Abstract
This review summarizes the data obtained with the aid of the recently introduced dual viral tracing technique, which uses isogenic recombinants of pseudorabies virus that express unique reporter gene. This approach made possible to explore simultaneously neural circuits of two organs. The results of these studies indicate: (1) there are neurons innervating exclusively a given organ; (2) left-sided predominance in the supraspinal innervation of the endocrine glands (adrenal, ovary) studied, so far; (3) viral co-infection of neurons, i.e., special neuronal populations coexist in different brain areas that are transsynaptically connected with both paired endocrine and non-endocrine organs, endocrine glands and non-endocrine organs, and organs of bodily systems other than the endocrine one. The number of common neurons seems to be related to the need of coordinating action of different systems. The data on co-infection of neurons suggest that the central nervous system has the capacity to coordinate different organ functions via common brain neurons providing supraspinal innervation of the organs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The nervous system plays a significant role in the control of all bodily systems including the endocrine one. In addition, the nervous system coordinates regulatory processes of different systems which enable the organism to respond properly to external and internal stimuli. The innervation of the endocrine organs and other viscera has been extensively studied. The autonomic and sensory nerves to and from the organs, together with the related pre- and postganglionic neurons have been described in detail. Because of the limitation of the classical anterograde and retrograde tracing methods, i.e., the techniques do not permit to follow a pathway beyond the first synapse, the neural connections of the internal organs with cerebral areas could not be studied.
The introduction of the transneuronal viral tracing technique overcame this problem. This multisynaptic tract-tracing method using pseudorabies virus, in most cases Bartha’s strain [1] of Aujeszky’s disease virus [2], became a commonly used tool for delineation of hierarchically organized neural pathways [3–5]. Injection of pseudorabies virus into a peripheral organ or into a brain area is followed by the uptake of the virus by nerve endings of the infected region. The virus then travels retrogradely along the axons to the cell bodies of the first-order neurons (peripheral ganglion), where reproductive infection takes place, subsequently the virus crosses synapses selectively and replicates in each successive retrogradely infected (second-, third-, fourth-order) neurons. The virus-infected neurons and thus the organization of neural circuitry can be visualized by immunocytochemical localization of the virus. With the aid of this retrogradely labeling technique the supraspinal innervation of different bodily system has been described.
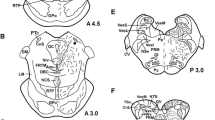
The results of these investigations indicated that several common cerebral cell groups are involved in the innervation of different organs. The virus-infected areas usually included cerebral structures known to project directly (third-order neurons) to the sympathetic preganglionic neurons of the intermediolateral cell column of the spinal cord or in the case of pelvic organs also to the sacral parasympathetic preganglionic neurons (second-order neurons) (Fig. 1). Third-order neurons include the ventrolateral medulla, caudal raphe nuclei, A5 cell group, locus coeruleus, the Barrington’s nucleus, periaqueductal gray, hypothalamic paraventricular nucleus, and the lateral hypothalamic area. Other common sites of supraspinal connections of the internal organs are the nucleus of the solitary tract and dorsal nucleus of the vagus that provide viscerosensory and visceromotor innervation, respectively, to several organs. Following infection of cerebral cell groups monosynaptically connected with the sympathetic and/or parasympathetic preganglionic neurons of the spinal cord, and the vagal nuclei, the infection progresses and fourth-order neurons, such as the medial preoptic area, hypothalamic arcuate nucleus, the suprachiasmatic nucleus, the perifornical region, zona incerta, bed nucleus of the stria terminalis, central amygdala, and in some cases cortical cell groups can be revealed.
Simplified schematic drawing illustrating the first-, second-, third-, and fourth-order of neurons involved in the innervation of the endocrine glands. A5 A5 catecholaminergic cell group; CR caudal raphe nuclei; GGL peripheral ganglion; IML intermediolateral cell column; LH lateral hypothalamus; PVN hypothalamic paraventricular nucleus; VLM ventrolateral medulla
On the basis of the observations that organs belonging to different bodily systems are transneuronally connected with neurons of the same cell group, together with physiological data that indicate the presence and need of coordinated action among different systems, the question raised as to whether there are distinct groups of neurons that are involved in the innervation of not just one organ, but of more organs. The use of the dual viral transsynaptic labeling technique employing recombinant strains of pseudorabies virus Bartha engineered to express different reporter genes [6] has made possible to study this question. Using genetically altered viruses, different strains of pseudorabies virus can be injected into various organs of the same animal and different multisynaptic pathways belonging to different organs or bodily systems can be identified (Fig. 2). In the present review, first we briefly deal with the dual viral tract-tracing technique and the field of application of this method. Then we summarize the findings indicating the presence of common neuronal populations in the innervation of paired endocrine and non-endocrine organs, and in the innervation of the adrenal gland and non-endocrine systems. Finally a few observations on the existence of cerebral neurons connected with two different organs/structures outside the endocrine system are reviewed.
