Abstract
Objective Growth hormone/insulin-like growth factor-1(GH/IGF-1) hypersecretion may influence risk factors contributing to the increased cardiovascular morbidity and mortality associated with acromegaly However, so far little is known about the impact of GH/IGF-1 on coagulation and fibrinolysis in acromegalic patients as possible risk factors for cardiovascular disease (CVD). To our knowledge, plasma tissue factor pathway inhibitor (TFPI) and thrombin-activatable fibrinolysis inhibitor (TAFI) levels in these patients have not been investigated. Therefore, the main purpose of this study was to evaluate the markers of endogenous coagulation/fibrinolysis, including TFPI and TAFI, and to investigate the relationships between GH/IGF-1 and these hemostatic parameters and serum lipid profile in patients with acromegaly. Research Methods and Procedures A total of 22 patients with active acromegaly and 22 age-matched healthy controls were included in the study. Fibrinogen, factors V, VII, VIII, IX, and X activities, von-Willebrand factor (vWF), antithrombin III (AT III), protein C, protein S, tissue plasminogen activator (t-PA), tissue plasminogen activator inhibitor-1 (PAI-1), TFPI and TAFI, as well as common lipid variables, were measured. The relationships between serum GH/IGF-1 and these hemostatic parameters were evaluated. Results Compared with the control subjects, fibrinogen, AT III, t-PA, and PAI-1 were increased significantly in patients with acromegaly (P < 0.0001, P < 0.05, P < 0.01, and P < 0.0001, respectively), whereas protein S activity and TFPI levels were decreased significantly (P < 0.05 and P < 0.01, respectively). Plasma TAFI Ag levels did not significantly change in patients with acromegaly compared with the controls. In patients with acromegaly, serum GH levels were inversely correlated with TFPI and apo AI levels (r: −0.514, P: 0.029 and r: −0.602, P: 0.014, respectively). There was also a negative correlation between insulin-like growth factor −1 (IGF-1) and PAI-1 (r: −0.455, P:0.045). Discussion We found some important differences in the hemostatic parameters between the patients with acromegaly and healthy controls. Increased fibrinogen, t-PA, PAI-1 and decreased protein S and TFPI in acromegalic patients may represent a potential hypercoagulable and hypofibrinolytic state, which might augment the risk for atherosclerotic and atherothrombotic complications. Thus, disturbances of the hemostatic system and dyslipidemia may contribute to the excess mortality due to CVD seen in patients with acromegaly.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acromegaly is associated with an increased cardiovascular morbidity and premature mortality, whose pathogenesis is not fully understood [1, 2]. The causes of increased cardiovascular risk associated with acromegaly are systemic arterial hypertension [3, 4], impaired glucose tolerance or overt diabetes [5], cardiomyopathy [6, 7], dyslipidemia [2, 8, 9], and endothelial dysfunction [10]; which contribute to a 2–4 times higher mortality rate than the expected rate [11]. Recenty, changes in coagulation and fibrinolytic systems have been reported in patients acromegaly, which might also have a role in the pathogenesis of cardiovascular disease (CVD) in this disease [12–15].
Growth hormone/insulin-like growth factor-1(GH/IGF-1) hypersecretion may influence risk factors contributing to the increased cardiovascular morbidity and mortality associated with acromegaly [12, 14, 16, 17]. However, so far little is known on the impact of GH/IGF-1 on coagulation and fibrinolysis in acromegalic patients as possible risk factors for CVD [14].
The thrombin-activatable fibrinolysis inhibitor (TAFI), an enzyme that may act as a link between coagulation and fibrinolysis, inhibits fibrinolysis by removing carboxyterminal residues from partially degraded fibrin, thus decreasing plasminogen binding on the surface of fibrin [18, 19]. Increased TAFI levels have been associated with several thrombotic conditions such as venous thromboembolism [20, 21] and ischemic stroke [22]. Tissue factor pathway inhibitor (TFPI) is secreted by the endothelium and stored in platelets [23]. TFPI binds directly and inhibits the earliest activation steps in the extrinsic pathway by binding tissue factor (TF)/factor VIIa and forming an inactive quaternary complex with factor Xa, the first step of the common pathway [24]. Low plasma TFPI levels have been reported in patients with ischemic stroke [25], thrombotic thrombocytopenic purpura [26] and in women taking combined oral contraceptives [27].
