Abstract
Purpose
Remission from Cushing disease (CD) after pituitary adenoma resection may be predicted by a postoperative reduction in serum cortisol level. A 2008 consensus statement recommends assessing morning cortisol levels during the first postoperative week, and replacing glucocorticoid (GC) if cortisol nadir of < 2 or < 5 µg/dL is achieved. We sought to evaluate adherence to consensus recommendations following adrenocorticotropic hormone (ACTH)-secreting pituitary adenoma resection at our tertiary medical center, and assess time to cortisol nadir to better define the window for assessment and intervention.
Methods
We retrospectively analyzed data extracted from in-hospital electronic medical records for CD surgeries between January 1991 and September 2015. We compared cortisol levels and collection times, ACTH measurement, and postoperative and discharge GC treatment before and after consensus statement publication in July 2008.
Results
107 surgeries were performed in 92 patients with CD. After 2008, more surgeries had at least one cortisol value assessed (67.9% before vs. 91.3% after, p = 0.033), with median initial cortisol measurement at 14 h post-surgery. However, ACTH measurement remained unchanged (42.9% vs. 43.5%; p > 0.99). Cortisol collection during GC treatment tended to increase (32.7% vs. 57.1%; p = 0.068). Of surgeries performed without prior GC treatment, 31.7 and 55.0% had a cortisol nadir of < 2 and < 5 µg/dL, respectively, within 72 h postoperative.
Conclusions
Our physicians were more diligent in measuring in-hospital postoperative cortisol levels consistent with 2008 consensus recommendations. Better management of cortisol measurements and their timing is an opportunity for improvement.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cushing disease (CD) is caused by endogenous hypercortisolism driven by an adrenocorticotropic hormone (ACTH)-secreting pituitary adenoma. CD accounts for 10–12% of all pituitary adenomas and 65–70% of patients with Cushing’s syndrome [1]. Surgical excision by an experienced neurosurgeon is the preferred treatment [2, 3], with remission rates of up to 65–98% reported after first surgery [3, 4].
Suppression of normal pituitary ACTH-secreting corticotrophs is caused by prolonged negative feedback inhibition due to high circulating cortisol levels [2]. Transient loss of ACTH secretion and related lack of adrenal glucocorticoid production, which may last for months, is seen upon complete pituitary tumor resection; the presence of this central adrenal insufficiency is suggestive of remission. Postoperative morning cortisol level nadir < 2 µg/dL is strongly associated with remission [1, 3, 5], and nadir < 5 µg/dL is suggestive of remission [2, 3, 6, 7]. The potential presence of adrenal insufficiency in these patients mandates glucocorticoid (GC) treatment until adrenal gland recovery occurs [2, 3, 8]. However, some advocate initiating GC replacement even in the absence of confirmatory biochemical testing [1, 6]. A consensus statement endorsed by the European Neuroendocrine Association and the Pituitary Society published in 2008 recommends measuring morning serum cortisol level during the first postoperative week while withholding GC treatment or using low doses of dexamethasone. When there is evidence of secondary adrenal insufficiency, initiating GC at doses equivalent to hydrocortisone 12–15 mg/m2 is recommended [3]. A more recent guideline from the Endocrine Society on the treatment of Cushing’s syndrome recommends a hydrocortisone dose of 10–12 mg/m2, but does not address timing of serum cortisol assessment [2].
As part of an effort to identify areas for quality improvement in our tertiary Pituitary Center, we evaluated the time and number of serum cortisol measurements after surgery and use of postoperative GC treatment, comparing practice standards before and after publication of the July 2008 consensus, and identifying potentially unnecessary tests. We also calculated median time to cortisol nadir reached during hospitalization to better define the critical time window for postoperative cortisol assessment and GC initiation.