Schematic drawing illustrating in a very simplified form the labeling by the dual viral tracing technique. Two different organs are inoculated with isogenic recombinants of pseudorabies virus that express unique reporter gene (organ 1 injected with virus expressing green fluorescence; organ 2 injected with virus expressing red fluorescence). The viruses are taken up by nerve terminals of the infected area, then they travel along the axons to the postganglionic neurons, where reproductive infection takes place. Subsequently, the viruses cross synapses selectively and replicate in each, successive retrogradely infected neurons (preganglionic neurons, cell groups of the brain stem, and the hypothalamus). “Green” and “red” neurons represent cells transneuronally connected exclusively with organ 1 and organ 2, respectively (organ-specific neurons). “Yellow” cells are special co-infected neurons that are involved in the innervation of both organs (coordinating neurons)
The dual viral transneuronal tracing technique and fields of application
As mentioned in the section Introduction, using two viruses expressing unique reporters, different circuitries belonging to different systems can be defined. There are, however, important considerations in the mapping of two different pathways in the same animal. Kim et al. [7] using two antigenetically distinct recombinants of the pseudorabies virus in single- and double-infection studies, observed that neurons previously infected with one strain (PRV D: expresses the PRV membrane protein gI) of the virus reduced invasiveness of the other strain (PRV-BaBlu: expresses beta-galactosidase). The authors concluded that the viruses to be used should express equivalent infection and transportation dynamics. These criteria can be achieved by generation of viruses that possess the same genetic background, but contain two distinct markers, whereby they can be distinguished. The new generation of tracing viruses, i.e., isogenic strains of viruses fulfills the crucial criteria of ability to co-infect neurons (similar invasiveness, the same rate of transport, similar replication) [8–14]. The most commonly used isogenic pseudorabies recombinants are: PRV-152, Ba-Blu (expressing beta-galactosidase) [9, 13, 15], Ba-PRV (expressing green fluorescence protein [10, 11], and PRV 614 (expressing red fluorescent reporter) [8].
The ability of isogenic recombinant viruses to investigate distinct multisynaptic neuronal circuits made possible: (a) to define connections of single cerebral neurons to multiple bodily systems, (b) to localize distinct cerebral connections of different organs, and (c) to reveal supraspinal connections of different areas of the same organ. The common neural innervation of different organs has been widely studied in the recent years and is dealt with in the present review focusing on the endocrine glands.
Findings indicating the existence of common neuronal populations participating in the innervation of paired endocrine glands/other symmetric structures
Left and right adrenal gland
The control of adrenal functions, catecholamine release, and steroid secretion, is complex and multifactorial, and includes both hormonal and neural regulatory processes. Besides the well-known corticotropin-releasing hormone-ACTH-cortisol/corticosterone system, adrenal cortical functions are also regulated by nerves to and from the gland [16–18]. In addition to the extremely rich sympathetic innervation of the adrenal medulla, sympathetic fibers supply also the cortical cells. Furthermore, parasympathetic fibers from the dorsal nucleus of the vagus project directly or via the celiac or suprarenal ganglion to the gland [19, 20]. Afferent (viscerosensory) fibers from the adrenal cortex towards the central nervous system have also been demonstrated [21]. Using the transneuronal virus labeling technique, following inoculation of neurotropic virus into the adrenal gland, infected neurons could be detected among others in the sympathetic preganglionic neurons of the spinal cord, in the ventromedial and rostral ventrolateral medulla, in the caudal raphe nuclei, in the A5 cell group, in the locus coeruleus, in the hypothalamic paraventricular nucleus, and in the lateral hypothalamus [14, 16, 22–24]. The demonstration of a multisynaptic pathway between the brain and the adrenal gland supports the view that the brain controls several functions of the organ including catecholamine release, compensatory adrenal hypertrophy [25], circadian rhythm of corticosterone secretion [26–28], and regeneration of the organ [29].
Using the double-viral transneuronal tracing technique, the supraspinal innervation of the left and right adrenal gland was investigated in the same animal. Isogenic recombinant viruses expressing different marker genes were injected into the left and right adrenal [30]. The pattern and distribution of virus-infected neurons following inoculation of the adrenal glands was similar to that previously demonstrated using Bartha strain of pseudorabies virus [14, 16, 22–24]. In addition, the labeling of cerebral structures transneuronally connected to the left adrenal gland was more abundant than that from the right organ indicating asymmetry in the supraspinal innervation of the left and right adrenal gland, i.e., the supraspinal innervation of the left adrenal predominates. Furthermore, double-labeled neurons could also be observed. Such nerve cells were detected in the ventrolateral medulla, in the nucleus of the solitary tract, in the raphe nuclei, in the A5 catecholaminergic cell group, and in the hypothalamic paraventricular nucleus.