Although several studies indicate that coagulation and fibrinolytic system is disturbed in the patients with acromegaly, the levels of plasma TAFI antigen and TFPI have not been investigated in patients with acromegaly. Therefore, in a case-control study, we determined the profile of a wide range of coagulation and fibrinolytic parameters including TAFI and TFPI and lipid profile in patients with active acromegaly. We also investigated the relationships between serum GH/IGF-1 levels and hemostatic parameters in these patients. A hypercoagulable state may increase the risk for thromboembolic complications and predispose to an increased prevalence of vascular disease.
Results
Table 1 summarizes the clinical characteristics and laboratory parameters in patients with acromegaly and control subjects. As expected, the levels of fasting blood glucose (FBG), total cholesterol (TC), low density lipoprotein cholesterol (LDL-C), TG, serum GH/IGF-1 were significantly higher in the patient group than those in controls, whereas serum apo AI levels were significantly decreased.
Compared with the control subjects, fibrinogen, AT III, t-PA, and plasminogen activator inhibitor-1 (PAI-1) were significantly increased in patients with acromegaly (P < 0.0001, P < 0.05, P < 0.01, and P < 0.0001, respectively), whereas protein S activity and TFPI levels were significantly decreased (P < 0.05 and P < 0.01, respectively). Plasma TAFI Ag levels did not significantly change in patients with acromegaly compared with the controls. The other coagulation/fibrinolysis parameters in patients with acromegaly were not different from the controls.
In patients with acromegaly, serum GH levels were inversely correlated with TFPI and apo AI levels (r: −0.514, P: 0.029 and r: −0.602, P: 0.014, respectively) (Figs. 1, 2). There was also a negative correlation between insulin-like growth factor −1 (IGF-1) and PAI-1 (r: −0.455, P:0.045) (Fig. 3). No significant correlation could be found between serum GH/IGF-1 and any other hemostatic parameters measured.
Discussion
The mortality rate of acromegalic patients is 2–4 times higher than the expected rate, primarily attributable to premature CVD [11]. Among 419 patients followed in the West Midlands Pituitary Database, increased mortality was ascribed primarily to elevated GH levels (above 2 ng/ml) and to previous radiotherapy [28]. In some studies, increased IGF-1 levels are associated with higher mortality [29, 30]. However, serum GH levels seem to be more consistently independent predictors of mortality than are serum IGF-1 levels [28].
Fibrinogen is a coagulation factor involved in thrombus formation [15]. Several prospective studies have consistently shown that a direct, independent, and statistically significant association exists between fibrinogen levels and the subsequent incidence of heart disease [31]. High fibrinogen increases the risk of CVD, and stroke in particular [32]. Moreover, among individuals who had increased serum LDL-C and increased fibrinogen levels, there was a 6.1-fold increase in coronary risk [33]. Increased fibrinogen levels have been reported in patients with active acromegaly [12, 15, 17, 34]. In the present study, we found a significant increase in fibrinogen levels in patients with acromegaly. The significantly increased fibrinogen we observed in the present study may reflect a tendency toward atherothrombosis and coagulation in acromegalic patients. Also, the elevated fibrinogen levels in acromegaly may be one explanation fort the increased incidence of vascular events and stroke, and as reported by Bengtsson et al. [35].
AT III is the most important anticoagulant molecule in mammalian systems [36]. It controls the activity of thrombin and inhibits activated FVII (in the presence of heparin), activated FX, activated FIX, and activated FXII [36]. The critical role that AT III plays in controlling coagulation is reflected in the strong correlation between AT III deficiency states and thrombosis [37]. To our knowledge, there is only one study to determine AT III levels in the acromegalic patients [38]. In this sudy, Vilar et al. reported that AT III levels in acromegalic patients were similar to control subjects. In the present study, interestingly, we found a significant increase in AT III levels in patients with acromegaly. The increase in AT III may be a protective mechanism and/or compensatory response versus hypercoagulable state (e.g., enhanced PAI-1 secretion) seen in acromegaly.
It is known that increased PAI-1 levels are associated with advanced atherosclerosis, new cardiovascular events, and increased risk of atherothrombosis [39]. Also, PAI-1 activity is a predictor of recurrent myocardial infarction [40]. Moreover, Padayatty et al. reported that the release of PAI-1 from hepatic cells was stimulated by IGF-1 in vitro [41]. In the present study, we found a significant increase in PAI-1 levels in patients with acromegaly. This result was consistent with the only one study in the literature [14]. In most of the studies, there was no significant differences in PAI-1 levels between patients with acromegaly and control subjects [12, 13, 15]. However, Delaroidis et al. reported that post-treatment plasma PAI-1 levels fell significantly in acromegalic patients. The PAI-1 levels observed in our study are expected to increase the risk for thromboembolic events by lowering fibrinolytic activity in patients with acromegaly.
