Abstract
The management of critically ill Cushing’s disease (CD) patients is extremely challenging. Pasireotide is indicated for the treatment of CD patients when pituitary surgery is unfeasible or has not been curative, but no data are available about the use of this drug as pre-operative treatment in critically ill patients. We report the effects of presurgical pasireotide therapy in CD patients in whom hypercortisolism caused life-threatening hypokalemia, alkalosis, and cardio-respiratory complications precluding surgical approach. Clinical, biochemical, and radiological data of two critically ill patients with ACTH-secreting pituitary macroadenoma, before and during first-line presurgical pasireotide treatment (600 μg s.c. bid). During the first 21 days of treatment, pasireotide therapy induced a rapid, partial decrease of plasma ACTH, serum cortisol, and urinary free cortisol levels, with the consequent normalization of serum potassium concentration and arterial blood gases parameters, in both the patients. They did not experience unmanageable side effects and underwent endoscopic transsphenoidal surgery after 4 weeks of effective treatment. Pre-operative MRI evaluation did not show pituitary tumor shrinkage. Surgical cure of CD was obtained in the first patient, while debulking allowed the pharmacological control of hypercortisolism in the second case. We suggest that pasireotide can induce a rapid improvement of clinical and metabolic conditions in critically ill CD patients in whom surgical approach is considered hazardous and need to be delayed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
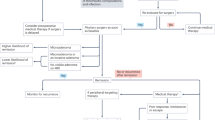
Cushing’s disease (CD) is a clinical condition associated with increased morbidity and mortality, caused by ACTH-secreting pituitary adenomas. Usually, these tumors are extremely small or even undetectable by post-contrast dynamic MRI, while macroadenomas are very rare [1, 2]. Pituitary surgery represents the first-line therapy, because it is a safe and effective approach that induces a dramatic and rapid ACTH and cortisol decrease in the majority of cases [3]. Patients not cured by surgery can undergo reintervention, radiosurgery, bilateral adrenalectomy, and/or medical therapy. Pharmacological treatment is also indicated when surgery is not feasible, to reach biochemical control while waiting for radiosurgery to be effective or in mild CD [4]. Medical therapies for CD include several drugs acting at pituitary (pasireotide, cabergoline, retinoic acid), adrenal (metyrapone, ketoconazole, mitotane, etomidate), or glucocorticoid receptor (mifepristone) level [5, 6]. At present, however, pasireotide is the only pituitary-directed drug registered in Europe and in USA for the treatment of CD patients when surgery is not curative or is unfeasible. It is a multireceptor-targeted somatostatin analog which induces antitumor effects and inhibits ACTH secretion in corticotroph adenomas, improving signs and symptoms of hypercortisolism in 30–50 % of CD patients [7, 8]. Nevertheless, few data are available about the use of pasireotide as first-line or presurgical treatment [9].
Clinical manifestations of CD include obesity—confined mainly to the face, neck, trunk, and abdomen—purple striae, muscle weakness, fatigue, easy bruising, hypertension, dyslipidemia, and diabetes mellitus. Acne, hirsutism, and menstrual irregularities are extremely common in women, while children usually show weight gain and growth retardation [10]. Neuropsychiatric disorders occur frequently in both the sexes and at all ages, due to the relevant effects of glucocorticoids on the central nervous system [11]. Osteoporosis and increased risk for low-energy bone fractures have been reported in adults [12]. The spectrum of clinical presentation is broad, ranging from mild/pauci-symptomatic to extremely severe forms that require alternative management strategy. Indeed, surgery is generally followed by rapid cortisol reduction, improving clinical conditions and decreasing mortality risk, but sometimes, patients in critical situations cannot be immediately operated because of very high anesthesiology risk. In these cases, some authors proposed alternative therapeutic approach based on the administration of drugs blocking cortisol production or antagonizing its receptor, alone or in association [13–15]. In patients with severe hypercortisolism, acute respiratory distress syndrome (ARDS), a life-threatening condition probably precipitated by hypophosphatemia, can also preclude surgery [16].