Left and right ovary
Several physiological studies indicated that the nerves to and from the ovary (superior ovarian nerve, ovarian plexus, vagus nerve) exert regulatory actions on ovarian functions including the timing of puberty, ovulation, development of compensatory hypertrophy, and in the pathogenesis of polycystic ovarian syndrome (see for review [31]). Furthermore, convincing experimental data have been accumulated suggesting that the brain controls ovarian functions also by a pituitary-independent, purely neural mechanism [31]. Using the transneuronal viral tracing technique, we demonstrated the existence of a multisynaptic pathway between the brain and the ovary [32]. To investigate the supraspinal innervation of the left and right ovary in individual rats [33], the left- and right-sided ovary were inoculated with genetically engineered pseudorabies virus expressing a red or a green fluorescent protein gene. The pattern of localization of infected neurons in the brain following injection of recombinant viruses into the ovary was similar to that observed earlier [32]. Infected neurons were present in the ventrolateral medulla, in the gigantocellular nucleus, in the area postrema, in vagal nuclei (nucleus of the solitary tract, dorsal nucleus of the vagus), in the A1 and A5 noradrenergic cell groups, in the caudal raphe nuclei, in the locus coeruleus, in the hypothalamic paraventricular nucleus, and in the lateral hypothalamus. In addition, viral infection of brain nuclei including the dorsal vagal nucleus, caudal raphe nuclei, A5 noradrenergic cell group, hypothalamic paraventricular nucleus was enhanced from the left ovary, when compared to labeling from the right ovary. Double-labeled cells, i.e., neurons transneuronally connected with both ovaries were also observed. Such neurons were detected in the nucleus of the solitary tract, in the dorsal nucleus of the vagus, in the A5 noradrenergic cell group, in the raphe magnus, and in the hypothalamic paraventricular nucleus.
Left and right kidney
Interestingly enough, the results of studies aimed to determine the extent of overlap of cerebral neurons involved in the transneuronal innervation of the two kidneys [13] showed pattern of infection similar to the supraspinal innervation of the adrenal gland or the ovary. Following injection of recombinants of pseudorabies virus into the left and right kidney infected neurons were observed in the classical sympathetic premotor regions (ventrolateral medulla, caudal raphe nuclei, ventromedial medulla, nucleus of the solitary tract, locus coeruleus, Barrington nucleus, periaqueductal gray, perifacial zone, A5 cell group, hypothalamic paraventricular nucleus, lateral hypothalamus). Analysis of double-labeled neurons at the intermediate survival interval indicated the presence of dual-infected neurons in all areas, but the percentage of them differed across infected structures. Highest incidence of double-labeled neurons (more than half of total infected neurons) was found in the rostral ventrolateral medulla, the A5 cell group and in the perifacial zone. In the hypothalamic paraventricular nuclei somewhat lower percentage (36–41%) of dual-infected neurons occurred.
Regulation of blood flow of the hindlimb muscles
The observations of Lee et al. [34] indicate that the regulation of blood flow in the hindlimb muscles is not exerted only by side-specific neurons, but also by special population of cells that provides bilateral innervation and coordinating action of the hindlimb muscles.
Functional considerations
The observations obtained by the use of viral transneuronal tracing technique using isogenic recombinants of pseudorabies virus in individual rats indicate that the supraspinal innervation of the endocrine (adrenal, ovary) and non-endocrine organs (kidney) or non-endocrine structures (vessels to hindlimb muscles) includes two populations of neurons. One population of neurons exhibits single labeling, i.e., these neurons are transneuronally connected with the left or the right organ. The other population includes dually labeled neurons that are co-infected from the circuitry innervating both the left and right organ. Single-labeled neurons are considered organ- and side-specific cells that control the function of the left or the right organ. By contrast, the co-infected neuronal population may coordinate the function of the paired organ. Side-specific and coordinating neurons are present at the same level of the circuitry; however, the ratio of single- and double-labeled neurons varies across regions. Physiological studies are needed to demonstrate the functional significance of the common neurons in the coordination of the left- and right-sided adrenal glands and ovaries.
The other observation of these studies is the asymmetry in the supraspinal innervation of the adrenal gland and the ovary. Both the left adrenal gland and the left ovary receive denser innervation from the brain stem and the hypothalamus than the right organs. Only a few data are available on the asymmetry of the adrenal gland and structures controlling the function of the organ. Gross anatomical asymmetry of the adrenal gland is well known (the left organ weighing more than the right one, the venous drainage of the two adrenals is different). Functional asymmetry of the medial prefrontal cortex that controls neuroendocrine and stress responses have been described [35]. There are clinical observations that indicate that adrenal tumors causing primary aldosteronism are 2 or 3 times more likely to occur on the left than on the right side [36]. Furthermore, several data indicate associations between cerebral dominance, the reactivity of the hypothalamo–pituitary–adrenal axis and the pathogenesis of certain immune-mediated diseases [37].
More data are available on the morphological, biochemical, functional asymmetry of the ovary and structures involved in the control of the organ (see for reviews [38, 39]). Asymmetry of the ovary, such as difference in venous drainage, response to hemicastration, and to transection of the superior ovarian nerve [40–43] has been described. Chavez et al. [44] provided evidence on the predominance of the left vagus nerve in the control of ovulation. Cerebral structures involved in the control of ovarian functions also exhibit asymmetry. Asymmetry in gonadotrope hormone-releasing hormone content in the hypothalamus, asymmetric expression of estrogen receptors in the preoptic and anterior hypothalamic area [45, 46], and functional asymmetry of the anterior hypothalamus [47, 48], and the preoptic area [49, 50] have been reported. The neuromorphological evidence of the asymmetry of the transneuronal innervation of the ovary completed our knowledge on the presence of asymmetry at all levels of the regulatory system of the ovary: asymmetry of the hypothalamus and extrahypothalamic structures, asymmetry of cerebral structures transneuronally connected with the ovary, and asymmetry of peripheral nerves supplying the gland.
