The following information was extracted: RCTs phase (II, III), age of participants, sample size, a summary of inclusion/exclusion criteria, years since menopause, types of interventions in active and control group, study duration, incidence of vertebral and non-vertebral fractures, mean percent change in lumbar spine BMD, and adverse events.
Quality Assessment
The methodological quality of the studies was assessed according to the standardized form of the CONSORT 2010 statement for randomized controlled trials (www.consort-statement.org) [13] by MK and HM independently.
Statistical Analysis
Due to different intervention and the different measure for mean percent change in lumbar spine BMD, we only reported each study result and did not report pooled mean percent change in BMD. Relative risk (RRs) with 95% confidence intervals (CIs) estimated for the association of BZA and vertebral and non-vertebral fractures compared with placebo were pooled. Adverse events data were also summarized with RRs.
Heterogeneity between studies was assessed using both the I2 statistic with a cutoff of ≥ 50% and the χ2 test with a P value < 0.10, to define a significant degree of heterogeneity. Also, in order to investigate the existence of publication bias, we used the Egger regression asymmetric test with 10% significant levels. Stata 14 (StataCorp, College Station, TX) was used for all the analyses and graphics. A two-sided P value ≤ 0.05 was considered statistically significant, if not otherwise specified.
Results
A total of 1318 articles were retrieved from five scientific databases (Cochrane Central Register of Controlled Trials, Embase, ISI Web of Sciences, PubMed, and Scopus). These papers were screened on the basis of title and abstract, and 1298 were excluded because they were irrelevant or duplicates. Thirty-four studies were eligible for full-text review. Twenty-five of these were subsequently excluded according to the criteria (Fig. 1). Finally, nine studies were selected for the systematic review of mean percent change in lumbar spine BMD [7, 11, 14,15,16,17,18,19,20]. Four of these studies reported incidence of vertebral and non-vertebral fracture compared with placebo, and they were included in the meta-analysis [7, 11, 14, 19]. One study was a phase II clinical trial [14], and to describe the long-term efficacy and safety of BZD, Silverman et al. continued the RCT on healthy post-menopausal women with osteoporosis for up to 7 years and reported findings in three papers [7, 11, 19]. Table 2 outlines the main study characteristics.
BMD
All of nine studies assessed BMD of the lumbar spine changing after taking BZA 20 mg compared with the control group. Furthermore, in three studies BZD was taken with conjugated estrogens (CEs) [15, 17, 18]. Study results showed that BZA significantly improves BMD [7, 11, 14,15,16,17,18,19,20], even in Lindsay et al.’s study, BMD increased significantly more in BZA/CE groups compared with controls [15]. In studies that women had been treated with only BZD [7, 11, 14, 16, 19, 20], mean percent change of lumbar spine BMD from baseline was greater than those that women had been treated with BZD/CE [15, 17, 18]. In three studies, women took BZD 40 mg [14, 16, 19], and in Lindsay et al.’s study, they took BZD 40/CE [15]. According to study results, both BZD doses 20 and 40 mg had a similar effect on BMD. Efficacy information is summarized in Table 3.
Vertebral and Non-Vertebral Fracture
Only four studies reported the efficacy of BZD 20 mg/day on the incidence of vertebral and non-vertebral fracture. We estimated the RR for each study. In all of the four studies, RR of BZD on vertebral fracture was protective and significant. Also, pooling under a fixed-effects model showed a significant association (RR = 0.63; 95% CI 0.48, 0.83; P = 0.001) (Fig. 2), with no evidence of statistical heterogeneity across studies (I2 = 0.0%; P = 0.798). In order to find an estimation about publication bias, a Begg’s funnel plot was drawn and the result showed a no significant publication bias (Egger test, t = − 0.33, P = 0.772).
Non-vertebral osteoporosis- related fractures were defined as fractures that were sustained after minimal or low-impact trauma, such as falling from standing height [19]. We observed no association between taking BZD 20 mg/kg and the incidence of non-vertebral fracture (RR = 0.97; 95% CI 0.83, 1.13; P = 0.683) in fixed model results (Fig. 3), and there was no significant heterogeneity across studies (I2 = 0.0%; P = 0.846). Also, we assessed publication bias. There was no publication bias according to the Egger test and Begg’s funnel plot in evaluating the effect BZD 20 mg/day on non-vertebral fracture (Egger test, t = 0.14, P = 0.903).