Materials and methods
Subjects and data collection
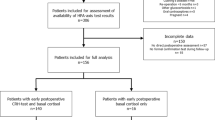
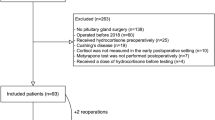
We retrospectively searched our electronic medical records (EMR) system (EPIC and WebVS) for patients with the ICD-9 code 255.0 (http://www.icd9data.com/2013/Volume1/240-279/249-259/255/255.0.htm), which includes the diagnosis of CD, and who also underwent pituitary surgery between January 1, 1991, and September 1, 2015. Of the 1030 patients with ICD-9 code 255.0, 101 patients also underwent pituitary surgery during this time frame; 929 patients did not undergo surgery and were excluded. Of the 101 patients identified, 9 were excluded due to pituitary surgery outside of our center, insufficient data in the EMR, or incorrectly documented diagnosis (e.g., Rathke’s cleft cyst) (Fig. 1). As some patients underwent more than one procedure, 107 pituitary surgeries among 92 patients with CD met inclusion criteria.
Data were collected from the time the patient was hospitalized for resection until discharge. We recorded the date and time of surgery; patient age and sex; whether it was the initial or a follow up surgery; length of hospital stay; operating neurosurgeon; preoperative biochemical assessment for CD including 24-h urinary free cortisol (24UFC), late night salivary cortisol (LNSC), and low and high dose dexamethasone suppression tests (DST); tumor size on pituitary magnetic resonance imaging (MRI); and whether inferior petrosal sinus sampling (IPSS) validated the diagnosis. Initial biochemical assessment, MRI, and IPSS data were used in the few patients with two close subsequent surgeries at our facility if no additional workup was done in the interim. Results of pituitary tissue immunohistochemistry were recorded.
The value, date, and time of all postoperative serum cortisol and ACTH measurements were collected, and results grouped by time after surgery (0–6, 7–12, 13–24, 25–48, and 49–72 h). Morning serum cortisol measurement was defined as any collection between 5 am and 10 am. We also recorded the type of GC used (hydrocortisone, prednisone, or dexamethasone) and specified whether it was administered intraoperatively (during surgery), postoperatively (any time after surgery but during hospitalization), or prescribed at discharge (to be initiated after release from the hospital).
Study endpoints, including time to first postoperative cortisol measurement, number of postoperative serum cortisol and in-hospital ACTH measurements, and use and type of postoperative GC treatment were compared for surgeries performed before and after publication of the consensus statement in July 2008 (identified below as the before and after study groups). Time to cortisol nadir was calculated for all patients not treated with GC during hospitalization.
Statistical analysis
Statistical analysis was done using SAS version 9.2 (SAS Institute, Cary, NC). Analyses were performed on individual surgeries. Categorical variables were compared across groups by the Fisher exact test and numerical variables were compared between groups by the Wilcoxon rank sum test. A 2-sided statistical significance level of < 0.05 was used throughout.
Results
Patient demographics
A total of 92 patients underwent 107 surgeries for CD between January 1991 and September 2015; 84 surgeries comprised the before group and 23 surgeries comprised the after group (Fig. 1; Table 1). Mean age at surgery was 41.9 years (range 16.0–71.0) and was similar in the before and after groups. A higher percentage of females was treated (86.9%). In total, 68.2, 21.5, 9.4, and 0.9% of surgeries were the first, second, third, and fourth surgeries, respectively, and the distribution was similar for the before and after groups. Four patients (3.7%) underwent total hypophysectomy, three of whom were in the before group (data not shown). Hospital length of stay was longer in the after group (4 days) as compared to the before group (3 days; p = 0.002).
