Abstract
In vitro the vasoactive intestinal peptide (VIP) stimulates progesterone, androgens, and estradiol secretion, and the effects are time-dependent. The present study analyzed the acute (1 h) and sub-acute (24 h) effects of unilateral injection of VIP into the ovarian bursa on each day of the estrous cycle on progesterone, testosterone, and estradiol serum levels. Cyclic 60-day-old virgin female rats on diestrus-1, diestrus-2, proestrus, or estrus were injected with saline or VIP 10−6 M into the left or right ovarian bursa. One hour after saline injection on each day of estrus cycle, progesterone levels were higher than in control animals. The acute effects of saline solution on testosterone and estradiol levels were asymmetric and varied during the estrous cycle. In comparison with saline groups, the effects of VIPergic stimulation on progesterone, testosterone, and estradiol serum levels depend on the time elapsed between treatment and autopsy and vary during the estrous cycle. An acute asymmetric response from the ovaries to the VIP was observed at diestrus-1, diestrus-2, and proestrus on progesterone and estradiol levels. The asymmetries on testosterone levels were observed at diestrus-1, diestrus-2, and estrus days. The present results suggest that in the cyclic rat, each ovary has different sensitivities to VIPergic stimulation which depends on the endocrine status of the animal.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
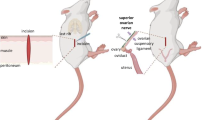
In the ovary, the ovulation and steroid hormones secretion are regulated by hormonal and neural signals [1–6]. The ovary receives sympathetic innervation through two neural pathways: the superior ovarian nerve (SON), which travels along the suspensory ligament and the ovarian plexus nerve (OPN) accompanying the ovarian artery [7]. The SON is the main source of sympathetic innervations [8] and provides to the ovary with fibers containing catecholamines and vasoactive intestinal peptide (VIP) [9–11]. The vagus nerve also provides VIPergic innervation to the ovaries [12].
VIP is a 28-amino acid peptide [13]. The VIP-mRNA has been observed in the ovaries of rats [14], and the presence of VIP immuno-reactivity in the granulosa layer of the pre-ovulatory follicles suggests that these cells may be the site where VIP is synthesized by the ovary, although it cannot be excluded that VIP is internalized by granulosa cells [15]. VIP immune-reactive fibers are associated to blood vessels, the interstitial tissue, and are found around pre-antral and antral follicles [11, 16].
VIP acts on target specific cells by binding to class II G protein-coupled receptors family, VPAC-1 and VPAC-2, which activates the adenylate cyclase pathway and stimulates cAMP production [17]. Using RT-PCR and immunofluorescence analysis, it has been shown that the VPAC-1 receptors are present in the theca/interstitial cells, while VPAC-2 receptors are present in both granulosa and theca/interstitial cells [18].
In the ovary, VIP stimulates the initiation of follicular growth [19, 20], the maturation of oocytes from isolated rat follicles [21], tissue-type plasminogen activator activity [22], and ovulation in PMSG-primed immature rat [23]. VIP also inhibits granulosa cells apoptosis [18, 24] and follicular atresia [24].
In female rabbits, injecting VIP intravenously increases progesterone plasma levels [25]. Adding VIP to cultured ovaries [16] or granulosa cells [26, 27] stimulates progesterone, androgens, and estradiol secretion; effects are dose- and time-dependent [27]. These effects have been explained by VIP’s multiple roles in the regulation of steroidogenic enzymes, including its ability to increase the expression of acute steroidogenic regulatory protein (StAR) [28]; the synthesis of mRNA for cytochrome P450 cholesterol side-chain cleavage enzyme complex (P450scc) and for 17α-hidroxilase (17α-OH) [26]. VIP also enhances the activity of 3β-hydroxysteroid dehydrogenase (3β-HSD) [27] and of aromatases [29].
These evidences indicate that VIP plays an important role in regulating female reproductive functions. The levels of VIP in the rat’s ovary vary along the estrous cycle [30]. In in vitro ovaries, VIP’s stimulating effects on androgen and estradiol secretion are higher in ovaries obtained from rats on proestrus [30].
Previously, using unilateral ovariectomized animals as a study model, we showed the ovaries’ capacity to secrete progesterone, testosterone, and estradiol is asymmetric, and that this asymmetry varies along the estrous cycle [31–35].
To our knowledge, there are no studies analyzing the acute and sub-acute effects of injecting VIP into the ovarian bursa of rats on each day of the estrous cycle. The aim of the present study was to analyze the effects on progesterone, testosterone, and estradiol serum levels resulting from the unilateral VIPergic stimulation of the ovaries along the estrous cycle of the rat. For this purpose, and to mainly stimulate the target ovary, VIP was micro-injected into the ovarian bursa of the left or right ovary of adult rats on diestrus-1, diestrus-2, proestrus, or estrus. Effects were evaluated 1 or 24 h after treatment.