Neurons contributing to the innervation of both the adrenal gland and other systems
Most physiological and behavioral responses of the organism are coordinated actions of different bodily systems. The autonomic changes of visceral functions are coordinated actions and are supposed to be controlled by central command neurons. The double-virus transneuronal technique was applied to localize common set of cerebral neurons transneuronally innervating organs that respond simultaneously to appropriate stimuli. Here we summarize the data on the presence of cerebral neurons that transneuronally innervate both the adrenal gland and other viscera and are presumably involved in the coordination of actions of different bodily systems that affect adrenal functions.
Adrenal gland and ovary
Previous studies have indicated that following inoculation of neurotropic virus into the adrenal gland [14, 22–24] or into the ovary [32, 51] infected neurons can be detected in similar central nervous system structures (intermediolateral cell column of the spinal cord, ventrolateral medulla, dorsal nucleus of the vagus, nucleus of the solitary tract, caudal raphe nuclei, A5 cell group, hypothalamic paraventricular nucleus, and the lateral hypothalamus).
Furthermore, it is well documented that the hypothalamo–pituitary–adrenal axis and the hypothalamo–pituitary–ovarian axis are closely related and different mechanisms are involved in the complex neuroendocrine interactions controlling adrenal and ovarian functions. Recently, we investigated whether there are populations of neurons in the brain which are transneuronally connected both with the adrenal gland and the ovary (isogenic viruses expressing two different fluorescence protein were injected into the adrenal and into the ovary) [52]. Double-infected neurons could be observed in the ventrolateral medulla, the nucleus of the solitary tract, the caudal raphe nuclei, the A5 cell group (Fig. 3), and in the hypothalamic paraventricular nucleus, but not in the dorsal nucleus of the vagus. The presence of double-labeled neurons at different levels of the neuronal circuitry innervating both the adrenal gland and the ovary provides the anatomical correlate to the mutual physiological and pathophysiological influence of the two neuroendocrine systems.
Fluorescent micrograph of a part of the rat brain stem (coronal section) showing the autofluorescence of the virus-infected neurons. The left ovary was injected with pseudorabies virus expressing green fluorescent protein (BDG), while the left adrenal was inoculated with a recombinant strain expressing DS-RED. Neurons connected solely with the left ovary (green) or the left adrenal gland (red) are well-distinguished. A few neurons (yellow, arrowheads) are double-labeled indicating that they project to both organs
Adrenal gland and heart
Stress is known to activate the sympathetic nervous system including stimulation of adrenal catecholamine release and cardiovascular function. In order to study the localization of common cerebral neurons transneuronally connected both with the adrenal gland and the heart, two different genetically engineered forms of Bartha strain of pseudorabies virus were used [14]. One virus was injected into the adrenal gland, while the other one into the ipsilateral stellate ganglion—the major sympathetic ganglion innervating the heart [53]. Double-labeled neurons were detected in the ventrolateral medulla, lateral paragigantocellular nucleus, the dorsal medulla (neurons exhibiting adrenaline immunochemistry), the dorsal raphe nuclei (identified for serotonin), the A5 area (noradrenaline positivity), and the paraventricular hypothalamic nucleus (less than 10% of double-labeled cells contained oxytocin immunopositivity), while in the spinal cord small number of infected neurons were observed in the sympathetic preganglionic neurons of the intermediolateral cell column and in the dorsal horn (T5–T7 spinal cord segments). These results indicate that the sympathetic innervation of the adrenal gland and the heart includes brain structures that contain neurons that project along synapses to both organs providing the neuromorphological basis of coordinated response to stressful stimuli.
Adrenal gland and submandibular gland
Another important question concerning the organization of the autonomic nervous system is whether cerebral nuclei connected transneuronally with different endocrine or non-endocrine organs and involved both in the sympathetic and parasympathetic innervation, have also common or just separate population of neurons belonging to the sympathetic and the parasympathetic output. The suprachiasmatic nucleus, site of the biological clock, plays a significant role in the control of daily rhythms of the organism. It has been demonstrated that the suprachiasmatic nucleus is involved in the transneuronal sympathetic innervation of several organs including the thyroid gland, adrenal medulla, adrenal cortex, pancreas, kidney, urinary bladder, brown adipose tissue, while parasympathetic neurons of the nucleus are transneuronally connected with the thyroid gland, pancreas, liver, and submandibular gland (see for review Bartness et al. [54]). To study whether the suprachiasmatic nucleus is connected to multiple autonomic motor outputs, double-virus tracing experiments using two Bartha pseudorabies mutants were performed [55]. When one of the viruses was injected into the adrenal gland and the other one into the ipsilateral stellate ganglion (major source of cardiac innervation), double-labeled neurons could be detected in the suprachiasmatic nucleus. If the submandibular gland, subjected previously to sympathetic or parasympathetic denervation, was injected with one isogenic virus, and the ipsilateral adrenal gland with the other one, common group of suprachiasmatic neurons was observed. These findings suggest that the same set of neurons of the suprachiasmatic nucleus transmit sympathetic input to different organs, and further that single suprachiasmatic neurons might belong to both the sympathetic and parasympathetic system.