Here we report on the clinical management of two patients with ACTH-secreting pituitary macroadenomas and secondary hypercortisolism, which caused severe alkalosis and cardio-respiratory complications rapidly reverted by presurgical pasireotide administration. We demonstrate for the first time that this pituitary-targeted therapy represents a valid option in these cases, as well as other drugs acting at adrenal level which have been used in combination or monotherapy [13, 14, 17].
Patients and methods
Patients
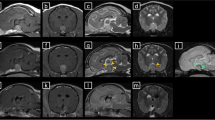
Patient no. 1 was a 53-year-old woman referred to the gynecological unit of our university hospital for a huge pelvic abscess causing sepsis in March 2013. She complained of typical cushingoid features, hypertension, diabetes mellitus, and depression for 2 years. Morning plasma ACTH, serum cortisol, and urinary free cortisol (UFC) levels were dramatically elevated (416 pg/ml, 553 ng/ml and 15.4 × ULN, respectively). Moreover, human CRH and DDAVP tests induced exaggerated ACTH increase (3.2- and 10.0-fold from baseline levels, respectively). Thyroid function was normal. Gadolinium contrast enhanced MRI showed an 18-mm (max diameter) sellar lesion with strong and dishomogeneous contrast enhancement. The lesion had a suprasellar expansion with partial invasion of the left cavernous sinus, without compression of the optic chiasm. Sepsis resolved within few days, but electrolytic, metabolic, and respiratory conditions dramatically worsened. Severe hypokalemia (2.1 mEq/L) and hypophosphatemia (2.4 mg/dL) developed, although oral and intravenous potassium supplementation was started since admission. The patient rapidly became tachypneic (respiratory rate 24 breaths/min) and dyspneic. Arterial blood gases evaluation showed severe metabolic alkalosis with a compensatory rise of pCO2 (pH 7.621, pCO2 53.1 mmHg, pO2 50.6 mmHg, SO2 83.4 %, HCO3 − 53.5 mEq/L). Clinical symptoms and laboratory examinations supported the diagnosis of ARDS. Despite mechanical ventilation, clinical status did not sufficiently improve during the following days. On the basis of the critical clinical conditions, surgical approach was considered hazardous, and pasireotide treatment was immediately started.
Patient no. 2 was a 35-year-old male referred to our neurosurgery unit in January 2014 because of multiple vertebral fractures at D7, D8, D9, L2, and L4 levels. He showed severe cushingoid features and complained of muscle weakness, depression, and hypertension for at least 10 months. Plasma ACTH, serum cortisol, and UFC levels were very high (218 pg/ml, 409 ng/ml and 17.1 × ULN, respectively). Biochemical evaluation showed also low testosterone (60.8 ng/dL, n.v. 175–781) and IGF-1 (−2.9 SDS) concentrations, whereas thyroid function was normal. Pituitary-MRI demonstrated a sellar lesion with a huge infrasellar and suprasellar extension, invading both cavernous sinuses and encasing internal carotid arteries. The tumor extended to the posterior clinoid processes and to the suprasellar cisterns and invaded the sphenoidal sinus. Severe hypokalemia (2.4 mmol/l), which had been documented at admission, persisted during the following days despite oral and parenteral potassium supplementation. Also in this case, arterial blood gases evaluation indicated metabolic alkalosis with a compensatory rise of pCO2 (pH 7.574, pCO2 51.0 mmHg, pO2 51.2 mmHg, SO2 88.5 %, HCO3 46 mEq/L). Because of patient’s very poor clinical status, surgery was delayed and pasireotide treatment was started 8 days after admission.
Biochemical evaluation
Plasma ACTH determination was performed by a chemiluminescent method (Immulite 2000, DPC, Los Angeles CA, USA). Serum cortisol and UFC levels were measured by a commercial immunoassay (Beckham Coulter, Inc.). Method sensitivity was 5 pg/ml for plasma ACTH, 10 ng/ml for serum cortisol, and 15 µg/24 h for UFC. Intra- and inter-assay variation coefficients were <7 and <9 % for plasma ACTH and <6 and <9 % for both urinary and serum cortisol levels, respectively. In our laboratory, normal ranges are 10–50 pg/ml for plasma ACTH and 50–250 ng/ml for morning serum cortisol levels. UFC (normal range 75–270 µg/24 h) have been expressed as times the upper limit of normal (ULN).