Materials and methods
Experiments were carried out in strict accordance with the Mexican Law of Animal Treatment and Protection Guidelines and the specifications in the Mexican Official Standard NOM-062-ZOO-1999. The Committee of the Facultad de Estudios Superiores Zaragoza approved the experimental protocols. All efforts were made to minimize the number of animals used and their suffering.
The study was performed using 60-day-old virgin female rats of the CIIZ-V strain from our own stock. The animals were kept under controlled lighting conditions 14/10 h light–dark cycle (lights on from 05:00 to 19:00 h), with free access to rat chow and tap water. Estrous cycles were monitored by cytological examination of daily vaginal smears performed at 09:00 h. Only rats showing at least two consecutive 4-day cycles were used in the experiment. All surgeries were performed between 10:30 and 11:30 h at the days of diestrus-1, diestrus-2, proestrus, or estrus. Ten animals were used in each experimental group.
Experimental groups
Rats were randomly assigned to one of the following groups:
Control group. Groups of cyclic-untouched rats were sacrificed between 10:30 and 11:30 h on the day of diestrus-1, diestrus-2, proestrus, or estrus.
VIP or saline solution treatment: The left or right ovary treatment consisted of injecting the saline solution (0.9 % w/v NaCl) or the VIP 10−6 M (Sigma Chem. Co., St. Luis, MO, USA) solution into the respective left or right ovarian bursa. The concentration of VIP was based on studies in rat performed by Ahmed et al. [16], and Davoren and Hsueh [27], where they showed that a dose of 10−6 M of VIP produces the maximum stimulation of progesterone, androgens, and estradiol secretion from granulosa cells [27] and the whole ovary [16].
Injection procedures were performed following previously described methodology [36–39]. In brief, animals on each day of the estrous cycle were anesthetized with ether, and subsequently a left or right dorso-lateral incision was performed two cm below the last rib. The incision affected skin, muscle, and peritoneum, allowing exposure of the left or right ovary. After exposing the left or right ovary, 20 µl of saline or VIP solution was injected into the left or right ovarian bursa with the aid of a 0.5 ml syringe with a 31G × 8 mm gauge needle. To prevent leakage of the saline or VIP solution and allow the injected solution to fully cover the ovary, the needle was kept in the bursa for 1 min after injection treatment. Subsequently, the ovary was carefully cleaned, dried, and returned to the abdominal cavity, and the wound was immediately sealed.
Autopsy procedures
Rats were sacrificed by decapitation either 1 h (acute effects) or 24-h after treatment (sub-acute effects). The blood of the trunk was collected, allowed to clot at room temperature for 30 min, and centrifuged at 3,000 rpm during 15 min. Serum was stored at −20 °C until progesterone, testosterone, and estradiol levels were measured using radioimmunoassay (RIA).
Hormone assay
Serum progesterone, testosterone, and estradiol concentration were determined in duplicate in a single assay using RIA, with solid-phase kits purchased from Diagnostic Products (Los Angeles, CA, USA). Results are expressed in ng/ml (progesterone) and pg/ml (testosterone and estradiol). The intra- and inter-assay coefficients of variation and their standard deviation were 8.35 ± 3.9 and 9.45 ± 0.09 % for progesterone, 9.65 ± 4.5 and 10.2 ± 0.1 % for testosterone, and 8.12 ± 3.8 and 9.28 ± 0.09 % for estradiol, respectively.
Statistical analyses
Data on progesterone, testosterone, and estradiol serum levels were analyzed using multivariate analysis of variance (MANOVA), followed by Tukey’s test. Differences in serum hormone levels between two groups were analyzed using the Student’s t test. A probability value, lower than 0.05, was considered statistically significant.
Results
Acute and sub-acute effects of unilaterally injecting a saline solution into the ovarian bursa on progesterone, testosterone, and estradiol serum levels
Acute effects on progesterone: Compared to the control group on any day of estrus cycle, 1 h after animals were treated with the saline solution into the left or right ovary, progesterone serum levels were higher. On diestrus-2, saline solution treatment in the left ovary yielded the highest progesterone level increase. A similar effect was observed on proestrus, when saline solution treatment was performed in the right ovary (Table 1).
Sub-acute effects on progesterone: Compared to the control group, 24 h after saline solution treatment to either ovary on diestrus-1 resulted in higher progesterone levels. On estrus, 24 h after saline solution treatment of the right ovary also resulted in higher progesterone levels (Table 1).
Acute effects on testosterone: Compared to control animals on proestrus, testosterone levels were higher 1 h after saline solution treatment of the right ovary. Animals on estrus treated with the saline solution on the left ovary showed lower testosterone levels 1 h after treatment (Table 2).
Sub-acute effects on testosterone: 24 h after rats on diestrus-1 were treated with the saline solution on the right ovary; testosterone levels were higher than its corresponding control group. On diestrus-2, the same treatment resulted in lower testosterone levels. In turn, 24 h after rats on proestrus were treated with the saline solution on the left ovary; testosterone levels were lower than its corresponding control group. Rats treated on estrus with saline solution into the left or right ovary showed lower testosterone levels (Table 2).