Averse Events
Seven articles contained safety data and the number of patients with AEs or any AE, and the number of patients with serious AEs or most common AEs were reviewed [8, 14, 16, 19, 20]. Meanwhile, Palacios et al. [11] reported AEs up to 7 years and contains AEs of Silverman et al. studies [7, 19] as well. Two studies were removed from the meta-analysis because their interventions were different (CE and BZD together). Therefore, we used 4 studies for the meta-analysis of BZD 20 mg/day and AEs. Table 4 shows the adverse effects in detail. Side effects such as endometrial carcinoma and hyperplasia, vaginal hemorrhage, breast cancer, myocardial infarction, stroke, and hot flushes were evaluated in these studies. In the BZD 20 mg/day group compared with placebo group, incidence of infectious and parasitic disease, headache, endometrial disorder, endometrial carcinoma, and hyperplasia decreased significantly, but the incidence of deep vein thrombosis, venous thromboembolism, and hot flushes increased significantly. As well as in the BZD 40 mg/day group, the risk of infectious and parasitic disease, arthralgia, and hot flushes increased significantly in comparison to the placebo group.
As shown in Fig. 4, there was no association between taking BZD 20 mg/day and incidence of any AE (RRfixed effect = 1.00; 95% CI 0.98, 1.02; P = 0.683). Heterogeneity across these studies was low (I2 = 22.7%; P = 0.275). In Fig. 5, pooled RRs show no association between BZD 20 mg/day and serious AEs (RR fixed effect = 1.00; 95% CI 0.98, 1.02; P = 0.683) either.
Discussion
Immediately after the menopause, bone mass decreases and increased bone turnover is associated with increased bone loss and the risk of fractures. This led to the use of estrogen therapy which was shown to prevent bone loss at menopause and to reduce the risk of important fragility fractures [21, 22]. Due to the adverse effects of estrogen in extra-skeletal organs, SERMs has been considered for treating osteoporosis in both sexes. SERMs contain non-steroidal synthetic compounds that have been developed to retain the beneficial effects of estrogens while eliminating unwanted side effects [21, 23]. This group includes raloxifene, arzoxifene, tamoxifene, lasofoxifene, and bazedoxifene [21,22,23]. Bazedoxifene acetate, the first of the third-generation SERMs, is chemically distinct SERM that was developed using stringent preclinical screening parameters, including favorable effects on the skeleton and lipid metabolism and demonstrable breast and uterine safety [16, 24]. BZA is available for the treatment of post-menopausal women at risk for, or presenting with, osteoporosis in Europe, Korea, and Japan [11, 12].
Due to its favorable preclinical effects, BZA has been selected to combine CE resulting in CE/BZA as a new progestin-free hormone therapy option for alleviating estrogen deficiency symptoms in post-menopausal women [12]. This treatment has shown the reduction of the incidence of serious adverse effect such as s myocardial infarction, cystic or fibrocystic breast diseases, venous thromboembolism, and back pain [15, 17, 18]. Also BZD/CE approved in the United States for the treatment of moderate to severe vasomotor symptoms associated with menopause and prevention of post-menopausal osteoporosis, and in the European Union for the treatment of estrogen deficiency symptoms in post-menopausal women with a uterus for whom treatment with progestin-containing therapy is not appropriate [25].
The objective of this study was to compare the efficacy of BZD versus placebo in terms of lumbar spine BMD improvement and prevention of vertebral and non-vertebral fracture in the post-menopausal women with an intact uterus. We also summarized the adverse events of BZD.
In this systematic review, studies showed that the BZD is effective on lumbar spine BMD improvement in healthy post-menopausal women. Even in three studies which the treatment group had taken BZD with CE, lumbar spine BMD increased from baseline compared to the placebo group [15, 17, 18]. Studies showed that the efficacy of BZA remained after 5 and 7 years of treatment. [7, 11, 19] Calcium and vitamin D supplementation may have contributed to this increase [11] as well. The increase in BMD with BZD and BZA/CE can be due to the decrease in bone turnover and bone loss as demonstrated by a significant decrease in osteocalcin and C-telopeptide plasma levels versus the baseline and placebo group levels [15, 26].