In concordance with some previous studies, t-PA levels were elevated in active acromegaly [12, 14]. The increase in t-PA may be a compensatory effect of enhanced PAI-1 secretion [14]. Moreover, interestingly, PAI-1 was inversely correlated with IGF-1 in the acromegalic patients (Fig. 3). Wildbrett et al. reported an inverse correlation between PAI-1 and GH [14].
Activated protein C (APC) cleaves and inhibits coagulation cofactors FVIIa and FVa, which result in down-regulation of the activity of the coagulation system. The 2 cofactors, protein S and the intact form of factor V, enhance the anticoagulant activity of APC [42]. Deficiency of protein S increases the risk of thrombosis and is associated with cerebral arterial ischemia [43, 44]. However, protein S deficiency is not a major risk factor for ischemic stroke [44]. Thus, although there is conflicting evidence, deficiency of protein S appears to have a mild association with arterial stroke [44]. In the present study, we found a significant decrease in protein S activity in patients with acromegaly. This result is consistent with only one study in the literature [38]. Decreased protein S activity in the patients may indicate a tendency to thrombosis and coagulation which is crucial in cardiovascular events.
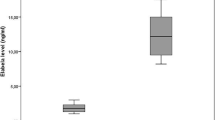
Tissue factor pathway inhibitor regulates FX activation. Low TFPI is a risk factor for a first venous thrombosis, recurrent venous thromboembolism, and stroke [25, 45, 46]. To our knowledge, this is the first study to determine TFPI levels in acromegalic patients. In the present study, we found a significant decrease in TFPI levels in patients with acromegaly. Decreased TFPI levels in patients with acromegaly may show a tendency to thrombosis and coagulation in these patients. Also, we found an inverse correlation between TFPI and serum GH levels (Fig. 1). This result indicates that extrinsic coagulation pathway is influenced by GH.
Thrombin-activatable fibrinolysis inhibitor, also known as procarboxypeptidase B, is a plasma zymogen that potently inhibits fibrinolysis [47, 48]. It protects the fibrin clots from breakdown by removing C-terminal lysine residues from partially degraded fibrin which are necessary for t-PA-mediated plasmin regeneration [48]. Increased activation of TAFI might exacerbate a prothrombotic disposition [49]. Increased plasma TAFI Ag levels were associated with a mild risk for venous thrombosis [20]. One study reported that patients with a recent myocardial infarction presented lower TAFI Ag values and that increased TAFI levels were actually protective against myocardial infarction [50]. On the other hand, high TAFI levels were reported to be associated with an increased risk of first ischemic stroke [51]. To our knowledge, this is the first study to evaluate TAFI Ag levels in patients with acromegaly. In the present study, TAFI Ag levels did not change in our patients with acromegaly.
A high prevalence of lipid profile abnormalities increased TG, LDL-C, Lp (a), apo B, and decreased high density lipoprotein cholesterol (HDL-C) and apo AI, whereas TC levels increased, unchanged, or even decreased have been reported in patients with acromegaly compared with the controls [2, 12, 15, 16, 34]. The lipid abnormalities may be, at least in part, mediated by the insulin resistance induced by GH excess [52]. TGs have been found to increase coagulation factor activity and to decrease fibrinolysis [53]. In the present study, we found increased TC, TG, and LDL-C levels and decreased apo AI levels in the patients with acromegaly. This condition may represent a situation with increased cardiovascular risk and the higher thromboembolic potential. Moreover, in our study, apo AI was inversely correlated with GH (Fig. 2). Our data regarding with apo AI are in agreement with those of Delaroudis et al., who recently found decreased apo AI levels in patients with active acromegaly [12].
In conclusion, we found some important differences in the hemostatic parameters between the patients with active acromegaly and healthy controls. Increased fibrinogen and PAI-1 and decreased TFPI and protein S in these patients represent a potential hypercoagulable and hypofibrinolytic state, which might augment the risk for atherosclerotic and atherothrombotic complications. Thus, disturbances of the hemostatic system and dyslipidemia may contribute to the excess mortality due to CVD seen in patients with acromegaly. However, our study comprised a small number of patients with acromegaly. A larger number of patients should be included in a prospective study to explain the association between GH, IGF-1, and TAFI.