Acute effects on estradiol: Compared to their respective control group, animals on diestrus-1 treated with saline solution in either ovary or those treated on diestrus-2 or proestrus in the left ovary showed higher estradiol levels 1 h after treatment. On estrus, 1 h after saline solution treatment to the left ovary resulted in lower estradiol levels (Table 3).
Sub-acute effects on estradiol: Compared to control animals, lower estradiol levels were observed; 24-h after rats on diestrus-1 were treated with the saline solution on either ovary. Lower estradiol levels were also observed in rats on diestrus-2 treated with the saline solution on the left ovary (Table 3).
Our results indicate that, compared to control groups, the saline solution treatment resulted in significant differences on progesterone, testosterone, and estradiol serum levels. Consequently, the effects of VIP treatment into the left or right ovarian bursa were compared with their respective saline treatment groups.
Acute and sub-acute effects on progesterone, testosterone, and estradiol serum levels resulting from unilateral VIPergic ovarian stimulation
VIP acute affects on progesterone: Compared to the corresponding saline solution group, animals on diestrus-1 with VIPergic stimulation to the right ovary showed lower progesterone levels while on diestrus-2 resulted in higher progesterone levels 1 h after treatment. In turn, animals on proestrus with VIPergic stimulation to the left ovary, sacrificed 1 h after treatment, showed higher progesterone serum levels (Fig. 1a).
Means ± SEM of progesterone serum level (ng/ml) in rats injected with saline or VIP into the left or right ovarian bursa on diestrus-1, diestrus-2, proestrus, or estrus. Animals were sacrificed 1 h (a) or 24 h (b) after treatment. *p < 0.05 vs. its respective saline solution group; ✧p < 0.05 vs. VIP solution group into the left ovary (Student’s t test)
VIP sub-acute effects on progesterone: 24 h after VIPergic stimulation of the right ovary of rats on diestrus-2 resulted in higher progesterone levels than its respective saline solution group (Fig. 1b).
VIP acute effects on testosterone: Compared to the corresponding saline solution group, VIPergic stimulation of the left ovary of rats on diestrus-1 or diestrus-2 resulted in higher testosterone level 1 h after treatment. VIPergic stimulation to either ovary of rats on estrus day also yielded higher testosterone levels (Fig. 2a).
Means ± SEM of testosterone serum level (pg/ml) in rats injected with saline or VIP into the left or right ovarian bursa on diestrus-1, diestrus-2, proestrus, or estrus. Animals were sacrificed 1 h (a) or 24 h (b) after treatment. *p < 0.05 vs. its respective saline solution group; ✧p < 0.05 vs. VIP solution group into the left ovary (Student’s t test)
VIP sub-acute effects on testosterone: Compared to the corresponding saline solution group, twenty-four hours after VIPergic stimulation to either ovary of rats on diestrus-1 or of left ovary of animals on estrus day yielded lower testosterone levels. VIPergic stimulation of the left ovary of rats on proestrus day resulted in higher testosterone levels (Fig. 2b).
VIP acute effects on estradiol: Compared to the corresponding saline solution group, 1 h after VIPergic stimulation of the right ovary of rats on diestrus-1 decreased estradiol serum levels. VIPergic stimulation to the left ovary of rats on diestrus-2 or proestrus also resulted in lower estradiol serum level (Fig. 3a).
Means ± SEM of estradiol serum level (pg/ml) in rats injected with saline or VIP into the left or right ovarian bursa on diestrus-1, diestrus-2, proestrus, or estrus. Animals were sacrificed 1 h (a) or 24 h (b) after treatment. *p < 0.05 vs. its respective saline solution group; ✧p < 0.05 vs. VIP solution group into the left ovary (Student’s t test)
VIP sub-acute effects on estradiol: Compared to the corresponding saline solution group, twenty-four hours after VIPergic stimulation of left ovary of rats on diestrus-2 or proestrus resulted in higher estradiol serum levels. In rats on diestrus-1 or proestrus, VIPergic stimulation of the right ovary also resulted in higher estradiol serum levels, while in rats on diestrus-2, VIPergic stimulation of the right ovary yielded lower estradiol serum levels (Fig. 3b).
Discussion
The results obtained in the present study show that in the adult rat, over-stimulating the VIPergic system of left or right ovary changes the secretion rates of steroid hormone (progesterone, testosterone and estradiol), and that these changes depend on the stimulated ovary, the day of the estrous cycle studied, and the time elapsed between the ovarian stimulation and autopsy.
Estradiol is the final product in the biosynthesis of steroid hormones by the ovary. In general, progesterone, testosterone, and estradiol levels are considered an activity index of key enzymes that participate in the synthesis of these three hormones: 3β-HSD for progesterone, P450c17α for testosterone, and aromatase for estradiol [3, 4].
In previous studies, we showed that the ovaries have an asymmetric capacity to release steroid hormones, and that this capacity varies along the estrous cycle [31, 33–35]. The ovaries’ asymmetric response has been explained by the neural information received by each ovary [3]. Results obtained in the present study indicate that unilaterally injecting saline solution or VIP into the ovarian bursa modified the secretion rate of steroid hormones in different ways. These results suggest that the neural signals received by the ovary are translated differently by the left and right gonads, and that the information, or its translation, depends on the endocrine status of the animal.