Another important result of this review was the assessment of the efficacy of BZD in preventing vertebral and non-vertebral fractures. Meta-analysis results showed significant reductions in the relative risks of vertebral fractures. After treatment with BZD 20 mg/day, the overall risk of vertebral fracture was around 30%. But BZA had no effect on the incidence of non-vertebral fractures. Even in a higher-risk subpopulation, there were 30% reductions in the risk of non-vertebral fracture at 7 years versus placebo, but they were not statistically significant [11]. Our findings are consistent with other meta-analysis that reported the vertebral fractures relative risk reduction for BZD was − 0.23 versus ibandronate, − 0.17 versus alendronate, and − 0.06 versus risedronate. They concluded that bazedoxifene is comparable to bisphosphonates in the overall post-menopausal osteoporosis (PMO) population and is at least as effective as bisphosphonates for preventing vertebral fractures among higher-risk PMO patients [6].
Changes in BMD have been shown to predict improvements in fracture risk reduction [7, 27], because reductions in bone turnover and/or improvements in bone properties/microarchitecture have been contribute to enhanced bone strength with osteoporosis treatments [11, 23, 28]. Significant reductions in bone turnover markers were seen after taking BZD [7, 14, 16, 18,19,20]. According to expert opinion, BZD could be considered as a second-line therapy for women < 65–70 years of age, where other drug such as bisphosphonates are contraindicated or not well tolerated [24, 29]. Furthermore, bazedoxifene could also have its place as a first-line therapy for younger post-menopausal patients in the management of menopause and prevention of osteoporosis [24].
We also assessed publication bias with the Egger test, Begg’s funnel plots, and trim tests. There was no evidence of small study effects (publication bias) according to visual inspection of the funnel plots and the Egger test.
The meta-analyses did not show any significant association between AEs and BZD 20 mg/day versus the placebo group. Also, in two studies in which the treatment group had taken CE and BZA, the treatment was safe and well tolerated [17, 18]. The overall rates of AEs, serious AEs, and discontinuations due to AEs were similar among the bazedoxifene and placebo groups. According to results of RCTs, BZA treatment showed no evidence of breast or endometrial stimulation. The incidence of endometrial carcinoma was significantly lower in the BZA group compared with the placebo group at 7 and at 5 years. The incidence of breast carcinoma was low and similar for BZA and placebo at 7 years [7, 11].
Conclusions
In summary, these results suggest that bazedoxifene is a safe and effective therapy for post-menopausal women with an intact uterus, seeking treatment for post-menopausal symptoms and prevention of bone loss. It significantly improves lumbar spine BMD, reduces incidence of vertebral fracture, and is well tolerated in post-menopausal women with osteoporosis.
References
Singer A, Exuzides A, Spangler L, O’Malley C, Colby C, Johnston K et al., editors. Burden of illness for osteoporotic fractures compared with other serious diseases among postmenopausal women in the United States. Mayo Clin Proc; 2015: Elsevier.
Detilleux J, Reginster JY, Chines A, Bruyere O. A Bayesian path analysis to estimate causal effects of bazedoxifene acetate on incidence of vertebral fractures, either directly or through non-linear changes in bone mass density. Stat Methods Med Res. 2016;25(1):400–12. https://doi.org/10.1016/j.bone.2017.01.024.
Moreira LDF, MLd O, Lirani-Galvão AP, Marin-Mio RV, RNd S, Lazaretti-Castro M. Physical exercise and osteoporosis: effects of different types of exercises on bone and physical function of postmenopausal women. Arq Bras Endocrinol Metabol. 2014;58(5):514–22. https://doi.org/10.1590/0004-2730000003374.
Curtis EM, Moon RJ, Harvey NC, Cooper C. The impact of fragility fracture and approaches to osteoporosis risk assessment worldwide. Bone. 2017;104:29–38. https://doi.org/10.1016/j.bone.2017.01.024.