Results
Response to treatment
Changes of hormone profiles, electrolytes levels, arterial blood gases analysis, blood pressure values, and fasting glucose levels of both patients during presurgical pasireotide treatment are summarized in Table 1.
Pasireotide therapy (600 µg s.c. bid) induced a rapid decrease of plasma ACTH, serum cortisol, and UFC levels during the first 21 days of treatment in both the cases (Fig. 1). Serum potassium concentration rapidly normalized as well as arterial blood gases parameters, allowing potassium supplementation reduction and, finally, withdrawal. At the same time, clinical status significantly improved in both the patients.
Effects of 4-week presurgical treatment with pasireotide on plasma ACTH, serum cortisol, and urinary free cortisol levels in patients no. 1 and no.2. The escape of ACTH and cortisol levels is evident during the 4th week of treatment in the first case. The dashed lines indicate the upper limit of normal
In patient no. 1, however, plasma ACTH, serum cortisol, and UFC levels increased again during the fourth week of pasireotide treatment (Fig. 1). Nonetheless, the amelioration of metabolic parameters and improvement of clinical conditions, with the consequent reduction of the anesthesiology risk, led us to decide to perform endoscopic transsphenoidal surgery. Pre-operative MRI evaluation did not show pituitary tumor shrinkage. Pasireotide treatment was withdrawn 2 days before surgery. Histological and immunohistochemical evaluations showed a densely granulated pituitary adenoma positive for ACTH and negative for GH, PRL, TSH, FSH, and LH. Ki-67 labeling index was 1 % and p53 was negative, but cytokeratin AE1-AE3 and chromogranin were strongly and diffusely positive. Three days after surgery, hypoadrenalism was documented, and hydrocortisone replacement was necessary for 6 months. Normalization of midnight cortisol concentration (13.2 ng/ml), UFC levels (0.2 × ULN) and cortisol suppression after overnight 1-mg dexamethasone administration (10.8 ng/ml) was documented 6 months after surgery and confirmed 1 year later.
In patient no. 2, endoscopic transsphenoidal approach and simultaneous vertebroplasty were performed 30 days after he started pasireotide. Also in this case, medical treatment did not shrink pituitary mass. At histology and immunohistochemistry, an atypical ACTH-secreting pituitary adenoma, negative for GH, PRL, TSH, FSH, and LH, with scattered mitoses (1 mitosis per 10 high-power fields) was proven. Ki-67 labeling index was 8 %, p53 was positive in 5 % of tumor cells and also cytokeratin AE1–AE3 and chromogranin were strongly and diffusely positive. Pasireotide was withdrawn 2 days before and restarted at the same dose 5 days after surgery, when the persistence of active CD was confirmed (plasma ACTH 198.0 pg/ml; serum cortisol 372 ng/ml; UFC 6.39 × ULN). Then, plasma ACTH rapidly decreased as well as serum cortisol and UFC levels, which reached normalization after few weeks. Nevertheless, cyber-knife stereotactic radiosurgery was performed 6 months after surgery because pituitary tumor remnant slightly grew.
In both patients, pasireotide treatment was started at the dose of 600 μg bid, in accordance with the drug data sheet, and no titration was performed until surgery. In patient no. 2, the dose was not titrated either after surgery because UFC levels quickly normalized.
In both cases, blood pressure decreased during pasireotide treatment (Table 1). At admission, patient no. 1 was treated with valsartan (320 mg/day), hydrochlorothiazide (25 mg/day), and doxazosin (4 mg/day). These drugs were continued during pasireotide therapy, but doxazosin dose was reduced to 2 mg/day. Finally, the anti-hypertensive therapy was withdrawn after surgery because of blood pressure normalization. Patient no. 2 was treated with canrenone (100 mg/day) and hydrochlorothiazide (25 mg bid) at entry, but hydrochlorothiazide was replaced with nebivolol (5 mg/day) immediately after admission to the neurosurgery unit. Unfortunately, serum aldosterone and plasma renin values were not evaluated in both the cases.