Adrenal gland and liver
Buijs et al. [22] studied whether neurons of the suprachiasmatic and hypothalamic paraventricular nucleus, transsynaptically connected both to the adrenal gland and to the liver, belong to the sympathetic or parasympathetic output or to both autonomic systems. Injecting one isogenic virus strain into the adrenal gland and the other one into the sympathetic denervated liver (forcing the virus to infect the brain via the vagus nerve), separate population of neurons exhibited infection in the hypothalamic paraventricular and in the suprachiasmatic nucleus. These data are not in line with the previously mentioned results of Ueyama et al. [55], and indicate specialization of function (sympathetic versus parasympathetic) within the central nervous system.
Adrenal gland and small intestine
Levatte et al. [56] studied in individual hamsters the localization of sympathetic preganglionic neurons connected with the small intestine and the adrenal medulla. Alkalinephosphatase-expressing herpes simplex virus was injected into the adrenal gland, while the muscular wall of the small intestine was inoculated with a β-galactosidase-expressing herpes simplex virus. After virus administration neurons labeled from the intestine were observed bilaterally along the intermediolateral cell column, while those related to the adrenal gland were found along the ipsilateral cell column. In addition, scattered labeled cells were present in the nucleus intermediolateralis, pars funicularis. The percentage of double-labeled cells was low: about 3% in the ipsilateral intermediolateral cell column and about 7% along the ipsilateral nucleus intermediolateralis, pars funicularis. These data suggest that the sympathetic preganglionic neurons involved in the innervation of the adrenal gland and the small intestine are almost completely distinct.
Adrenal gland and gastrocnemius muscle
It is well established that the motor and autonomic activity is coordinated by the nervous system [57, 58], however, the neural circuit that is dedicated to coordinate motor and sympathetic output was unknown before the observations of Kerman et al. [59]. They investigated whether there are overlapping neuron populations that are involved in the coordination of the motor and the autonomic system. Following injection of distinct recombinants of pseudorabies virus into the adrenal gland and the ipsilateral gastrocnemius muscle (subjected previously to sympathectomy), double-labeled neurons were recorded in several brain areas including the rostral ventromedial medullary areas, the A5 cell groups, and the dorsolateral pons (A7 cell group, locus coeruleus, locus subcoeruleus). The greater number of co-infected neurons was localized in the ventromedial medullary nuclei (gigantocellular nucleus pars alpha, ventral gigantocellular nucleus, raphe obscurus, and raphe magnus) indicating that a substantial number of neurons from this region project both to sympathetic preganglionic neurons, as well as to motoneurons. These cells are supposed to act as central command neurons to regulate the concomitant motor and autonomic activity.
Functional considerations
The results mentioned suggest that there are neural pathways involved in the coordinated action of adrenal functions and other bodies systems, such as the reproductive, the cardiovascular, and motor system, and organs of the digestive system.
It is well established that dysfunction of the hypothalamo–pituitary–adrenal axis might result in failure in female reproductive processes [60–62]. Multiple mechanisms are responsible for this inhibitory action: gonadotrope hormone-releasing hormone directly suppresses ACTH release [63], ACTH reduces LH and FSH secretion [64], and corticoids are capable to inhibit gonadotropin release [65, 66]. On the other hand, ovarian hormones have been reported to be involved in the activity of the hypothalamo–pituitary–adrenal axis and can alter stress response [67]. The demonstration of common set of neurons projecting transneuronally both to the adrenal gland and ovary suggests that the interaction between the two neuroendocrine axes includes, besides the mutual hormonal effects, a novel mechanism: integration at the level of specific sets of cerebral neurons, i.e., regulatory action at cellular level.
The neural mechanism that contributes to the integrated response to stressful stimuli includes also neural circuits that are dedicated to coordination of adrenal and cardiac functions, and adrenal and motor functions. Furthermore, in the integration of the cardiovascular and the somatomotor system special group of neurons (in the lateral hypothalamus and in the lateral parafascicular thalamic nucleus) are involved [68]. In addition, the presence of neurons, co-linked to the sympathetic outflow to the heart and the medial prefrontal cortex involved in mental stress and mood disorders [69], might provide the morphological substrate of the underlying mechanism involved in the blood pressure changes during stress and in mood disorders.
Interestingly enough, the innervation of the organs of the digestive system and that of the adrenal gland includes only a modest number of overlapping neurons suggesting that the common neural circuitry might play only a minor role in the integrated control of the two systems.
Neurons involved in the innervation of two structures outside the endocrine system
Experimental data indicate that the integrated control is characteristic not only for endocrine glands but also for structures outside the endocrine system. To illustrate this a few examples are mentioned.
The central coordination of different bodily systems seems to be involved in visceral functions and in visceral pathology. Coexistence of multiple pelvic symptoms (colonic symptoms, irritable bladder, pelvic pain, colonic hypersensitivity) [70–73] suggests that specific neuronal populations could participate in the innervation of pelvic viscera. Following injection of two antigenetically distinct recombinant strains of pseudorabies virus into the bladder and distal colon, three populations of neurons were observed in the Barrington’s nucleus: one transneuronally connected with both the bladder and the colon and the other two sets of neurons specifically innervated the two organs. In addition, single-labeled neurons from either viscera exhibited a viscerotropic organisation [74]. These findings provide morphological evidence for integrated control of the two viscera.