A total of 75.7% (81/107) of surgeries [84.5% (71/84) before and 43.5% (10/23) after] had a documented diagnosis of Cushing’s syndrome with two of three positive tests (24UFC, LNSC, DST). The remaining surgeries were performed on patients diagnosed with CD by an endocrinologist outside our center without full EMR documentation of biochemical results confirming the diagnosis. MRI results were available for 93 of 107 surgeries. Among these, 7.5% (7/93) were pituitary macroadenomas (largest diameter ≥ 1 cm). Median adenoma diameter was 4.0 mm for all tumors (range 1.4–37.2). A total of 16.1% (15/93) had no identifiable adenoma on MRI [14.3% (10/70 before) and 21.7% (5/23) after], with equivocal results suggesting the possibility of an adenoma reported in 8.6% (8/93) of these surgeries [10.0% (7/70) before and 4.3% (1/23) after]. Data on whether IPSS was performed were available for 94 of 107 surgeries; 36.2% (34/94) of surgeries had preoperative IPSS, all of which localized an ACTH-secreting adenoma. Four surgeries without documented IPSS also had negative MRI results (three before and one after 2008). Eight neurosurgeons performed 84 surgeries before and two neurosurgeons performed the 23 surgeries after. However, most surgeries were performed by two neurosurgeons: one performed 61.7% (66/107) of all surgeries (78.6% [66/84] before and 0% [0/23] after) and the second performed 26.2% (28/107) of all surgeries (8.3% [7/84] before and 91.3% [21/23] after; data not shown). Positive tumor ACTH immunohistochemistry was observed in 52.8% of resected tumors (56/106), with higher rates seen in the after group (45.2% [38/84] before vs 81.8% [18/22] after) compared with combined normal and negative pathology, p = 0.003. A total of 42.5% (45/106) had normal pituitary tissue on surgical pathology (48.8% [41/84] before vs. 18.2% [4/22] after), and 4.7% had negative tumor ACTH immunohistochemistry (6.0% [5/84] before vs. 0% [0/22] after).
Postoperative cortisol measurement
Routine postoperative measurement of cortisol level was more diligent after publication of the consensus statement (Table 2): postoperative cortisol was measured at least once irrespective of GC exposure in 67.9% (57/84) of surgeries before versus 91.3% (21/23) after (p = 0.033). These measurements were taken within the first 24 h following surgery in 93.0% (53/57) before and 100.0% (21/21) after (p = 0.57). Overall median time to first postoperative cortisol measurement irrespective of prior GC exposure (n = 78) was 14 h [range 2.0–44.0] (data not shown).
Among the 76 surgeries with documented GC exposure during hospitalization (defined as hydrocortisone or prednisone in the prior 24 h or dexamethasone in the prior 48 h) and at least one assessed cortisol, more postoperative cortisol measurements were taken after (57.1% [12/21] vs. before (32.7% [18/55]; p = 0.068), but there was no change in postoperative measurement of ACTH (42.9% [36/84] before vs. 43.5% [(10/23] after; p > 0.99; data not shown).
Among the 60 surgeries with at least one serum cortisol collected without prior GC exposure, 59 (98.3%) were collected within 24-h of surgery, with a similar distribution before (97.7%; 43/44) versus after (100.0%; 16/16; data not shown). As depicted in Fig. 2a, first cortisol measurement was undertaken mostly at 13–24 h after surgery (60.0%; 36/60), followed by 7–12 h after surgery (31.7%; 19/60). Fewer measurements were taken within the first 6 postoperative hours (6.7%; 4/60), and the fewest after 25 h (1.7%; 1/60).
Postoperative cortisol measurement. a Time to first cortisol measurement at each time interval after surgery. Black, percentage of total surgeries without glucocorticoid exposure (n = 60) with first cortisol assessment; light gray, percentage of first cortisol measurements collected at each time interval achieving nadir < 2 µg/dL; dark gray, percentage of first cortisol measurements collected at each time interval achieving nadir < 5 µg/dL. b Time to cortisol nadir at each time interval after surgery. Light gray, cortisol nadir < 2 µg/dL; dark gray, cortisol nadir < 5 µg/dL. c Time of day of cortisol measurement. Black, percentage of total cortisol measurements (n = 136); light gray, before group (n = 82); dark gray, after group (n = 54). *p = 0.048 for both comparisons
Among those that reached one of two nadir cortisol level thresholds suggested in the consensus statement (Fig. 2a), 5.3% (1/19) of those with a first cortisol level measured at 7–12 h postoperative attained a nadir of < 2 µg/dL, and 15.8% (3/19) attained a nadir of < 5 µg/dL, while 16.7% (6/36) and 38.9% (14/36) attained cortisol nadir of < 2 or < 5 µg/dL at 13–24 h postoperative, respectively (p = 0.58 and p = 0.146, respectively).