Morphological evidences of a multisynaptic neural pathway between the brain and the adrenals, and between the brain and the ovaries have been reported [40]. Tóth et al. [41] described the existence of neurons in the central nervous system receiving direct neural communication from the adrenals and the ovaries. The unilateral perforation of the peritoneum to pre-pubertal [5, 6] or cyclic rats [31, 33] resulted in higher progesterone serum levels. In the present study, unilaterally injecting the saline solution into the bursa of either ovary on any day of the estrous cycle resulted in higher progesterone levels 1 h after treatment. Since the adrenals seem to be the main source of progesterone secretion [42, 43], it is possible that the observed increase in progesterone levels to be mainly adrenal origin and result of a neural signal between ovaries and adrenals. Another possibility is that the distention of the ovarian bursa by saline solution activated a neural pathway connecting the ovaries and adrenals [41].This assumption must be further elucidated in detail.
VIP enhanced in vitro progesterone release in granulosa cell [27] or the whole ovary from pre-pubertal and puberal rats [16]. Such effects have been associated with increases in StAR mRNA levels and the protein phosphorylation [28]; higher P450scc mRNA and protein levels [26, 44]; and with greater rates of pregnenolone to progesterone conversion via 3β-HSD and lower metabolism of progesterone by 20α-hydroxysteroid dehydrogenase [27]. In vitro, adding VIP to hemi-ovaries from adult rats on diestrus-1 decreased the release of progesterone while increased it when the hemi-ovaries were obtained from rats on diestrus-2 [45]. In the present study, VIPergic stimulation yielded similar results, with an acute asymmetric response of progesterone secretion that varied along the estrus cycle. According to Hu et al. [46] and Domínguez et al. [3], the acute steroidogenic response is characterized by the phosphorylation (and hence the activation) of enzymes, a rapid mobilization of cholesterol esters and cholesterol delivery to the inner mitochondrial membrane, and its subsequent conversion to pregnenolone. Since VIP increases the expression and activity of key enzymes participating in the biosynthesis of progesterone [26–28, 44], it is possible that VIP inhibits or stimulates the activity of these enzymes depending on the neuroendocrine state of the animal.
During the estrous cycle of the rat, the ovaries are the main source of testosterone secretion, with only a moderate contribution by the adrenals [42, 47]. The acute effects on testosterone serum levels resulting from the unilateral perforation of the peritoneum depend on the side where the incision is made and the stage of the estrous cycle when surgery was performed [31, 34]. Similar results were obtained in the present study, where an asymmetric response on testosterone secretion was observed after unilateral saline solution treatment, which depends on the day of the cycle studied. Uchida et al. [48] showed that mechanical stimulation of abdominal skin result in the activation of spinal segmental reflex pathways that increase SON activity. According to Flores et al. [42], neural information arises from the dorsal-lateral peritoneum and from the ventral wall play different roles in the mechanism regulating testosterone secretion. Based on these evidences, it is possible that neural pathways arising from the abdominal skin or peritoneum that regulates enzymatic activity are activated during surgical procedures.
In in vitro rat ovaries, VIP has a stimulatory effect on androgen release [16, 30]. In cultured hen granulosa cells, the stimulating effects of VIP on androstenedione secretion are explained by the increase in 17α-OH mRNA levels [26]. VIP induces a maximum increase in androgen secretion in cultured rat ovaries obtained on early proestrus [30]. In the present study, comparing the effects of VIPergic stimulation with saline solution treatment indicates that VIP has an acute stimulatory effect on testosterone secretion and that the effect was asymmetric (between the left and right ovary) and depended on the day of the cycle studied.
On proestrus VIP’s stimulatory effects on testosterone secretion are evident when comparing testosterone levels from VIPergic stimulated rats to those of intact animals, particularly in the right ovary. Compared to the mechanical/neural stimulation induced by the saline solution treatment on proestrus, the apparent lack of effects on testosterone serum levels resulting from VIPergic treatment suggests that the stimulation induced by the ovarian bursa distention reached the ovaries’ maximum response capacity. This idea is supported by the fact that in rats anesthetized with ether, the increase in progesterone levels is similar to those in rats submitted to laparotomy [33]. Such results suggest that the hypothalamus–pituitary–adrenal axis’ stress response capacity, manifested by increasing progesterone secretion, reaches its peak with the effects of ether anesthesia [33].
During the rat’s estrous cycle, the intra-ovarian nerve growth factor (NGF) and VIP levels vary in an inverse relationship, suggesting that the local production of VIP is regulated by NGF [30]. According to Johnson et al. [26], in in vitro granulosa cells, VIP stimulates 17α-OH mRNA levels, and such effects are blocked with the addition of transforming growth factor α. Since the ovaries have an asymmetric capacity for testosterone release and this capacity varies along the estrous cycle [31, 34], we propose that the absence of acute effects on testosterone levels resulting from VIPergic stimulation to rats on diestrus-1 (right ovary), diestrus-2 (right ovary), or proestrus (both ovaries) could be explained by the action of local neuro-trophic factors that modulate the effects of VIP in the ovary.