The integration of the somatomotor and cardiovascular system occurs during locomotion [75]. Physiological studies indicate that the stimulation of the lateral hypothalamus or the mesencephalic locomotor region induces both cardiovascular and locomotor responses [76, 77]. Using the double-virus tracing technique, Krout et al. [68] investigated whether the simultaneous coordinated alterations in the locomotor and cardiovascular system are due, at least in part, to neuronal populations that are transsynaptically linked to both systems. For this purpose, one strain of isogenic Bartha pseudorabies virus was injected into the primary motor cortex and the other strain into the stellate ganglion. Many double-labeled cells were observed in the lateral hypothalamus and in the lateral parafascicular thalamic nucleus (connected with both the motor cortex and the striatum). Other regions exhibiting double-labeled neurons were the amygdalohippocampal transition zone and the lateral entorhinal cortex. These results indicate that a special group of neurons in the lateral hypothalamus plays an integrative role in adjustment of cardiovascular functions during locomotion. Furthermore, these findings also suggest that a set of neurons in the lateral parafascicular thalamic nucleus may coordinate cardiovascular changes and locomotor activity.
It is known that during a number of behaviours, such as vomiting and postural adjustment, the diaphragm and the abdominal muscles are simultaneously activated. Studies in which one strain of recombinant isogenic virus was injected into the diaphragm and the other strain into the rectus abdominis muscle, immunofluorescence localization of the unique reporter genes of each virus in the magnocellular part of the medullary reticular formation revealed labeled neurons transneuronally connected with the diaphragm or the rectus abdominis muscle. A third population of neurons exhibited double-labeling. These observations indicate that under certain conditions when the inspiratory and expiratory muscles work together, the coordination of muscle activity is elicited by a particular cell group in the medullary reticular formation, whose axons project, through collateralized projections, to motoneurons innervating both the diaphragm and the abdominal muscles [9].
Special neuronal populations participating in the innervation of pelvic viscera [74], and different muscle groups [9, 78] indicate that these neural circuitries might have functional and/or pathological role (development of multiple pelvic symptoms) in the central control of the pelvic organs, while the discrete common descending pathways to different muscles (muscle groups) seem to participate in the coordination of muscle action (working together or independently).
Taken together the results of these studies indicate a new type of control mechanism: regulation of different organs by common cerebral neurons. However, the data available are sporadic, it seems that the novel type of control mechanism might be a general phenomenon.
Conclusions
The dual viral transneuronal tracing technique has been proved to be a powerful and at present, a unique tool to reveal central neural circuitry involved in the innervation of two different organs. Using this technique novel information became available on the complexity of the cerebral control systems regulating and integrating visceral functions and activity of other bodily systems. The most important observation of these studies is that in different areas of the brain there are special populations of neurons transneuronally connected to more than one organ. We learned that the organs studied so far (adrenal gland, ovary, kidney, heart, etc.) are transneuronally connected with neurons that innervate exclusively the given organ (organ-specific neurons) and with neurons that innervate more than one organ or in the case of paired organs, the organ on the other side (coordinating neurons). Data suggest that the neural input to the endocrine glands and other internal organs is an integrated signal that is formed, at least in part, by cerebral cell groups that are involved in the supraspinal innervation of multiple organs. The ratio of neurons projecting uniquely or simultaneously to a bodily system(s) largely depends on whether the central neuronal circuitries in question are co-activated or not for homeostatic adjustment. The findings obtained by the dual transneuronal tracing technique also demonstrate asymmetry in the intensity of the supraspinal innervation of the adrenal gland and the ovary: the left organs receive more cerebral neural inputs than the contralateral ones.