Time to cortisol nadir in the subgroup of surgeries without GC exposure is depicted in Fig. 2b. Of the 60 surgeries included, 31.7% (19/60) and 55.0% (33/60) reached a nadir of < 2 µg/dL and < 5 µg/dL, respectively, within the first 72 h (data not shown). All cortisol nadirs were achieved more than 7 h post-surgery, and the majority did so by 25–48 h. There was no significant difference in the percentage of surgeries attaining nadir of < 2 µg/dL (29.5% [13/44] before vs. 37.5% [6/16] after; p = 0.55), nor in attaining nadir of < 5 µg/dL (50.0% [22/44] before vs. 68.8% [11/16] after; p = 0.25) (data not shown). Of note, among surgeries in which patients received prior dexamethasone (> 1 mg/dL), serum cortisol suppression below 5 and 2 µg/dL was observed in 46.2% (6/13), and 15.4% (2/13), respectively (data not shown).
Endogenous cortisol levels peak in the early morning in healthy subjects, which is why measuring morning serum cortisol measurements are recommended [3]. Of the 136 cortisol measurements for surgeries in patients not previously treated with GC (Fig. 2c), 61.0% (83/136) were performed between 5 and 10 am; in the before group, 68.3% (56/82) were performed during this interval versus 50% (27/54) in the after group (p = 0.048). The remaining tests were performed at other times (39.0% [53/136] overall; 31.7% [26/82] before vs. 50.0% [27/54] after; p = 0.048).
GC use
Perioperative GC treatment (IV, IM, or PO) is depicted in Table 3. In-hospital postoperative GC was administered to 71.6% (58/81) before versus 82.6% (19/23) after (p = 0.42), and GC prescription at discharge was given to 72.1% (31/43) before versus 78.3% (18/23) after (p = 0.77). Of the 29 surgeries with no in-hospital cortisol assessment, 62.1% (18/29) were discharged with GC, 63.0% (7/27) before versus 50.0% (1/2) after (p > 0.99; data not shown). Intraoperative GC use was rare, and administered to 7.7% (8/104) of patients overall, yet its use increased significantly, from 3.7% (3/81) before to 21.7% (5/23) after (p = 0.007).
We observed a shift from dexamethasone to hydrocortisone use in both the in-hospital postoperative period and at discharge (Fig. 3). In the postoperative period, in the before group, 74.2% of surgeries (43/58) were followed with dexamethasone treatment versus only 15.8% (3/19) of surgeries after. In contrast, 24.1% of surgeries (14/58) were followed with hydrocortisone treatment before, and 84.2% (16/19) were followed with hydrocortisone after. The shift from postoperative dexamethasone to hydrocortisone was highly significant (p < 0.0001). A similar shift was observed in discharge GC prescribed. While dexamethasone prescriptions were provided in 19.4% (6/31) of surgeries at discharge before, only 11.1% (2/18) were prescribed dexamethasone after. In contrast, hydrocortisone prescriptions at discharge increased from 51.6% (16/31) before to 88.9% (16/18) after. This shift from dexamethasone to hydrocortisone prescription at discharge was also significant (p = 0.01). Prednisone was prescribed in 1.7% (1/58) postoperative and in 29.0% (9/31) of surgeries at discharge before, and was not prescribed at all after at either time point. Of the 27% (29/107) of patients who had no postoperative cortisol assessment, 62.1% (18/29) were discharged with GC treatment, 31.0% (9/29) had unknown GC discharge status, and 6.9% (2/29) had no GC prescribed upon discharge (data not shown).