Research methods and procedures
Patients and study design
The study was performed at Karadeniz Technical University Medical Faculty, Department of Internal Medicine. We prospectively evaluated 22 untreated patients with active acromegaly (14 women and 8 men; mean age, 39.6 ± 7.5 years). The diagnosis of acromegaly was established by the typical clinical signs and symptoms (acral enlargement, hypertension, soft tissue overgrowth, hyperhidrosis, headache, weight gain, acanthosis nigricans), by the GH nadir value during the oral glucose tolerance test >1 ng/ml, by the absence of physiologic circadian rhythm and by the presence of elevated gender-and age-related serum IGF-1 levels. In all patients, imaging of pituitary was obtained by magnetic resonance imaging (MRI). MRI confirmed the presence of pituitary adenoma (18 macro-and 4 microadenomas). Endocrine study documented a pure GH-secreting adenoma in 21 of the patients and GH- and PRL-secreting pituitary macroadenoma (mammosomatotropinoma) in 1 patient. All patients were affected by active acromegaly at the time of the study (duration of disease since onset of symptoms 7.7 ± 4.1 years). All patients underwent microsurgical selective resection of the pituitary adenomas by the transsphenoidal approach; immunohistochemistry confirmed the diagnosis in all patients. Adjuvant radiotherapy and/or medical therapy (octreotide-LAR and cabergoline) were given to patients with elevated GH and IGF-1 levels after surgery.
Patients neither received any medical treatments (e.g., estrogen therapy) nor had any known diseases (e.g., thyroid dysfunction, coronary heart disease, collagen disease, liver cirrhosis, atrial fibrillation, or renal disease) that might affect blood coagulation or fibrinolysis, and, lipid profile at the time of the study. At diagnosis, risk factors for coagulation and thromboembolism, including known cancer, pregnancy, known thrombophilia, recent childbirth, and use of oral contraceptives were excluded from patient group. Also, no medication known to influence the serum lipid concentration was administered. A total of 22 healthy age- and-sex-matched subjects (15 women and 7 men, mean age 38.2 ± 8.6 years) were used as controls. Their biochemical values were within normal ranges. None of the controls were taking any drugs that may affect the levels of serum cortisol and hemostatic parameters and lipid levels. All participants including patients and control subjects were non-smokers, and there were neither minor illness (e.g., viral infections), nor family history of clotting disorders in either the patients or the control subjects.
Laboratory analysis
Blood was collected in the morning between 08:00–09:00 h after an overnight fast to avoid the differences of diurnal variation, especially for hormonal and hemostatic parameters. Serum GH and IGF-1 levels were measured by a chemiluminescent immunometric assay (Immulite 2000 DPC, Diagnostic Product Corporation, 5210 Pacific Concourse, Los Angeles, USA). The normal range for serum GH was <5 ng/ml. The normal range for IGF-1 was age-, but not sex-adjusted (21–25 years: 116–358, 26–30 years: 117–329, 31–35 years: 115–307, 36–40 years: 109–284, 41–45 years: 101–267, 46–50 years: 94–252, 51–55 years: 87–238, 56–60 years: 81–225, 61–65 years: 75–212, 66–70 years: 69–200, 71–75 years: 64–188, 76–80 years: 59–177 μg/l).
Serum TC was measured using a cholesterol oxidase enzymatic method; triglycerides (TG), by a glycerol oxidase enzymatic method; HDL-C, by a cholesterol oxidase enzymatic method in supernatant after precipitation with phosphotungstic acid-MgCl2. These routine analyses were carried out by autoanalyzer (Technicon AXON). LDL-C was calculated by the Friedewald’s formula. FBG was measured using an enzymatic (glucose oxidase) colorimetric method. Apolipoproteins AI and B were determined by an immunonephelometric assay method (Dode Behring, Marburg, GmBH Emil-Von-Behring-Str 76 35041 Marburg, Germany). Normal ranges are 125–215 mg/dl for apo AI and 55–125 mg/dl for apo B. All determinations were performed with an autoanalyzer (Roche, Modular, Switzerland). Reagents used were supplied by the same manufacturer.