Christiansen C, Chesnut CH 3rd, Adachi JD, Brown JP, Fernandes CE, Kung AW, et al. Safety of bazedoxifene in a randomized, double-blind, placebo- and active-controlled phase 3 study of postmenopausal women with osteoporosis. BMC Musculoskelet Disord. 2010;11(1):130. https://doi.org/10.1186/1471-2474-11-130.
Ellis AG, Reginster JY, Luo X, Bushmakin AG, Williams R, Sutradhar S, et al. Indirect comparison of bazedoxifene vs oral bisphosphonates for the prevention of vertebral fractures in postmenopausal osteoporotic women. Curr Med Res Opin. 2014;30(8):1617–26. https://doi.org/10.1185/03007995.2014.908279.
Silverman SL, Chines AA, Kendler DL, Kung AW, Teglbjaerg CS, Felsenberg D, et al. Sustained efficacy and safety of bazedoxifene in preventing fractures in postmenopausal women with osteoporosis: results of a 5-year, randomized, placebo-controlled study. Osteoporos Int : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2012;23(1):351–63. https://doi.org/10.1007/s00198-011-1691-1.
Palacios S, de Villiers TJ, Nardone Fde C, Levine AB, Williams R, Hines T, et al. Assessment of the safety of long-term bazedoxifene treatment on the reproductive tract in postmenopausal women with osteoporosis: results of a 7-year, randomized, placebo-controlled, phase 3 study. Maturitas. 2013;76(1):81–7. https://doi.org/10.1016/j.maturitas.2013.06.008.
Xu B, Lovre D, Mauvais-Jarvis F. The effect of selective estrogen receptor modulators on type 2 diabetes onset in women: basic and clinical insights. J Diabetes Complications. 2017;31(4):773–779.https://doi.org/10.1016/j.jdiacomp.2016.12.010
An K-C. Selective estrogen receptor modulators. Asian Spine Journal. 2016;10(4):787–91. https://doi.org/10.4184/asj.2016.10.4.787.
Palacios S, Silverman SL, de Villiers TJ, Levine AB, Goemaere S, Brown JP, et al. A 7-year randomized, placebo-controlled trial assessing the long-term efficacy and safety of bazedoxifene in postmenopausal women with osteoporosis: effects on bone density and fracture. Menopause. 2015;22(8):806–13. https://doi.org/10.1097/GME.0000000000000419.
Jover-Mengual T, Castelló-Ruiz M, Burguete MC, Jorques M, López-Morales MA, Aliena-Valero A, et al. Molecular mechanisms mediating the neuroprotective role of the selective estrogen receptor modulator, bazedoxifene, in acute ischemic stroke: a comparative study with 17β-estradiol. J Steroid Biochem Mol Biol. 2017;171:296–304. https://doi.org/10.1016/j.jsbmb.2017.05.001.
Schulz KF, Altman DG, Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMC Med. 2010;8(1):18. https://doi.org/10.1136/bmj.c332.
Itabashi A, Yoh K, Chines AA, Miki T, Takada M, Sato H, et al. Effects of bazedoxifene on bone mineral density, bone turnover, and safety in postmenopausal Japanese women with osteoporosis. J Bone Miner Res : Off J Am Soc Bone Mineral Res. 2011;26(3):519–29. https://doi.org/10.1002/jbmr.252.
Lindsay R, Gallagher JC, Kagan R, Pickar JH, Constantine G. Efficacy of tissue-selective estrogen complex of bazedoxifene/conjugated estrogens for osteoporosis prevention in at-risk postmenopausal women. Fertil Steril. 2009;92(3):1045–52. https://doi.org/10.1016/j.fertnstert.2009.02.093.
Miller PD, Chines AA, Christiansen C, Hoeck HC, Kendler DL, Lewiecki EM, et al. Effects of bazedoxifene on BMD and bone turnover in postmenopausal women: 2-yr results of a randomized, double-blind, placebo-, and active-controlled study. J Bone Miner Res : Off J Am Soc Bone and Mineral Res. 2008;23(4):525–35. https://doi.org/10.1359/JBMR.071206.
Mirkin S, Komm BS, Pan K, Chines AA. Effects of bazedoxifene/conjugated estrogens on endometrial safety and bone in postmenopausal women. Climacteric : J Int Menopause Soc. 2013;16(3):338–46. https://doi.org/10.3109/13697137.2012.717994.