Type of postoperative and discharge glucocorticoid used. Postoperative, glucocorticoid treatment following surgery during hospitalization; at discharge, glucocorticoid treatment prescribed upon hospital discharge. Before group was compared to after group. Black, prednisone; light gray, dexamethasone; dark gray, hydrocortisone. *p < 0.0001; **p = 0.01
Discussion
The 2008 consensus statement on treatment of CD recommends measuring morning serum cortisol in the first week after resection of the adenoma while withholding GC treatment or treating with low-dose dexamethasone. Cortisol level < 2 µg/dL is associated with remission and a recurrence rate of only 10% over 10 years, with a slightly higher recurrence rate of 15–30% seen if cortisol nadir is < 5 µg/dL [3]. A recent review from the American Association of Clinical Endocrinologists focusing on CD recurrence noted that there is no agreement on the exact postoperative cortisol nadir predictive of remission, but that lower cutoffs, i.e., < 2 or 2–5 µg/dL, are associated with lower risk of recurrence [8].
We analyzed our tertiary center’s practice of in-hospital assessment of CD patients for postoperative remission before and after the 2008 consensus statement was published to determine whether it affected perioperative management. Use of our EMR afforded us an advantage, as details of laboratory tests, medications, pathology reports, and imaging studies were available if performed in our hospital.
Our study sample size of 107 surgeries was relatively small due to the rarity of CD, with an incidence rate of approximately 1.2–2.4 per million per year and a prevalence of up to 40 cases per million [9, 10]. Moreover, not all surgeries could be included for each analysis, due to incomplete reporting by the treating physician or partial data uploaded to the EMR. We found that, of the 60 surgeries without IPSS, 4 surgeries also had negative MRI. Three patients before 2008 were directly referred for surgery by outside endocrinologists, and the lack of IPSS either represents variable practices by practitioners outside of a large pituitary center, or IPSS was performed but not documented in our EMR. One patient who had a second surgery after 2008 for cyclical CD had confirmatory IPSS done prior to the first surgery 7 years prior. Importantly, we found that one-fourth of surgeries were performed on patients without definitive documentation of CD. Although this may be due, at least in part, to poor data collection from outside endocrinologists, it also highlights the danger of potentially unnecessary pituitary surgery on patients without complete preoperative evaluation.
As adenomas are not always visualized on MRI [5,6,7,8, 11], pathologic confirmation of an ACTH-secreting pituitary adenoma is not always available. Our observation of nearly 50% of surgical specimens without confirmatory ACTH staining was higher than the approximately one-fourth of surgeries reported by one retrospective study [12]. Our rate of pathology confirmation improved over time, increasing from 50% of specimens before to 80% after, likely because a single specialized and highly experienced neurosurgeon (A.M.) performed nearly all surgeries after 2007 [13]. We also note that no surgical specimens were ACTH-negative adenomas after. Possible explanations for negative histological staining for ACTH, including incomplete submission of resected specimen, partial staining, or failed resection of the adenoma [12], may also be affected by near-exclusive use of a specialized neurosurgeon.
We found that, after publication of the 2008 consensus, cortisol level was measured after more surgeries during hospitalization, consistent with the recommendation, although more measurements were performed per patient and at times other than in the morning. Despite the consensus recommendations, it is unclear whether cortisol collection time should indeed be restricted to the morning in hospitalized patients immediately after resection. Multiple factors may alter cortisol levels in these patients, including the possibility that the endogenous CRH-ACTH-cortisol diurnal rhythm may not have yet recovered, the potential for hospitalization-related altered sleep-wake cycles, surgery or hospitalization stress-related effects, and, perhaps more common in our referral hospital, disruptions in the circadian rhythm due to patients travelling across time zones [11]. Studies targeting cortisol measurement timing postoperatively are needed to address the question whether cortisol levels can reflect likelihood of remission at any time of the day in the immediate post-surgical period.