For coagulation and fibrinolysis, a venous blood sample (9 vol) was collected into Vacutainer tubes (Becton Dickinson, Mountain View, CA) containing 0.129 mol/l trisodium citrate (1 vol). Platelet-poor plasma was obtained by centrifugation 3,500 × g at 10°C for 20 min. Platelet count, mean platelet volume (MPV), fibrinogen, antithrombin III (AT III), factors V, VII, VIII, IX, and X measurements were performed immediately. Aliquots of plasma were transferred into plastic tubes without delay and frozen at −80°C until assays for the determination of von-Willebrand factor (vWF), protein C, protein S, t-PA, and plasminogen activator inhibitor-1 (PAI-1). Platelet count and MPV were measured with automatic cell counter (Coulter Micro Diff II). Fibrinogen was determined using a nephelometric assay by commercial kits for fibrinogen (Cat No.OSCA 09, Dade Behring Marburg GmbH, Germany). D-Dimer measurement was performed using a NycoCard assay by commercial kits for D-Dimer. Factors V, VII, VIII, IX, and X activities were measured with coagulometer (Diagnostica Stago) using commercial kits of Diagnostica Stago. AT III assay was performed with spectrophotometric method (Behring turbitimer, Turbiquant, AT III, Dade Behring). Normal ranges are 200–400 mg/dl for fibrinogen, <0.3 ng/ml for D-Dimer, 50–150% for factors V, FVII, FVIII, FIX, and FX. Protein C and Protein S activity assays were performed with ELISA method using commercial kits of Biopool International. VWF activity was determined by ELISA method using commercial kits of Imtec Immundiagnostica GmBH. T-PA, PAI-1, TAFI Ag, and TFPI assays were performed with ELISA using commercial kits of American Diagnostica. According to our hematology laboratory, normal ranges are 22–39 mg/dl for AT III, 70–150% for vWF, 72–160% for protein C activity, 60–150% for protein S activity, 1–20 ng/ml for t-PA Ag, 20–44 ng/ml for PAI-1 Ag, 40–250% for TAFI, and 75–120 ng/ml for TFPI Ag.
Statistical analysis
Data normality was assessed by the Kolmogorov-Smirnov test. All data except that FBG, MPV, GH, and t-PA were analyzed as normally distributed. Statistical analyses were performed by Student’s t test for normal distribution data and Mann Whitney U test for not normal distribution data. In patient group, correlations among biochemical parameters and GH/IGF-1 and coagulation and lipid profile were carried out using Pearson (normal distribution data) and Spearman (not normal distribution data) correlation analysis. Results are cited as mean ± standard deviation, P < 0.05 was accepted as statistically significant.
References
S.M. Orme, R.J. McNally, R.A. Cartwright, P.E. Belchetz, Mortality and cancer incidence in acromegaly: a retrospective cohort study. United Kingdom Acromegaly Study Group. J. Clin. Endocrinol. Metab. 83, 2730–2734 (1998)
A. Colao, D. Ferone, P. Marzullo, G. Lombardi, Systemic complications of acromegaly: epidemiology, pathogenesis, and management. Endocr. Rev. 25, 102–152 (2004)
G. Vitale, R. Pivonello, R.S. Auriemma, E. Guerra, F. Milone, S. Savastano, G. Lombardi, A. Colao, Hypertension in acromegaly and in the normal population: prevalence and determinants. Clin. Endocrinol. (Oxf) 63, 470–476 (2005)
L. Saccà, A. Cittadini, S. Fazio, Growth hormone and the heart. Endocr. Rev. 15, 555–573 (1994)
M.L. Jaffrain-Rea, C. Moroni, R. Baldelli, C. Battista, P. Maffei, M. Terzolo, M. Correra, M.R. Ghiggi, E. Ferretti, A. Angeli, N. Sicolo, V. Trischitta, A. Liuzzi, R. Cassone, G. Tamburrano, Relationship between blood pressure and glucose tolerance in acromegaly. Clin. Endocrinol. (Oxf) 54, 189–195 (2001)