VIPergic innervation arrives to the ovaries mainly via the SON [11]. VIP enhances estradiol secretion in ovarian and granulosa cell cultures [16, 27, 30]. On estrus, electrical stimulation to the right SON during 5 min resulted in lower estradiol secretion rates in the ovarian venous blood [2], while bilateral section of the SON on proestrus day decreased estradiol secretion, with no apparent effects observed when SON surgery was performed on estrus day [49]. In the present study, the increase in estradiol serum levels that followed the unilateral injection of saline solution into the ovarian bursa on diestrus-2 or proestrus was inhibited with the VIPergic stimulation. Based on these results we propose that VIP has an asymmetric inhibitory effect on estradiol secretion at the ovarian level. It should be noted that the inhibitory effect of VIP on estradiol levels, observed 1 h after treatment, can also be related to hypothalamic control of ovarian VIP levels, as has been shown by Advis et al. [50] in pre-pubertal rats, the left, right, or bilateral lesions of the preoptic-anterior hypothalamic area resulted in higher VIP levels in the left ovary, suggesting the existence of a marked asymmetry in the hypothalamic control of ovarian VIP.
In the ovary, the follicle-stimulating hormone (FSH) stimulates proliferation of granulosa cells and the aromatization of testosterone to estrogens [51]. In fetal ovaries, VIP increases aromatase activity within 24 h and reached its maximal effect at 48 h [29]. The stimulating effects of VIP on estradiol production are time-dependent and of smaller magnitude than those induced by the FSH [27]. In the present study, unilateral overstimulation of the ovary with VIP on diestrus-1 or diestrus-2 resulted in asymmetric effects on estradiol secretion 24 h after treatment. The highest stimulatory effect of VIP on estradiol secretion was observed on proestrus day, similarly to the results reported by Parra et al. [30]. The ovarian follicle has a subpopulation of granulosa cells that are predominantly sensitive to VIP and another subpopulation that only responds to FSH stimulation [52]. Based on this information, we suggest that the different effects of VIP on each day of estrous cycle on estradiol serum levels observed in the present study are explained by the different sensitivities of the granulosa cell to the VIP in each stage of the estrus cycle.
According to Uchida et al. [48, 53, 54], the mechanical stimulation of the hindpaw or the abdominal wall decreased estradiol secretion rate by the ovary as a consequence of the reflex increase in SON activity. The decrease of the estradiol secretion rate from the ovary did not immediately result in changes in systemic blood [53]. In pre-pubertal rats, unilateral or bilateral laparotomy results in an acute (30 min) increase of progesterone and testosterone serum levels, while estradiol levels were lower. One hour after surgery, the serum levels of the hormones were higher than in untouched control animals [5, 6]. In present study, the sub-acute effects of unilateral saline injection into either ovarian bursa on steroid hormone levels could reflect the changes produced by neural signals arising from abdominal wall [48], ovaries, and the adrenals [40, 41, 55], which were activated during the surgery.
According to Hu et al. [46], long-term steroidogenesis stimulation induces the transcription of genes codifying for steroidogenic enzymes. Such increase in the synthetic capacity of the cells could explain the results observed 24 h after overstimulation of ovarian VIPergic system observed in the present study.
The asymmetries in hormone synthesis between the left and right ovaries observed in the present study could be explained by differences in the innervation received by each ovary. According to Tóth et al. [55], the supra-spinal innervation of the left ovary is more abundant than in the right ovary. Klein and Burden [56] showed a slight asymmetry in the number of neural fibers projecting into the ovary through the SON and OPN from the celiac-superior mesenteric ganglia, with a higher number of inputs into the right ovary than in the left. Additionally, Gerendai et al. [40] and Tóth et al. [41] showed that the supra-spinal innervations of the ovaries and adrenals have left side predominance.
Taken together, present results indicate that in the adult cyclic rat each ovary has a different sensitivity to VIPergic stimulation. It is possible that the ovarian asymmetric response to VIP is modulated, at least in part, by the innervation received by each ovary. The validity of this hypothesis awaits experimental analysis.