Our physicians continued their practice of early postoperative assessment of first cortisol within the first 24 h after the 2008 guideline. Whether cortisol should be measured during the first 24 h is unclear [1, 3, 6], but is supported by the short half-life of cortisol [5] and our observation that < 2 and < 5 µg/dL nadirs are attained within 24 h in approximately a tenth and a third of surgeries, respectively. Others have proposed delaying assessment. One study of patients in whom GCs were given only upon clinical signs of adrenal insufficiency found that cortisol levels may not approach 2 µg/dL until 30 h post-surgery, even though a decline could be observed as early as 1 h [1]. Based on this experience, they recommend postoperative cortisol assessment every 6 h for 3 days while withholding GC until there is clinical evidence of adrenal insufficiency or a nadir of < 2 µg/dL is achieved [1, 5]. Another prospective study demonstrated that morning serum cortisol can be safely assessed on postoperative days 1 and 2 while withholding GC replacement until there is biochemical or clinical evidence of hypocortisolemia; using this approach, early remission was seen in 80% of patients [6]. Measuring cortisol within the first week after surgery allows for a longer time for diagnosis of adrenal insufficiency, but may miss it due to lack of follow up after discharge. In our experience, of the nearly one-fourth of patients who did not have a cortisol assessment during hospitalization, approximately two-thirds were discharged on GC therapy without explicit mention of endocrinology follow up within the week after surgery. Presumably, GC was prescribed empirically, but it may have been unnecessary. It led to missed opportunities to assess remission following discharge from the hospital, and could have led to harm if given to patients with persistent endogenous hypercortisolemia.
As cortisol levels can be falsely elevated due to cross-reaction of exogenous GC with the cortisol assay or can be falsely suppressed by treatment with dexamethasone, we considered only patients without prior GC treatment as having properly collected cortisol levels [14,15,16]. Within this group, approximately one-third reached a nadir of < 2 µg/dL during postoperative hospitalization and more than half had a nadir of < 5 µg/dL. Administration of “low-dose” dexamethasone, i.e., below the test dose of 1 mg, while evaluating cortisol for remission can potentially create falsely positive suppressed cortisol levels [14, 15]. None of our patients received low-dose dexamethasone in the hospital; however, nearly 50% of patients previously administered dexamethasone doses > 1 mg/dL either intra- or postoperatively had cortisol levels < 5 µg/dL. This is especially important as providers may prescribe dexamethasone believing that it will not interfere with the cortisol assay or to minimize intracranial edema [17]. Avoiding any GC treatment while evaluating for cortisol nadir and tightly following for signs and symptoms of adrenal insufficiency is recommended [1, 5, 6].
Several factors could have affected our measured total cortisol levels. Cortisol-inhibiting treatments prior to surgery, such as ketoconazole, dopamine agonists, metyrapone, and pasireotide, may have reduced pre-surgery cortisol levels and shortened time to nadir or masked the real nadir levels. Alterations in corticosteroid binding globulin and albumin due to concomitant hormonal treatment affect cortisol levels, and kidney or liver failure affect cortisol metabolism and clearance [1, 5, 11]. We could not analyze potentially confounding medications and comorbidities as many patients were referred from outside facilities and such data were missing from their electronic records.
It is unclear whether our continued measurement of ACTH is truly necessary. Early ACTH levels are considered a poor predictor of sustained remission [1, 3]. Although one study showed its utility by demonstrating that none of 38 patients with plasma ACTH levels < 20 ng/L had disease recurrence after a mean follow-up of 83 months [18], others have found that low early postoperative ACTH levels may predict early cortisol nadirs, but is not always reflective of long-term remission [19]. The true utility of measuring postoperative ACTH for assessment of CD remission remains inconclusive [8].
Without a clear agreement on the most efficient schedule for postoperative cortisol testing, we similarly cannot conclude that our observed rise in cortisol measurements after GC treatment suggests overuse. However, a practice of scheduling recurring blood biochemical measurements even after a nadir level of < 2 µg/dL is reached would likely contribute to wasted resources.