Adverse events
Pasireotide administration induced a rapid impairment of glycemic control in both patients, which was corrected in few days by insulin treatment. Only patient no. 1 was already diabetic and treated with metformin. Patient no. 2 complained of nausea and intestinal discomfort during the first 2 weeks of pasireotide treatment. Liver function was unchanged during all the treatment period in both cases. Patients did not report other adverse effects related to treatment.
Discussion
Transsphenoidal approach is considered the first-line treatment for patients with ACTH-secreting pituitary adenoma, since its cure rate is very high when surgery is performed by skilled neurosurgeons. However, the cure rate decreases significantly in patients with macroadenomas especially if they are invasive or aggressive [1, 2]. Adjunctive treatments, based on reintervention, radiosurgery, drugs, or bilateral adrenalectomy can be necessary when pituitary surgery is ineffective [4, 18]. Management of CD becomes extremely challenging when patients are in very poor clinical conditions due to opportunistic infections, hearth failure, psychiatric disturbances, and metabolic or respiratory complications. In these cases, the patient’s status can hamper surgical management, being not compatible with general anesthesia or lengthy surgical procedures. Indeed, although endoscopic approach has reduced surgical risk, operation remains potentially dangerous in critically ill patients [13–15]. For this reason, it can be necessary to delay intervention and to treat hypercortisolism by other approaches.
Our patients were symptomatic for several months before diagnosis but an opportunistic infection, in the first case, and multiple vertebral fractures, in the second one, suddenly induced metabolic decompensation. In these conditions, surgical risk was very high, and we opted for pharmacological treatment of hypercortisolism, delaying operation. At our knowledge, the only prospective study concerning alternative treatment in critical patients with ACTH-dependent hypercortisolism has been published by Kamenický et al. few years ago [13]. These authors reported a marked clinical improvement induced by combined treatment with metyrapone, ketoconazole, and mitotane, three drugs which block steroidogenesis, in 4 CD patients and in seven cases with ACTH-secreting neuroendocrine tumors. The control of hypercortisolism and its life-threatening complications was rapidly achieved by combined drugs administration and then maintained by low-dose mitotane alone in all CD patients, who were finally able to undergo pituitary surgery. Nevertheless, this approach is at risk of major adverse events, especially for the potential consequences on liver function [13]. In another study, Feelders et al. evaluated the effect of combined treatment with pasireotide, cabergoline, and ketoconazole in CD patients [19]. The stepwise pasireotide dose increase, followed by the addition of the other drugs in resistant patients, needed a long period of time for reaching UFC normalization in 88 % of cases. However, this study aimed to define a therapeutic strategy to control hypercortisolism in patients with refractory CD, rather than addressing the management of patients in critical conditions. In 2008, a consensus statement on the treatment of ACTH-dependent Cushing’s syndrome included a recommendation for the use of etomidate when a rapid control of cortisol levels is required and other therapies are unfeasible [20]. Etomidate is a carboxylated imidazole used as intravenous hypnotic agent, which inhibits the mitochondrial cytochrome p450-dependent adrenal enzyme 11β-hydroxylase that catalyzes the production of cortisol from deoxycortisol. However, etomidate should be used in an intensive care setting and a close serum cortisol monitoring is necessary to ensure that adrenal insufficiency does not occur [21]. Another drug proposed for the treatment of critically ill patients with endogenous hypercortisolism is mifepristone, which blocks cortisol action at level of glucocorticoid receptors [15]. In patients with severe Cushing’s syndrome, mifepristone administration induces a considerable improvement of hyperglycemia and hypertension; nevertheless, until recently, most of the information on the use and effectiveness of this drug was based only on case reports and small case series [22]. Management of mifepristone treatment is challenging, due to the lack of biochemical markers of treatment response and the frequent occurrence of adverse events related to the mechanism of action.