References
M.L. Forneris, L.I. Aguado, Neonatal superior ovarian nerve transection disturbs the cyclic activity of the female rats. J. Steroid Biochem. Mol. Biol. 82, 75–82 (2002)
F. Kagitani, S. Uchida, H. Hotta, Effects of electrical stimulation of the superior ovarian nerve and the ovarian plexus nerve on the ovarian estradiol secretion rate in rats. J Physiol Sci. 58, 133–138 (2008)
R. Domínguez, A. Flores, S.E. Cruz-Morales, in Hormonal and Neural Mechanisms Regulating Hormone Steroids Secretion, ed. by A. Hassan. Steroids Basic Science, Chaper 1, 1st edn. (In Tech, Croacia, Rijeka, 2011), pp. 3–34. ISBN 978-953-307-866-3 (2011)
W.L. Miller, R.J. Auchus, The molecular biology, biochemistry, and physiology of human steroidogenesis and its disorders. Endocr. Rev. 32, 81–151 (2011)
L. Morales-Ledesma, D.A. Ramírez, E. Vieyra, A. Trujillo, R. Chavira, M. Cárdenas, R. Domínguez, Effects of acute unilateral ovariectomy to pre-pubertal rats on steroid hormones secretion and compensatory ovarian responses. Reprod. Biol. Endocrinol. 9, 41 (2011)
L. Morales-Ledesma, E. Vieyra, D.A. Ramírez, A. Trujillo, R. Chavira, M. Cárdenas, R. Domínguez, Effects on steroid hormones secretion resulting from the acute stimulation of sectioning the superior ovarian nerve to pre-pubertal rats. Reprod. Biol. Endocrinol. 10, 88 (2012)
I.E. Lawrence Jr, H.W. Burden, The origin of the extrinsic adrenergic innervation to the rat ovary. Anat. Rec. 196, 51–59 (1980)
B. Baljet, J. Drukker, The extrinsic innervation of the abdominal organs in the female rat. Acta Anat. (Basel) 104, 243–267 (1979)
J.M. Bahr, N. Ben-Jonathan, Preovulatory depletion of ovarian catecholamines in the rat. Endocrinology 108, 1815–1820 (1981)
L.I. Aguado, S.R. Ojeda, Prepubertal ovarian function is finely regulated by direct adrenergic influences. Role of noradrenergic innervation. Endocrinology. 114, 1845–1853 (1984)
W.L. Dees, C.E. Ahmed, S.R. Ojeda, Substance P- and vasoactive intestinal peptide-containing fibers reach the ovary by independent routes. Endocrinology 119, 638–641 (1986)
S.I. Said, R.N. Rosenberg, Vasoactive intestinal peptide: abundant immunoreactivity in neural cell lines and normal nervous tissue. Science 192, 907–908 (1976)
S.I. Said, V. Mutt, Polypeptide with broad biological activity: isolation from small Intestine. Science 169, 1217–1218 (1970)
I. Gozes, A. Tsafriri, Detection of vasoactive intestinal peptide-encoding messenger ribonucleic acid in the rat ovaries. Endocrinology 119, 2606–2610 (1986)
S.C.J. Hulshof, G. Dijkstra, E.M. Van Der Beek, M.M. Bevers, J.R. Figueiredo, J.F. Beckers, R. Van Den Hurk, Immunocytochemical localization of vasoactive intestinal peptide and neuropeptide Y in the bovine ovary. Biol. Reprod. 50, 553–560 (1994)
C.E. Ahmed, W.L. Dees, S.R. Ojeda, The immature rat ovary is innervated by vasoactive intestinal peptide (VIP)-containing fibers and responds to VIP with steroid secretion. Endocrinology 118, 1682–1689 (1986)
S. Onoue, S. Misaka, S. Yamada, Structure-activity relationship of vasoactive intestinal peptide (VIP): potent agonists and potential clinical applications. Naunyn-Schmiedeberg’s Arch. Pharmacol. 377, 579–590 (2008)
S. Vaccari, S. Latini, M. Barberi, A. Teti, M. Stefanini, R. Canipari, Characterization and expression of different pituitary adenylate cyclase-activating polypeptide/vasoactive intestinal polypeptide receptors in rat ovarian follicles. J. Endocrinol. 191, 287–299 (2006)
A. Mayerhofer, G.A. Dissen, M.E. Costa, S.R. Ojeda, A role for neurotransmitters in early follicular development: induction of functional follicle-stimulating hormone receptors in newly formed follicles of the rat ovary. Endocrinology 138, 3320–3329 (1997)
N. Chen, Y. Li, W. Wang, Y. Ma, D. Yang, Q. Zhang, Vasoactive intestinal peptide can promote the development of neonatal rat primordial follicles during in vitro culture. Biol. Reprod. 88, 12 (2013)
J. Törnell, B. Carlsson, T. Hillensjö, Vasoactive intestinal peptide stimulates oocyte maturation, steroidogenesis, and cyclic adenosine 3′, 5′-monophosphate production in isolated preovulatory rat follicles. Biol. Reprod. 39, 213–220 (1988)
Y.X. Liu, B.G. Kasson, K.D. Dahl, A.J.W. Hsueh, Vasoactive intestinal peptide stimulates plasminogen activator activity by cultured rat granulosa cells and cumulus-oocyte complexes. Peptides 8, 29–33 (1987)