We found no difference in the percentage of surgeries that included postoperative GCs before or after 2008. However, we did find that hydrocortisone replaced dexamethasone and prednisone as the preferred postoperative and discharge GC treatment, which may also be attributed to a more uniform treatment approach of a dedicated neurosurgeon. Hydrocortisone use is preferred as it allows for a close extrapolation to the normal diurnal variation of endogenous cortisol production due to its short half-life and 2–3 times a day weight-based dosing [2]. By contrast, dexamethasone and prednisone are longer acting and more potent, and therefore carry a greater risk for over-replacement and long-term adverse effects such as insulin resistance, bone loss, weight gain, and sleep disturbances, in addition to the prolonged attenuation of hypothalamic–pituitary–adrenal axis recovery [20]. We also observed an unexpected dramatic increase in intraoperative GC use in the after group. GCs are frequently used by neurosurgeons to manage peritumoral brain edema [17], but it is unclear whether this practice is beneficial in preventing adrenal crisis. While not specifically commented on by the 2008 consensus statement, it is presumed to not be supported, as withholding of GC is recommended prior to cortisol assessment.
Conclusions
Following publication of the 2008 consensus statement, and in accordance with its recommendations, providers at our tertiary referral center more often assessed initial early serum cortisol after pituitary tumor resection during hospitalization in patients with CD. Hydrocortisone became the GC of choice both postoperative and at discharge. However, we also found continued postoperative ACTH measurements, which may be unnecessary, and an increase in cortisol measurement during GC exposure, which represents wasteful testing. Our findings have been helpful in the design of a new standardized protocol that will be implemented in our institute to improve quality and efficiency of perioperative in-hospital care of patients undergoing ACTH-secreting pituitary adenoma resection. Our study results support the importance of perioperative management of patients with CD by an integrated team of experienced endocrinologists, pituitary neurosurgeons, and neuroradiologists. Such practice standards can minimize unnecessary surgery, identify patients with possible remission, and avoid inappropriate postoperative GC use.
References
Krikorian A, Abdelmannan D, Selman WR, Arafah BM (2007) Cushing disease: use of perioperative serum cortisol measurements in early determination of success following pituitary surgery. Neurosurg Focus 23(3):E6. doi:10.3171/foc.2007.23.3.8
Nieman LK, Biller BM, Findling JW, Murad MH, Newell-Price J, Savage MO, Tabarin A, Endocrine S (2015) Treatment of Cushing’s syndrome: an endocrine society clinical practice guideline. J Clin Endocrinol Metab 100(8):2807–2831. doi:10.1210/jc.2015-1818
Biller BM, Grossman AB, Stewart PM, Melmed S, Bertagna X, Bertherat J, Buchfelder M, Colao A, Hermus AR, Hofland LJ, Klibanski A, Lacroix A, Lindsay JR, Newell-Price J, Nieman LK, Petersenn S, Sonino N, Stalla GK, Swearingen B, Vance ML, Wass JA, Boscaro M (2008) Treatment of adrenocorticotropin-dependent Cushing’s syndrome: a consensus statement. J Clin Endocrinol Metab 93(7):2454–2462. doi:10.1210/jc.2007-2734
Dallapiazza RF, Oldfield EH, Jane JA Jr (2015) Surgical management of Cushing’s disease. Pituitary 18(2):211–216. doi:10.1007/s11102-015-0646-5
AbdelMannan D, Selman WR, Arafah BM (2010) Peri-operative management of Cushing’s disease. Rev Endocr Metab Disord 11(2):127–134. doi:10.1007/s11154-010-9140-6
Esposito F, Dusick JR, Cohan P, Moftakhar P, McArthur D, Wang C, Swerdloff RS, Kelly DF (2006) Clinical review: early morning cortisol levels as a predictor of remission after transsphenoidal surgery for Cushing’s disease. J Clin Endocrinol Metab 91(1):7–13. doi:10.1210/jc.2005-1204