References
A. Bartha, Magyar Állatorvosok Lapja 16, 42–45 (1961)
A. Aujeszky, Veterinarius 25, 387–396 (1902)
P. Card, in Viral Vectors: Gene Therapy and Neuroscience Applications, ed. by M.G. Kaplitt, A.D. Loewy (Academic Press, New York, 1995), pp. 319–347
J.P. Card, L.W. Enquist, in Methods in Molecular Genetics, ed. by K.W. Adolph (Academic Press, New York, 1994), pp. 363–382
G. Ugolini, in Viral Vectors: Gene Therapy and Neuroscience Application, ed. by M.G. Kaplitt, A.D. Loewy (Academic Press, New York, 1995), pp. 293–317
J.P. Card, L.W. Enquist, Curr. Protocol. Neurosci. unit 1.5, 1–28 (1999)
J.S. Kim, L.W. Enquist, J.P. Card, J. Virol. 73, 9521–9531 (1999)
B.W. Banfield, J.D. Kaufman, J.A. Randall, G.E. Pickard, J. Virol. 77, 10106–10112 (2003)
I. Billig, J.M. Foris, L.W. Enquist, J.P. Card, B.J. Yates, J. Neurosci. 20, 7446–7454 (2000)
Zs. Boldogkői, A. Reichart, I.E. Tóth, A. Sik, F. Erdélyi, I. Medveczky, C. Llorens-Cortes, M. Palkovits, Zs. Lenkei, Mol. Brain Res. 109, 105–118 (2002)
Zs. Boldogkői, A. Sik, Á. Dénes, A. Reichart, J. Toldi, I. Gerendai, K.J. Kovács, M. Palkovits, Prog. Neurobiol. 72, 417–445 (2004)
G. Cano, A.F. Sved, L. Rinaman, B.S. Rabin, J.P. Card, J. Comp. Neurol. 439, 1–18 (2001)
G. Cano, J.P. Card, A.F. Sved, J. Comp. Neurol. 471, 462–481 (2004)
A.S.P. Jansen, X.V. Nguyen, V. Karpitskiy, T.C. Mettenleiter, A.D. Loewy, Science 270, 644–646 (1995)
I. Billig, K. Hartge, J.P. Card, B.J. Yates, Brain Res. 912, 24–32 (2001)
R.M. Buijs, J. Wortel, J.J. Heerikhuize, M.G.P. Feenstra, G.J.T. Horst, H.J. Romijn, A. Kalsbeek, Eur. J. Neurosci. 11, 1535–1544 (1999)
W.K. Kesse, T.L. Parker, R.E. Coupland, J. Anat 157, 33–41 (1988)
N. Kleitman, M.A. Holzwarth, Cell Tissue Res. 241, 139–147 (1985)
H.R. Berthoud, T.L. Powley, J. Auton. Nerv. Syst. 42, 153–170 (1993)
R.E. Coupland, T.L. Parker, W.K. Kesse, A.A. Mohamed, J. Anat. 163, 173–181 (1989)
A.A. Mohamed, T.L. Parker, R.E. Coupland, J. Anat. 160, 51–58 (1988)
R.M. Buijs, S.E. La Fleur, J. Wortel, C. Van Heyningen, L. Zuiddam, T.C. Mettenleiter, A. Kalsbeek, K. Nagai, A. Niijima, J. Comp. Neurol. 464, 36–48 (2003)
A.M. Strack, W.B. Sawyer, K.B. Platt, A.D. Loewy, Brain Res. 491, 274–296 (1989)
I.E. Tóth, Horm. Metab. Res. 30, 329–333 (1998)
M.F. Dallman, W.C. Engeland, J. Shinsako, Am. J. Physiol. 231, 408–414 (1976)
M. Kaneko, T. Hiroshige, J. Shinsako, M.F. Dallman, Am. J. Physiol. 239, 309–316 (1980)
M. Kaneko, K. Kaneko, J. Shinsako, M.F. Dallman, Endocrinology 109, 70–75 (1981)
Y.M. Ulrich-Lai, M.M. Arnhold, W.C. Engeland, Am. J. Physiol. 290, 1128–1135 (2006)
Y.M. Ulrich-Lai, W.C. Engeland, Neuroendocrinology 71, 107–123 (2000)
I.E. Tóth, O. Wiesel, D.E. Tóth, Zs. Boldogkői, B. Halász, I. Gerendai, Microsc. Res. Tech. 71, 503–509 (2008)
I. Gerendai, P. Banczerowski, B. Halász, Endocrine 28, 309–318 (2005)
I. Gerendai, I.E. Tóth, Zs. Boldogkői, I. Medveczky, B. Halász, Neuroendocrinology 68, 244–256 (1998)
I.E. Tóth, O. Wiesel, Zs. Boldogkői, K. Bálint, Zs. Tapaszti, I. Gerendai, Microsc. Res. Tech. 70, 710–718 (2007)
T.K. Lee, J.H. Lois, J.H. Troupe, T.D. Wilson, B.J. Yates, Am. J. Physiol. Regul. Integr. Comp. Physiol. 292, 1532–1541 (2007)
E.M. Sullivan, A. Gratton, J. Neurosci. 19, 2834–2840 (1999)
A.M. Neville, A.M. Mackay, Clin. Endocrinol. Metab. 1, 361–395 (1972)
J.M. Martins, J. Alves, A. Trinca, B. Grima, S. do Vale, T. Vasconcelos, N. Riso, V. Riscado, J.C. da Costa, Brain Behav. Immun. 16, 383–397 (2002)
I. Gerendai, B. Halász, Front. Neuroendocrinol. 18, 354–381 (1997)
I. Gerendai, B. Halász, News Physiol. Sci. 16, 92–95 (2001)
A.I. Barco, A. Flores, R. Chavira, P. Damian-Matzumura, R. Dominguez, M.E. Cruz, Endocrine 21, 209–215 (2003)