G. Schmidt, J. Jörgensen, P. Kannisto, F. Liedberg, B. Ottesen, Ch. Owman, Vasoactive intestinal polypeptide in the PMSG-primed immature rat ovary and its effect on ovulation in the isolated rat ovary perfused in vitro. J. Reprod. Fertil. 90, 465–472 (1990)
J.A. Flaws, A. DeSanti, K.I. Tilly, R.O. Javid, K. Kugu, A.L. Johnson, A.N. Hirshfield, J.L. Tilly, Vasoactive intestinal peptide-mediated suppression of apoptosis in the ovary: potential mechanisms of action and evidence of a conserved antiatretogenic role through evolution. Endocrinology 136, 4351–4359 (1995)
C.M. Fredericks, L.E. Lundquist, R.S. Mathur, S.H. Ashton, S.C. Landgrebe, Effects of vasoactive intestinal peptide upon ovarian steroids, ovum transport, and fertility in the rabbit. Biol. Reprod. 28, 1052–1060 (1983)
A.L. Johnson, Z. Li, J.A. Gibney, S. Malamed, Vasoactive intestinal peptide-induced expression of cytochrome P450 cholesterol side-chain cleavage and 17α-hydroxylase enzyme activity in hen granulosa cells. Biol. Reprod. 51, 327–333 (1994)
J.B. Davoren, A.J.W. Hsueh, Vasoactive intestinal peptide: a novel stimulator of steroidogenesis by cultured rat granulosa cells. Biol. Reprod. 33, 37–52 (1985)
M.P. Kowalewski, M.T. Dyson, A. Boos, D.M. Stocco, Vasoactive intestinal peptide (VIP)-mediated expression and function of steroidogenic acute regulatory protein (StAR) in granulosa cells. Mol. Cell. Endocrinol. 328, 93–103 (2010)
F.W. George, S.R. Ojeda, Vasoactive intestinal peptide enhances aromatase activity in the neonatal rat ovary before development of primary follicles or responsiveness to follicle-stimulating hormone. Proc. Natl. Acad. Sci. USA 84, 5803–5807 (1987)
C. Parra, J.L. Fiedler, S.L. Luna, M. Greiner, V. Padmanabhan, H.E. Lara, Participation of vasoactive intestinal polypeptide in ovarian steroids production during the rat estrous cycle and in the development of estradiol valerate-induced polycystic ovary. Reproduction 133, 147–154 (2007)
A.I. Barco, A. Flores, R. Chavira, P. Damián-Matsumara, R. Domínguez, M.E. Cruz, Asymmetric effects of acute hemiovariectomy on steroid hormone secretion by the in situ ovary. Endocrine 21, 209–215 (2003)
M.E. Cruz, A. Flores, M.T. Palafox, G. Meléndez, J.O. Rodríguez, R. Chavira, R. Domínguez, The role of the muscarinic system in regulation estradiol secretion varies during the estrous cycle: the hemiovariectomized rat model. Reprod. Biol. Endocrinol. 4, 43 (2006)
A. Flores, G. Meléndez, M.T. Palafox, J.O. Rodríguez, A.I. Barco, R. Chavira, R. Domínguez, M.E. Cruz, The participation of the cholinergic system in regulating progesterone secretion through the ovarian-adrenal crosstalk varies along the estrous cycle. Endocrine 28, 145–151 (2005)
A. Flores, J.O. Rodríguez, M.T. Palafox, G. Meléndez, A.I. Barco, R. Chavira, M.E. Cruz, R. Domínguez, The acute asymmetric effects of hemiovariectomy on testosterone secretion vary along the estrous cycle. The participation of the cholinergic system. Reprod. Biol. Endocrinol. 4, 11 (2006)
A. Flores, J. Velasco, A.I. Gallegos, F.D. Mendoza, P.M. Everardo, M.E. Cruz, R. Domínguez, Acute effects of unilateral sectioning the superior ovarian nerve of rats with unilateral ovariectomy on ovarian hormones (progesterone, testosterone and estradiol) levels vary during the estrous cycle. Reprod. Biol. Endocrinol. 9, 34 (2011)
R. Domínguez, M.E. Cruz, C. Morán, Differential effects of ovarian local anaesthesia during pro-oestrus on ovulation by the right or left ovary in normal and hemi-ovariectomized adult rats. J. Reprod. Fertil. 113, 185–190 (1998)
C. Morán, A. Franco, J.L. Morán, A. Handal, L. Morales, R. Domínguez, Neural activity between ovaries and the prevertebral celiac-superior mesenteric ganglia varies during the estrous cycle of the rat. Endocrine 26, 147–152 (2005)
C. Morán, F. Zarate, J.L. Morán, A. Handal, R. Domínguez, Lateralization of the connections of the ovary to the celiac ganglia in juvenile rats. Reprod. Biol. Endocrinol. 7, 50 (2009)
M.J. Moran, M.E. Ayala, E. Gallegos, J. Romero, R. Chavira, P. Damián-Matsumura, R. Domínguez, Effects of systemic administration or intrabursal injection of serotonin on puberty, first ovulation and follicular development in rats. Reprod. Fertil. Dev. 25, 1105–1114 (2013)