When we treated our patients with pasireotide, ketoconazole, metyrapone, or mifepristone were not immediately available in our hospital. On the other hand, mitotane needs several weeks and progressive dose increase to control hypercortisolism. On the contrary, Trementino et al. recently reported that pasireotide administration induces a very rapid decrease of plasma ACTH, serum cortisol, and UFC levels in responder patients, allowing the immediate identification of candidates to treatment [23]. At present, pasireotide is the only drug registered in Italy for treatment of adult Cushing’s disease. It is a multireceptor-targeted somatostatin analog, effective in reducing biochemical markers as well as in improving signs and symptoms of hypercortisolism [4, 6, 24]. Data reported until now show that long-term pasireotide administration induced UFC normalization in 26 % of patients treated with 900 μg bid and decreased median UFC levels by 50 % compared to baseline in both the groups of patients treated with 600 or 900 μg bid [8]. The control rate is higher in patients with mild Cushing’s disease than in those with severe hypercortisolism, since UFC normalization is reached only in 8 % of patients with UFC > 5 × ULN after 6-month treatment [8]. It is noteworthy that in our patients, the partial decreases of serum cortisol and UFC levels were associated with a rapid, dramatic improvement of clinical conditions, which allowed in few weeks surgical management. Few years ago, we reported that octreotide administration induced a rapid resolution of alkalosis and ARDS in a woman with CRH-secreting neuroendocrine adrenal tumor [16]. In that case, as well as in patient no. 1, ARDS may have been precipitated by hypophosphatemia, which normalized rapidly during somatostatin analogs' treatment. Nevertheless, a direct effect of these drugs on alkalosis cannot be ruled out.
Safety profile of pasireotide is similar to other somatostatin analogs, but the incidence of moderate or severe hyperglycemia is dramatically higher [24–26]. Accordingly, our patients showed impairment of glycemic profile nevertheless reduced by insulin administration. Persistent abdominal discomfort, complained by patient no. 2, is also frequently reported during pasireotide administration and is resistant to treatment but rarely causes withdrawal. In both the patients, our anesthesiologist requested pasireotide withdrawal 2 days before surgery because no safety data were available. However, no adverse events have been reported until now in patients operated during somatostatin analogs treatment.
Pasireotide as first-line treatment in a patient with ACTH-secreting macroadenoma has been reported by Shimon et al. [9]. In this case, however, the reason for first-line medical treatment was the refusal of surgery. The patient was treated for 39 months with high doses of pasireotide (900–1200 µg bid s.c.), reaching a satisfactory control of symptoms and signs of hypercortisolism after few months although UFC levels never normalized. Interestingly, tumor volume progressively decreased during 2-year treatment, reducing maximum diameter from 15 to 11 mm [9]. Recently, Simeoli et al. reported a significant reduction (>25 %) of tumor volume in 62.5 % and in 100 % of eight CD patients after 6 and 12 months, respectively, of pasireotide treatment at the dose of 600–1200 μg bid [27]. In our cases, tumor shrinkage was not demonstrated by MRI, but treatment was very short in the first case, while patient no. 2 harbored a giant tumor with a very high Ki67 labeling index, which slightly increased during pasireotide treatment and finally required radiosurgery.
In conclusion, pasireotide can induce a rapid improvement of clinical conditions and metabolic abnormalities in critical patients with Cushing’s disease, in whom transsphenoidal approach is considered hazardous and should be delayed. Indeed, even a partial decrease of cortisol concentrations can be associated with serum potassium increment and alkalosis resolution. For this reason, a short-term presurgical treatment with pasireotide can be a therapeutic option for selected patients with very high anesthesiology risk.