Pinkerton JV, Harvey JA, Lindsay R, Pan K, Chines AA, Mirkin S, et al. Effects of bazedoxifene/conjugated estrogens on the endometrium and bone: a randomized trial. J Clin Endocrinol Metab. 2014;99(2):E189–98. https://doi.org/10.1210/jc.2013-1707.
Silverman SL, Christiansen C, Genant HK, Vukicevic S, Zanchetta JR, de Villiers TJ, et al. Efficacy of bazedoxifene in reducing new vertebral fracture risk in postmenopausal women with osteoporosis: results from a 3-year, randomized, placebo-, and active-controlled clinical trial. J Bone Miner Res : Off J Am Soc Bone Miner Res. 2008;23(12):1923–34. https://doi.org/10.1359/jbmr.080710.
Xu L, Tsai KS, Kim GS, Wu Y, Vincendon P, Chines AA, et al. Efficacy and safety of bazedoxifene in postmenopausal Asian women. Osteoporos Int : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2011;22(2):559–65. https://doi.org/10.1007/s00198-010-1259-5.
McClung MR. New management options for osteoporosis with emphasis on SERMs. Climacteric : J Int Menopause Soc. 2015;18(sup2):56–61. https://doi.org/10.3109/13697137.2015.1104010.
Maximov PY, Lee TM, Jordan VC. The discovery and development of selective estrogen receptor modulators (SERMs) for clinical practice. Curr Clin Pharmacol. 2013;8(2):135–55. https://doi.org/10.2174/1574884711308020006.
Tabatabaei-Malazy O, Salari P, Khashayar P, Larijani B. New horizons in treatment of osteoporosis. DARU J Pharm Sci. 2017;25(1):2. https://doi.org/10.1186/s40199-017-0167-z.
Gatti D, Rossini M, Sblendorio I, Lello S. Pharmacokinetic evaluation of bazedoxifene for the treatment of osteoporosis. Expert Opin Drug Metab Toxicol. 2013;9(7):883–92. https://doi.org/10.1517/17425255.2013.794221.
McKeand W. Pharmacokinetics, dose proportionality, and bioavailability of bazedoxifene in healthy postmenopausal women. Clin Ther. 2017;39(9):1769–79. https://doi.org/10.1016/j.clinthera.2017.07.012.
Rossini M, Lello S, Sblendorio I, Viapiana O, Fracassi E, Adami S, et al. Profile of bazedoxifene/conjugated estrogens for the treatment of estrogen deficiency symptoms and osteoporosis in women at risk of fracture. Drug Des Dev Ther. 2013;7:601–10. https://doi.org/10.2147/DDDT.S47807.
Reginster J-Y, Ferrari S, Hadji P. Current challenges in the treatment of osteoporosis: an opportunity for bazedoxifene. Curr Med Res Opin. 2014;30(6):1165–76. https://doi.org/10.1185/03007995.2014.890927.
Komm BS, Chines AA. Bazedoxifene: the evolving role of third-generation selective estrogen-receptor modulators in the management of postmenopausal osteoporosis. Ther Adv Musculoskelet Disease. 2012;4(1):21–34. https://doi.org/10.1177/1759720X11422602.
Komm BS, Morgenstern D, Yamamoto LA, Jenkins SN. The safety and tolerability profile of therapies for the prevention and treatment of osteoporosis in postmenopausal women. Expert Rev Clin Pharmacol. 2015;8(6):769–84. https://doi.org/10.1586/17512433.2015.1099432.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
The article does not contain any studies with human or animal subjects performed by any of the authors.
Informed Consent
The article does not contain any studies with human subjects performed by any of the authors.
Rights and permissions
About this article
Cite this article
Khalili, M., Hosseinzadeh, A., Kiavandani, H.M. et al. Effects of Bazedoxifene on Bone Mineral Density and Fracture in Post-Menopausal Osteoporotic Women: a Systematic Review and Meta-Analysis. Clinic Rev Bone Miner Metab 16, 22–32 (2018). https://doi.org/10.1007/s12018-018-9241-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12018-018-9241-4