G. Lombardi, M. Galdiero, R.S. Auriemma, R. Pivonello, A. Colao, Acromegaly and the cardiovascular system. Neuroendocrinology 83, 211–217 (2006)
G. Vitale, R. Pivonello, G. Lombardi, A. Colao, Cardiovascular complications in acromegaly. Minerva Endocrinol. 29, 77–88 (2004)
G.F. Maldonado Castro, H.F. Escobar-Morreale, H. Ortega, D. Gómez-Coronado, J.A. Balsa Barro, C. Varela, M.A. Lasunción, Effects of normalization of GH hypersecretion on lipoprotein(a) and other lipoprotein serum levels in acromegaly. Clin. Endocrinol. (Oxf) 53, 313–319 (2000)
M. Mishra, P. Durrington, M. Mackness, K.W. Siddals, K. Kaushal, R. Davies, M. Gibson, D.W. Ray, The effect of atorvastatin on serum lipoproteins in acromegaly. Clin. Endocrinol. (Oxf) 62, 650–655 (2005)
G. Brevetti, P. Marzullo, A. Silvestro, R. Pivonello, G. Oliva, C. Di Somma, G. Lombardi, A. Colao, Early vascular alterations in acromegaly. J. Clin. Endocrinol. Metab. 87, 3174–3179 (2002)
AACE Acromegaly Guidelines Task Force, AACE Medical Guidelines for Clinical Practice for the diagnosis and treatment of acromegaly. Endocr. Pract. 10, 213–225 (2004)
S.P. Delaroudis, Z.A. Efstathiadou, G.N. Koukoulis, M.D. Kita, D. Farmakiotis, O.G. Dara, D.G. Goulis, A. Makedou, P. Makris, A. Slavakis, A.I. Avramides, Amelioration of cardiovascular risk factors with partial biochemical control of acromegaly. Clin. Endocrinol. (Oxf). 69, 279–284 (2008)
A. Sartorio, M. Cattaneo, P. Bucciarelli, B. Bottasso, S. Porretti, P. Epaminonda, G. Faglia, M. Arosio, Alterations of haemostatic and fibrinolytic markers in adult patients with growth hormone deficiency and with acromegaly. Exp. Clin. Endocrinol. Diabetes 108, 486–492 (2000)
J. Wildbrett, M. Hanefeld, K. Fücker, T. Pinzer, S. Bergmann, G. Siegert, M. Breidert, Anomalies of lipoprotein pattern and fibrinolysis in acromegalic patients: relation to growth hormone levels and insulin-like growth factor I. Exp. Clin. Endocrinol. Diabetes 105, 331–335 (1997)
K. Landin-Wilhelmsen, L. Tengborn, L. Wilhelmsen, B.A. Bengtsson, Elevated fibrinogen levels decrease following treatment of acromegaly. Clin. Endocrinol. (Oxf) 46, 69–74 (1997)
S. Melmed, Medical progress: Acromegaly. N. Engl. J. Med. 355, 2558–2573 (2006)
G. Sesmilo, W.P. Fairfield, L. Katznelson, K. Pulaski, P.U. Freda, V. Bonert, E. Dimaraki, S. Stavrou, M.L. Vance, D. Hayden, A. Klibanski, Cardiovascular risk factors in acromegaly before and after normalization of serum IGF-I levels with the GH antagonist pegvisomant. J. Clin. Endocrinol. Metab. 87, 1692–1699 (2002)
E. Rooth, H. Wallen, A. Antovic, M. von Arbin, G. Kaponides, N. Wahlgren, M. Blombäck, J. Antovic, Thrombin activatable fibrinolysis inhibitor and its relationship to fibrinolysis and inflammation during the acute and convalescent phase of ischemic stroke. Blood Coagul. Fibrinolysis 18, 365–370 (2007)
A. Redlitz, A.K. Tan, D.L. Eaton, E.F. Plow, Plasma carboxypeptidases as regulators of the plasminogen system. J. Clin. Invest. 96, 2534–2538 (1995)
N.H. van Tilburg, F.R. Rosendaal, R.M. Bertina, Thrombin activatable fibrinolysis inhibitor and the risk for deep vein thrombosis. Blood 95, 2855–2859 (2000)
S. Eichinger, V. Schönauer, A. Weltermann, E. Minar, C. Bialonczyk, M. Hirschl, B. Schneider, P. Quehenberger, P.A. Kyrle, Thrombin-activatable fibrinolysis inhibitor and the risk for recurrent venous thromboembolism. Blood 103, 3773–3776 (2004)