References
H.R. Kakade, R. Kasaliwal, K.S. Khadilkar, S. Jadhav, A. Bukan, S. Khare, S.R. Budyal, A. Goel, A.R. Lila, T. Bandgar, N.S. Shah, Clinical, biochemical and imaging characteristics of Cushing’s macroadenomas and their long-term treatment outcome. Clin. Endocrinol. 81, 336–342 (2014)
S. Cannavo, B. Almoto, C. Dall’Asta, S. Corsello, R.M. Lovicum, E. De Menis, F. Trimarchi, B. Ambrosi, Long-term results of treatment in patients with ACTH-secreting pituitary macroadenomas. Eur. J. Endocrinol. 149, 195–200 (2003)
X. Bertagna, L. Guignat, Approach to the Cushing’s disease patient with persistent/recurrent hypercortisolism after pituitary surgery. J. Clin. Endocrinol. Metab. 98, 1307–1318 (2013)
A. Colao, M. Boscaro, D. Ferone, F.F. Casanueva, Managing Cushing’s disease: the state of the art. Endocrine 47, 9–20 (2014)
R.A. Feelders, L.J. Hofland, Medical treatment of Cushing’s disease. J. Clin. Endocrinol. Metab. 98, 425–438 (2013)
D. Ferone, C. Pivonello, G. Vitale, M.C. Zatelli, A. Colao, R. Pivonello, Molecular basis of pharmacological therapy in Cushing’s disease. Endocrine 46, 181–198 (2014)
M. Boscaro, W.H. Ludlam, B. Atkinson, J.E. Glusman, S. Petersenn, M. Reincke, P. Snyder, A. Tabarin, B.M. Biller, J. Findling, S. Melmed, C.H. Darby, K. Hu, Y. Wang, P.U. Freda, A.B. Grossman, L.A. Frohman, J. Bertherat, Treatment of pituitary-dependent Cushing’s disease with the multireceptor ligand somatostatin analog pasireotide (SOM230): a multicenter, phase II trial. J. Clin. Endocrinol. Metab. 94, 115–122 (2009)
A. Colao, S. Petersenn, J. Newell-Price, J.W. Findling, F. Gu, M. Maldonado, U. Schoenherr, D. Mills, L.R. Salgado, B.M. Biller, Pasireotide B2305 Study Group: a 12-month phase 3 study of pasireotide in Cushing’s disease. N. Engl. J. Med. 366, 914–924 (2012)
I. Shimon, L. Rot, E. Inbar, Pituitary-directed medical therapy with pasireotide for a corticotroph macroadenoma: pituitary volume reduction and literature review. Pituitary 15, 608–613 (2012)
G. Arnaldi, T. Mancini, G. Tirabassi, L. Trementino, M. Boscaro, Advances in the epidemiology, pathogenesis, and management of Cushing’s syndrome complications. J. Endocrinol. Invest. 35, 434–448 (2012)
A.M. Pereira, J. Tiemensma, J.A. Romijn, Neuropsychiatric disorders in Cushing’s syndrome. Neuroendocrinology 92(Suppl 1), 65–70 (2010)
L. Trementino, L. Ceccoli, C. Concettoni, G. Marcelli, G. Michetti, M. Boscaro, G. Arnaldi, Fracture risk assessment before and after resolution of endogenous hypercortisolism: is the FRAX® algorithm useful? J. Endocrinol. Invest. 37, 957–965 (2014)
P. Kamenický, C. Droumaguet, S. Salenave, A. Blanchard, C. Jublanc, J.F. Gautier, S. Brailly-Tabard, S. Leboulleux, M. Schlumberger, E. Baudin, P. Chanson, J. Young, Mitotane, metyrapone, and ketoconazole combination therapy as an alternative to rescue adrenalectomy for severe ACTH-dependent Cushing’s syndrome. J. Clin. Endocrinol. Metab. 96, 2796–2804 (2011)
V.A. Preda, J. Sen, N. Karavitaki, A.B. Grossman, Etomidate in the management of hypercortisolaemia in Cushing’s syndrome: a review. Eur. J. Endocrinol. 167, 137–143 (2012)
F. Castinetti, M. Fassnacht, S. Johanssen, M. Terzolo, P. Bouchard, P. Chanson, C. Do Cao, I. Morange, A. Picó, S. Ouzounian, J. Young, S. Hahner, T. Brue, B. Allolio, B. Conte-Devolx, Merits and pitfalls of mifepristone in Cushing’s syndrome. Eur. J. Endocrinol. 160, 1003–1010 (2009)