I. Gerendai, I.E. Tóth, Z. Boldogkoi, B. Halász, Recent findings on the organization of central nervous system structures involved in the innervation of endocrine glands and other organs; observations obtained by the transneuronal viral double-labeling technique. Endocrine 36, 179–188 (2009)
I.E. Tóth, P. Banczerowski, Z. Boldogkoi, J.S. Tóth, A. Szabó, B. Halász, I. Gerendai, Cerebral neurons involved in the innervation of both the adrenal gland and the ovary: a double viral tracing study. Brain Res. Bull. 77, 306–311 (2008)
A. Flores, A.I. Gallegos, J. Velasco, F.D. Mendoza, C. Montiel, P.M. Everardo, M.E. Cruz, R. Domínguez, The acute effects of bilateral ovariectomy or adrenalectomy on progesterone, testosterone and estradiol serum levels depend on the surgical approach and the day of the estrous cycle when they are performed. Reprod. Biol. Endocrinol. 6, 48 (2008)
J. Kalász, E.P. Tóth, B. Bódi, M. Fagyas, A. Tóth, B.H. Pal, S.G. Vári, M. Balog, S. Blažetić, M. Heffer, Z. Papp, A. Borbély, Single acute stress-induced progesterone and ovariectomy alter cardiomyocyte contractile function in female rats. Croat. Med. J. 55, 239–249 (2014)
W.H. Trzeciak, C.E. Ahmed, E.R. Simpson, S.R. Ojeda, Vasoactive intestinal peptide induces the synthesis of the cholesterol side-chain cleavage enzyme complex in cultured rat ovarian granulosa cells. Proc. Natl. Acad. Sci. USA 83, 7490–7494 (1986)
M.H. Garraza, L.I. Aguado, M.A. De Bortoli, In vitro effect of neuropeptides on ovary or celiac ganglion affects the release of progesterone from ovaries in the rat. Med. Sci. Monit. 10, 440–446 (2004)
J. Hu, Z. Zhang, W.J. Shen, S. Azhar, Cellular cholesterol delivery, intracellular processing and utilization for biosynthesis of steroid hormones. Nutr. Metab. 7, 47 (2010)
F. Stahl, F. Götz, G. Dörner, The influence of fetal adrenals on the androgen levels during brain differentiation in human subjects and rats. Exp. Clin. Endocrinol. 98, 131–139 (1991)
S. Uchida, F. Kagitani, H. Hotta, T. Hanada, Y. Aikawa, Cutaneous mechanical stimulation regulates ovarian blood flow via activation of spinal and supraspinal reflex pathways in anesthetized rats. Jpn. J. Physiol. 55, 265–277 (2005)
L.I. Aguado, S.R. Ojeda, Ovarian adrenergic nerves play a role in maintaining preovulatory steroid secretion. Endocrinology 114, 1944–1946 (1984)
J.P. Advis, C.E. Ahmed, S.R. Ojeda, Direct hypothalamic control of vasoactive intestinal peptide (VIP) levels in the developing rat ovary. Brain Res. Bull. 22, 605–610 (1989)
J.K. Findlay, K. Britt, J.B. Kerr, L. O’Donnell, M.E. Jones, A.E. Drummond, E.R. Simpson, The road to ovulation: the role of oestrogens. Reprod. Fertil. Dev. 13, 543–547 (2001)
B.G. Kasson, R. Meidan, J.B. Davoren, A.J. Hsueh, Identification of subpopulations of rat granulosa cells: sedimentation properties and hormonal responsiveness. Endocrinology 117, 1027–1034 (1985)
S. Uchida, F. Kagitani, H. Hotta, Reflex modulation of ovarian estradiol secretion by noxious mechanical stimulation of a hindpaw in anesthetized rats. Auton. Neurosci. 171, 14–20 (2012)
S. Uchida, F. Kagitani, Autonomic nervous regulation of ovarian function by noxious somatic afferent stimulation. J. Physiol. Sci. (2014). doi:10.1007/s12576-014-0324-9
I.E. Tóth, O. Wiesel, Z. Boldogkoi, K. Bálint, Z. Tapaszti, I. Gerendai, Predominance of supraspinal innervation of the left ovary. Microsc. Res. Tech. 70, 710–718 (2007)
C.M. Klein, H.W. Burden, Anatomical localization of afferent and postganglionic sympathetic neurons innervating the rat ovary. Neurosci. Lett. 85, 217–222 (1988)
Acknowledgments
This work was supported by grant UNAM-DGAPA-PAPIIT IN211813. We want to thank for the support given to in the realization of this study to the “Posgrado en Ciencias Biológicas, UNAM” and CONACyT. This work is a requirement for obtaining the degree of Doctor of Biological Sciences. We also thank Biol. R. Chavira having participated in performing the RIA’s to measure the hormones levels. We also want to thank M Sc A. Domínguez-González for the revision of the manuscript in English.
Conflict of interest
The authors declare that there is no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rosas, G., Ramírez, M.I., Linares, R. et al. Asymmetric steroidogenic response by the ovaries to the vasoactive intestinal peptide. Endocrine 48, 968–977 (2015). https://doi.org/10.1007/s12020-014-0449-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-014-0449-x