J. Montaner, M. Ribó, J. Monasterio, C.A. Molina, J. Alvarez-Sabín, Thrombin-activable fibrinolysis inhibitor levels in the acute phase of ischemic stroke. Stroke 34, 1038–1040 (2003)
T.M. Ravindranath, M. Goto, O. Iqbal, M. Florian-Kujawski, D. Hoppensteadt, R. Hammadeh, M.M. Sayeed, J. Fareed, Plasma thrombin activatable fibrinolysis inhibitor and tissue factor pathway inhibitor changes following sepsis. Clin. Appl. Thromb. Hemost. 13, 362–368 (2007)
G.J. Broze Jr, The role of tissue factor pathway inhibitor in a revised coagulation cascade. Semin. Hematol. 29, 159–169 (1992)
T. Abumiya, T. Yamaguchi, T. Terasaki, T. Kokawa, K. Kario, H. Kato, Decreased plasma tissue factor pathway inhibitor activity in ischemic stroke patients. Thromb. Haemost. 74, 1050–1054 (1995)
M. Kobayashi, H. Wada, Y. Wakita, M. Shimura, T. Nakase, K. Hiyoyama, S. Nagaya, N. Minami, T. Nakano, H. Shiku, Decreased plasma tissue factor pathway inhibitor levels in patients with thrombotic thrombocytopenic purpura. Thromb. Haemost. 73, 10–14 (1995)
G.M. Harris, C.L. Stendt, B.J. Vollenhoven, T.E. Gan, P.G. Tipping, Decreased plasma tissue factor pathway inhibitor in women taking combined oral contraceptives. Am. J. Hematol. 60, 175–180 (1999)
J. Ayuk, R.N. Clayton, G. Holder, M.C. Sheppard, P.M. Stewart, A.S. Bates, Growth hormone and pituitary radiotherapy, but not serum insulin-like growth factor-I concentrations, predict excess mortality in patients with acromegaly. J. Clin. Endocrinol. Metab. 89, 1613–1617 (2004)
N.R. Biermasz, F.W. Dekker, A.M. Pereira, S.W. van Thiel, P.J. Schutte, H. van Dulken, J.A. Romijn, F. Roelfsema, Determinants of survival in treated acromegaly in a single center: predictive value of serial insulin-like growth factor I measurements. J. Clin. Endocrinol. Metab. 89, 2789–2796 (2004)
B. Swearingen, F.G. Barker 2nd, L. Katznelson, B.M. Biller, S. Grinspoon, A. Klibanski, N. Moayeri, P.M. Black, N.T. Zervas, Long-term mortality after transsphenoidal surgery and adjunctive therapy for acromegaly. J. Clin. Endocrinol. Metab. 83, 3419–3426 (1998)
T.W. Meade, S. Mellows, M. Brozovic, G.J. Miller, R.R. Chakrabarti, W.R. North et al., Haemostatic function and ischaemic heart disease: principal results of the Northwick Park Heart Study. Lancet 2, 533–537 (1986)
L. Wilhelmsen, K. Svärdsudd, K. Korsan-Bengtsen, B. Larsson, L. Welin, G. Tibblin, Fibrinogen as a risk factor for stroke and myocardial infarction. N. Engl. J. Med. 311, 501–515 (1984)
D. Feinbloom, K.A. Bauer, Assessment of hemostatic risk factors in predicting arterial thrombotic events. Arterioscler. Thromb. Vasc. Biol. 25, 2043–2053 (2005)
A. Colao, L. Spinelli, A. Cuocolo, S. Spiezia, R. Pivonello, C. di Somma, D. Bonaduce, M. Salvatore, G. Lombardi, Cardiovascular consequences of early-onset growth hormone excess. J. Clin. Endocrinol. Metab. 87, 3097–3104 (2002)
B.A. Bengtsson, S. Edén, I. Ernest, A. Odén, B. Sjögren, Epidemiology and long-term survival in acromegaly. A study of 166 cases diagnosed between 1955 and 1984. Acta. Med. Scand. 223, 327–335 (1988)
N.S. Quinsey, A.L. Greedy, S.P. Bottomley, J.C. Whisstock, R.N. Pike, Antithrombin: in control of coagulation. Int. J. Biochem. Cell Biol. 36, 386–389 (2004)
T.A. Bayston, D.A. Lane, Antithrombin: molecular basis of deficiency. Thromb. Haemost. 78, 339–343 (1997)
L. Vilar, L.A. Naves, S.S. Costa, L.F. Abdalla, C.E. Coelho, L.A. Casulari, Increase of classic and nonclassic cardiovascular risk factors in patients with acromegaly. Endocr. Pract. 13, 363–372 (2007)