R. Chavez, M.E. Cruz, R. Dominguez, J. Endocrinol. 113, 397–401 (1987)
R. Chavez, R. Dominguez, J. Endocrinol. 140, 197–201 (1994)
A. Flores, J.O. Rodriguez, M.T. Palafox, G. Melendez, A.I. Barco, R. Chavira, M.E. Cruz, R. Dominguez, Reprod. Biol. Edocrinol. 1, 4–11 (2006)
R. Chavez, S. Sanchez, A. Ulloa-Aguirre, R. Dominguez, J. Endocrinol. 123, 441–444 (1989)
I. Gerendai, W. Rotsztejn, B. Marchetti, C. Kordon, U. Scapagnini, Neurosci. Lett. 9, 333–336 (1978)
P.R. Arteaga-Lopez, R. Dominguez, M.A. Cerbon, C.A. Mendoza-Rodriguez, M.E. Cruz, Endocrine 21, 251–260 (2003)
M. Fukuda, K. Yamanouchi, Y. Nakano, H. Furuya, Y. Arai, Neurosci. Lett. 51, 365–370 (1984)
M.E. Cruz, L.P. Jamarillo, R. Dominguez, J. Endocrinol. 123, 437–439 (1989)
J.P. Advis, C.E. Ahmed, S.R. Ojeda, Brain Res. Bull. 22, 605–610 (1989)
J.L. Moran, M.E. Cruz, R. Dominguez, Brain Res. Bull. 33, 663–668 (1994)
I. Gerendai, I.E. Tóth, Zs. Boldogkői, I. Medveczky, B. Halász, J. Auton. Nerv. Syst. 80, 40–45 (2000)
I.E. Tóth, P. Banczerowski, Zs. Boldogkői, J.S. Tóth, A. Szabó, B. Halász, I. Gerendai, Brain Res. Bull. 77, 306–311 (2008)
B.J. Pardini, D.D. Lund, P.G. Schmid, J. Auton. Nerv. Syst. 28, 193–201 (1989)
T.J. Bartness, C.K. Song, G.E. Demas, J. Biol. Rhythms 16, 196–204 (2001)
T. Ueyama, K.E. Krout, X.V. Nguyen, V. Karpitskiy, A. Kollert, T.C. Mettenleiter, A.D. Loewy, Nat. Neurosci. 2, 1051–1053 (1999)
M.A. Levatte, P.J. Mabon, L.C. Weaver, G.A. Dekaban, Neuroscience 82, 1253–1267 (1998)
S.C. Gandevia, K. Killian, D.K. McKenzie, M. Crawford, G.M. Allen, R.B. Gorman, J.P. Hales, J. Physiol. 470, 85–107 (1993)
T.G. Waldrop, F.L. Eldridge, G.A. Iwamoto, J.H. Mitchell, in Handbook of Physiology, Section 12, Exercise: Regulation and Integration of Multiple Systems, ed. by L.B. Rowell, J.T. Stepherd (Oxford University Press, New York, 1996)
I.A. Kerman, L.W. Enquist, S.J. Watson, B.J. Yates, J. Neurosci. 23, 4657–4666 (2003)
S. Kalantaridou, A. Makrigiannakis, E. Zoumakis, G. Chrousos, J. Reprod. Immunol. 62, 61–68 (2004)
M.A. Magiakou, G. Mastorakos, E. Webster, G.P. Chrousos, Ann. N. Y. Acad. Sci. 816, 42–56 (1997)
A.I. Turner, P.H. Hemsworth, B.J. Canny, A.J. Tilbrook, Biol. Reprod. 61, 614–620 (1999)
M.D. Chen, K.T. O’Byrne, S.E. Chiappini, J. Hotchkiss, E. Knobil, Neuroendocrinology 56, 666–673 (1992)
C. Baravalle, N.R. Salvetti, G.A. Mira, J.A. Lorente, H.H. Ortega, Physiol. Res. 56, 67–78 (2007)
F. Kamel, C.L. Kubajak, Endocrinology 121, 561–568 (1987)
M. Saketos, N. Sharma, N.F. Santoro, Biol. Reprod. 49, 1270–1276 (1993)
E.A. Young, M. Altemus, Ann. N. Y. Acad. Sci. 1021, 124–133 (2004)
K.E. Krout, T.C. Mettenleiter, A.D. Loewy, Neuroscience 118, 853–866 (2003)
K.E. Krout, T.C. Mettenleiter, V. Karpitskiy, X.V. Nguyen, A.D. Loewy, Brain Res. 1050, 199–202 (2005)
G.F. Longstreth, D.B. Preskill, L. Youkeles, Dig. Dis. Sci. 35, 1285–1290 (1990)
G.F. Longstreth, Obstet. Gynecol. Surv. 49, 505–507 (1994)
P.J. Whorwell, E.W. Lupton, D. Erduran, K. Wilson, Gut 27, 1014–1017 (1986)
P.J. Whorwell, M. McCallum, F.H. Creed, C.T. Roberts, Gut 27, 37–40 (1987)
M.L. Rouzade-Dominguez, R. Miselis, R.J. Valentino, Eur. J. Neurosci. 18, 3311–3324 (2003)
J.M. Kramer, E.D. Plowey, J.A. Beatty, H.R. Little, T.G. Waldrop, Brain Res. Bull. 53, 77–85 (2000)
F.L. Eldridge, D.E. Millhorn, T.G. Waldrop, Science 211, 844–846 (1981)
A.M. Motekaitis, M.P. Kaufman, Respir. Physiol. 106, 263–271 (1996)
J.B. Travers, L. Rinaman, Neuroscience 115, 1139–1151 (2002)
Acknowledgments
This work was supported in part by grants from the National Research Fund (OTKA T046624 to I.G.), the Ministry of Health (ETT 448/2006 to I.G.), and the Hungarian Academy of Sciences. The authors are grateful to Miss Mariann Akócsi for preparation of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gerendai, I., Tóth, I.E., Boldogkői, Z. et al. Recent findings on the organization of central nervous system structures involved in the innervation of endocrine glands and other organs; observations obtained by the transneuronal viral double-labeling technique. Endocr 36, 179–188 (2009). https://doi.org/10.1007/s12020-009-9189-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-009-9189-8