S. Mondello, V. Fodale, S. Cannavò, C. Aloisi, B. Almoto, M. Buemi, L.B. Santamaria, Hypophosphatemia as unusual cause of ARDS in Cushing’s syndrome secondary to ectopic CRH production. A case report. ScientificWorldJournal. 8, 138–144 (2008)
J.B. Corcuff, J. Young, P. Masquefa-Giraud, P. Chanson, E. Baudin, A. Tabarin, Rapid control of intense neoplastic hypercortisolism with metyrapone and ketoconazole. Eur. J. Endocrinol. 172, 473–481 (2015)
A. Ayala, A.J. Manzano, Detection of recurrent Cushing’s disease: proposal for standardized patient monitoring following transsphenoidal surgery. J. Neurooncol. 119, 235–242 (2014)
R.A. Feelders, C. de Bruin, A.M. Pereira, J.A. Romijn, R.T. Netea-Maier, A.R. Hermus, P.M. Zelissen, R. van Heerebeek, F.H. de Jong, A.J. van der Lely, W.W. de Herder, L.J. Hofland, S.W. Lamberts, Pasireotide alone or with cabergoline and ketoconazole in Cushing’s disease. N. Engl. J. Med. 362, 1846–1848 (2010)
B.M. Biller, A.B. Grossman, P.M. Stewart, S. Melmed, X. Bertagna, J. Bertherat, M. Buchfelder, A. Colao, A.R. Hermus, L.J. Hofland, A. Klibanski, A. Lacroix, J.R. Lindsay, J. Newell-Price, L.K. Nieman, S. Petersenn, N. Sonino, G.K. Stalla, B. Swearingen, M.L. Vance, J.A. Wass, M. Boscaro, Treatment of adrenocorticotropin dependent Cushing’s syndrome: a consensus statement. J. Clin. Endocrinol. Metab. 93, 2454–2462 (2008)
V.A. Preda, J. Sen, N. Karavitaki, A.B. Grossman, Etomidate in the management of hypercortisolaemia in Cushing’s syndrome: a review. Eur. J. Endocrinol. 167, 137–143 (2012)
J.D. Carmichael, M. Fleseriu, Mifepristone: is there a place in the treatment of Cushing’s disease? Endocrine 44, 20–32 (2013)
L. Trementino, M. Zilio, G. Marcelli, G. Michetti, M. Barbot, F. Ceccato, M. Boscaro, C. Scaroni, G. Arnaldi, The role of an acute pasireotide suppression test in predicting response to treatment in patients with Cushing’s disease: findings from a pilot study. Endocrine (2014). doi:10.1007/s12020-014-0499-0
G. Golor, K. Hu, M. Ruffin, A. Buchelt, E. Bouillaud, Y. Wang, M. Maldonado, A first-in-man study to evaluate the safety, tolerability, and pharmacokinetics of pasireotide (SOM230), a multireceptor-targeted somatostatin analog, in healthy volunteers. Drug. Des. Devel. Ther. 6, 71–79 (2012)
R.R. Henry, T.P. Ciaraldi, D. Armstrong, P. Burke, M. Ligueros-Saylan, S. Mudaliar, Hyperglycemia associated with pasireotide: results from a mechanistic study in healthy volunteers. J. Clin. Endocrinol. Metab. 98, 3446–3453 (2013)
A. Colao, C. De Block, M.S. Gaztambide, S. Kumar, J. Seufert, F.F. Casanueva, Managing hyperglycemia in patients with Cushing’s disease treated with pasireotide: medical expert recommendations. Pituitary 17, 180–186 (2014)
C. Simeoli, R.S. Auriemma, F. Tortora, M. De Leo, D. Iacuaniello, A. Cozzolino, M.C. De Martino, C. Pivonello, C.G. Mainolfi, R. Rossi, S. Cirillo, A. Colao, R. Pivonello, The treatment with pasireotide in Cushing’s disease: effects of long-term treatment on tumor mass in the experience of a single center. Endocrine. (2015). doi:10.1007/s12020-015-0557-2
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cannavo, S., Messina, E., Albani, A. et al. Clinical management of critically ill patients with Cushing’s disease due to ACTH-secreting pituitary macroadenomas: effectiveness of presurgical treatment with pasireotide. Endocrine 52, 481–487 (2016). https://doi.org/10.1007/s12020-015-0601-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-015-0601-2