M. Cortellaro, E. Cofrancesco, C. Boschetti, L. Mussoni, M.B. Donati, M. Cardillo, M. Catalano, L. Gabrielli, B. Lombardi, G. Specchia et al., Increased fibrin turnover and high PAI–1 activity as predictors of ischemic events in atherosclerotic patients. A case-control study. The PLAT Group. Arterioscler. Thromb. 13, 1412–1417 (1993)
A. Hamsten, U. de Faire, G. Walldius, G. Dahlén, A. Szamosi, C. Landou, M. Blombäck, B. Wiman, Plasminogen activator inhibitor in plasma: risk factor for recurrent myocardial infarction. Lancet 2, 3–9 (1987)
S.J. Padayatty, S. Orme, P.D. Zenobi, M.H. Stickland, P.E. Belchetz, P.J. Grant, The effects of insulin-like growth factor–1 on plasminogen activator inhibitor–1 synthesis and secretion: results from in vitro and in vivo studies. Thromb. Haemost. 70, 1009–1013 (1993)
B. Dahlback, B.O. Villoutreix, The anticoagulant protein C pathway. FEBS Lett. 579, 3310–3316 (2005)
B. Dahlbäck, Blood coagulation. Lancet 355, 1627–1632 (2000)
M.L. Moster, Coagulopathies and arterial stroke. J. Neuroophthalmol. 23, 63–71 (2003)
B.A. Lwaleed, P.S. Bass, Tissue factor pathway inhibitor: structure, biology and involvement in disease. J. Pathol. 208, 327–339 (2006)
M. Hoke, P.A. Kyrle, E. Minar, C. Bialonzcyk, M. Hirschl, B. Schneider, M. Kollars, A. Weltermann, S. Eichinger, Tissue factor pathway inhibitor and the risk of recurrent venous thromboembolism. Thromb. Haemost. 94, 787–790 (2005)
J. Monasterio, P. Bermúdez, D. Quiroga, E. Francisco, B. Meneses, J. Montaner, Plasma thrombin-activatable fibrinolytic inhibitor (TAFI) among healthy subjects and patients with vascular diseases: a validation study. Pathophysiol. Haemost. Thromb. 33, 382–386 (2003)
L. Bajzar, R. Manuel, M.E. Nesheim, Purification and characterization of TAFI, a thrombin-activable fibrinolysis inhibitor. J. Biol. Chem. 270, 14477–14484 (1995)
L. Bajzar, M. Kalafatis, P. Simioni, P.B. Tracy, An antifibrinolytic mechanism describing the prothrombotic effect associated with factor V Leiden. J. Biol. Chem. 271, 22949–22952 (1996)
I. Juhan-Vague, P.E. Morange, H. Aubert, M. Henry, M.F. Aillaud, M.C. Alessi, A. Samnegård, E. Hawe, J. Yudkin, M. Margaglione, G. Di Minno, A. Hamsten, S.E. Humphries, HIFMECH Study Group. Plasma thrombin-activatable fibrinolysis inhibitor antigen concentration and genotype in relation to myocardial infarction in the north and south of Europe. Arterioscler. Thromb. Vasc. Biol. 22, 867–873 (2002)
F.W. Leebeek, M.P. Goor, A.H. Guimaraes, G.J. Brouwers, M.P. Maat, D.W. Dippel, D.C. Rijken, High functional levels of thrombin-activatable fibrinolysis inhibitor are associated with an increased risk of first ischemic stroke. J. Thromb. Haemost. 3, 2211–2218 (2005)
F. Pazos, J.J. Alvarez, J. Rubiés-Prat, C. Varela, M.A. Lasunción, Long-term thyroid replacement therapy and levels of lipoprotein(a) and other lipoproteins. J. Clin. Endocrinol. Metab. 80, 562–566 (1995)
N. Garcia de la Torre, J.A. Wass, H.E. Turner, Parathyroid adenomas and cardiovascular risk. Endocr. Relat. Cancer 10, 309–322 (2003)
Acknowledgment
We are grateful to Yildiray Karayavuz from Hematology Laboratory for help in laboratory analyses.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Erem, C., Nuhoglu, İ., Kocak, M. et al. Blood coagulation and fibrinolysis in patients with acromegaly: increased plasminogen activator inhibitor-1 (PAI-1), decreased tissue factor pathway inhibitor (TFPI), and an inverse correlation between growth hormone and TFPI. Endocr 33, 270–276 (2008). https://doi.org/10.1007/s12020-008-9088-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-008-9088-4