Shimon I, Ram Z, Cohen ZR, Hadani M (2002) Transsphenoidal surgery for Cushing’s disease: endocrinological follow-up monitoring of 82 patients. Neurosurgery 51(1):57–61
Fleseriu M, Hamrahian AH, Hoffman AR, Kelly DF, Katznelson L, Neuroendocrine A (2016) Pituitary Scientific Committee: American Association of Clinical Endocrinologists and American College of Endocrinology disease state clinical review: diagnosis of recurrence in cushing disease. Endocr Pract 22(12):1436–1448. doi:10.4158/EP161512.DSCR
Etxabe J, Vazquez JA (1994) Morbidity and mortality in Cushing’s disease: an epidemiological approach. Clin Endocrinol 40(4):479–484
Lindholm J, Juul S, Jorgensen JO, Astrup J, Bjerre P, Feldt-Rasmussen U, Hagen C, Jorgensen J, Kosteljanetz M, Kristensen L, Laurberg P, Schmidt K, Weeke J (2001) Incidence and late prognosis of cushing’s syndrome: a population-based study. J Clin Endocrinol Metab 86(1):117–123. doi:10.1210/jcem.86.1.7093
Bansal V, Asmar El, Selman N, Arafah WR (2015) B.M.: Pitfalls in the diagnosis and management of Cushing’s syndrome. Neurosurg Focus 38(2):E4. doi:10.3171/2014.11.FOCUS14704
Pouratian N, Prevedello DM, Jagannathan J, Lopes MB, Vance ML, Laws ER Jr (2007) Outcomes and management of patients with Cushing’s disease without pathological confirmation of tumor resection after transsphenoidal surgery. J Clin Endocrinol Metab 92(9):3383–3388. doi:10.1210/jc.2007-0208
Mamelak AN, Carmichael J, Bonert VH, Cooper O, Melmed S (2013) Single-surgeon fully endoscopic endonasal transsphenoidal surgery: outcomes in three-hundred consecutive cases. Pituitary 16(3):393–401. doi:10.1007/s11102-012-0437-1
Refetoff S, Van Cauter E, Fang VS, Laderman C, Graybeal ML, Landau RL (1985) The effect of dexamethasone on the 24-hour profiles of adrenocorticotropin and cortisol in Cushing’s syndrome. J Clin Endocrinol Metab 60(3):527–535. doi:10.1210/jcem-60-3-527
Rittmaster RS, Loriaux DL, Cutler GB Jr (1985) Sensitivity of cortisol and adrenal androgens to dexamethasone suppression in hirsute women. J Clin Endocrinol Metab 61(3):462–466. doi:10.1210/jcem-61-3-462
Krasowski MD, Drees D, Morris CS, Maakestad J, Blau JL, Ekins S (2014) Cross-reactivity of steroid hormone immunoassays: clinical significance and two-dimensional molecular similarity prediction. BMC Clin Pathol 14:33. doi:10.1186/1472-6890-14-33
Dietrich J, Rao K, Pastorino S, Kesari S (2011) Corticosteroids in brain cancer patients: benefits and pitfalls. Expert Rev Clin Pharmacol 4(2):233–242. doi:10.1586/ecp.11.1
Abdelmannan D, Chaiban J, Selman WR, Arafah BM (2013) Recurrences of ACTH-secreting adenomas after pituitary adenomectomy can be accurately predicted by perioperative measurements of plasma ACTH levels. J Clin Endocrinol Metab 98(4):1458–1465. doi:10.1210/jc.2012-3910
Salmon PM, Loftus PD, Dodd RL, Harsh G, Chu OS, Katznelson L (2014) Utility of adrenocorticotropic hormone in assessing the response to transsphenoidal surgery for Cushing’s disease. Endocr Pract 20(11):1159–1164. doi:10.4158/EP14140.OR
Debono M, Ross RJ, Newell-Price J (2009) Inadequacies of glucocorticoid replacement and improvements by physiological circadian therapy. Eur J Endocrinol 160(5):719–729. doi:10.1530/EJE-08-0874
Acknowledgements
We thank Ms. Shira Berman for her help in editing this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
For this type of study formal consent is not required.
Rights and permissions
About this article
Cite this article
Stolyarov, Y., Mirocha, J., Mamelak, A.N. et al. Consensus-driven in-hospital cortisol assessment after ACTH-secreting pituitary adenoma resection. Pituitary 21, 41–49 (2018). https://doi.org/10.1007/s11102-017-0845-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11102-017-0845-3