Abstract
Background
Although the bioactive peptides associated with the apelinergic system are known to be associated with heart failure and ischemic heart disease, there are no data on their association with acromegaly.
Aim
We aimed to investigate the change in serum Elabela levels, a novel peptide of the apelinergic system, in patients with acromegaly.
Methods
Our study included 30 treatment naive patients who were recently diagnosed with acromegaly, and 50 age-and-sex-matched healthy controls. In addition to routine history, physical examination and laboratory examinations, serum Elabela level was measured. Participants were divided into two groups as individuals with and without acromegaly and compared to each other.
Results
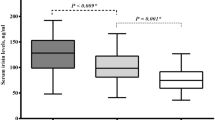
Diastolic blood pressure (DBP) and systolic blood pressure (SBP) were found to be higher in patients with acromegaly. Serum glucose, Hs-CRP, NT-proBNP, insulin-like growth factor-1, growth hormone and serum Elabela levels were higher in patients with acromegaly (p < 0.05 for each). Left ventricular ejection fraction (LV-EF) was found to be lower in patients with acromegaly than the patients in healthy control group (p < 0.05). In multivariate analysis; age, systolic blood pressure, NT-proBNP, Insulin-like growth factor 1 and growth hormone levels were found to be very closely and positively related to serum Elabela level (p < 0.05 for each).
Conclusions
Serum Elabela level can be used as an early and objective indicator of early cardiovascular involvement in patients with acromegaly. Further research is needed to clarify the role of serum Elabela levels on cardiovascular system in acromegaly patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acromegaly is a chronic disease characterized by growth hormone (GH) secreting pituitary adenoma which leads to increased Insulin-like growth factor 1 (IGF-1) synthesis in the liver resulting in excessive protein synthesis and abnormal tissue growth [1]. Chronic high levels of IGF-1 in acromegaly patients cause functional and structural changes specific to the disease [1]. Acromegaly causes left ventricular hypertrophy (LVH), which results in diastolic and systolic dysfunction of the heart [2, 3]. If left untreated, it causes mortality. In patients with acromegaly, the most common causes of mortality are cardiovascular diseases and malignancies [1, 4].
Apelinergic system has been reported to play an important role in the modulation of the cardiovascular system through the peptide called Apelin and the APJ receptor [5, 6]. Although bioactive peptides associated with the apelinergic system are known to be associated with and protective of heart failure and ischemic heart disease, there are no data on acromegaly patients [7, 8]. Recently, a peptide called Elabela, which binds to the same receptor as Apelin and has similar effects, was found [9]. The positive cardiac effect of Elabela is more prominent than Apelin. Studies have reported that there is a relationship between Elabela and many cardiovascular conditions and diseases. [10]. In patients with acromegaly, elabela level may be an important predictor for the development of cardiovascular complications. As far as we have investigated, there is no data on this subject in the literature. Therefore, in our study, it was aimed to investigate the changes in serum Elabela level — a novel apelinergic system peptide — and its relationship with clinical, echocardiographic, and laboratory parameters in patients who were recently diagnosed with acromegaly (Fig. 1).
Methods
This prospective study included 50 patients (31 men, 19 women, and average age 45.1 ± 6.7) who were recently diagnosed with acromegaly and were followed up with that disease, and 50 healthy individuals similar in age and gender as a control group (33 men, 17 women, and average age 44.5 ± 5.3). The duration of the disease before the acromegaly diagnosis was not known. The patients were included in the study before receiving their treatment for acromegaly. Patients who were diagnosed with acromegaly in the primary and secondary health care centers and then referred to our center for treatment were included in the study. The diagnosis and the treatment of acromegaly patients who were included in the study were carried out according to the latest guidelines [1]. Patients with moderately and severely reduced glomerular filtration rate (eGFR) <60 mL/min/1.73 m2 or >30 mg/L proteinuria were excluded. Patients with familial hyperlipidemia, severe and moderate valvular heart disease, known nephrectomy and heart failure history were also excluded. This study was approved by the local ethics committee and conducted according to the recommendations of the ethical principles published in the Declaration of Helsinki developed by the World Medical Association (WMA). All relevant information was explained to the patients in detail, and patients were included in the study after obtaining written consent.
Medical history was taken and detailed physical examinations were performed. Cardiovascular risk factors were noted. Diastolic blood pressure (SBP) and systolic blood pressure (DBP) were measured, and the resting pulse rate was determined. Demographic data including age, sex, hypertension, presence of diabetes mellitus, and history of smoking were recorded. Weight and height of all individuals were measured, and then body mass indexes were calculated.
Laboratory parameters
Routine laboratory parameters [glucose, high-sensitive troponin I, N terminal pro-brain natriuretic peptide (NT-ProBNP), renal functions, lipid parameters, high-sensitive C reactive protein (Hs-CRP) and complete blood count] were analyzed from all individuals included in the study. Serum GH level was detected by an automated chemistry analyzer (Abbott Aeroset, MN, USA) using appropriate commercial kits (Abbott) and reference value of GH level was between 0.014 and 5.219 ng/mL. Serum total IGF-1 level was detected by an automated chemistry analyzer (Abbott Aeroset, MN, USA) using appropriate commercial kits (Abbott) and IGF-1 level references value adjusted according to age and sex. Serum IGF-1 and GH levels were measured at the same time with USG examination for each patient with acromegaly.
Serum Elabela levels were measured using commercial kits (Sunred Biological Technology, Shanghai, China). According to the manufacturer; this assay has inter-assay coefficients of variation less than 12% and intra-assay coefficients of variation of less than 10%. Elabela−32 isoform was measured. The kit used a double-antibody sandwich enzyme-linked immunosorbent assay (ELISA) to analyze the level of Elabela in samples.
Echocardiographic evaluation
Two-dimensional and Doppler echocardiographic evaluations were performed using an echocardiography device (EPIQ 7; Philips Healthcare, Andover, Massachusetts, USA). The American Society of Echocardiography standards were used for all measurements. LV ejection fraction (LVEF) calculation was made by using Biplane Simpson’s method [11] (Fig. 2).
Statistical analysis
SPSS 22.0 (Chicago, IL, USA) statistical software package was used to perform all analyses. Whether the distribution of continuous variables is normal or not was evaluated by the Kolmogorov–Smirnov test. In group data, continuous variables were expressed as mean ± standard deviation. Numbers and percentages were used to specify categorical variables. Categorical variables were compared by the chi-square (χ2) test. Pearson’s and Spearman’s correlation analysis was used to evaluate the existence of relationship between countable parameters. Serum Elabela levels were statistically significant in the univariate analysis so that multivariate linear regression analysis was performed. Statistically significant p value was defined as < 0.05 for all comparisons.
Results
Serum Elabela measurement was successfully obtained from all participants included in the study. The study population was divided into two groups as acromegaly group and healthy control group, and all parameters were compared. When the demographic findings between the acromegaly group and the healthy control group were compared, all parameters except SBP and DBP were found to be similar between the two groups (Table 1). SBP and DBP values were higher in patients with acromegaly. Considering laboratory data, serum glucose, HS-CRP, NT-proBNP, Insulin-like growth factor 1, GH and serum Elabela levels were higher in patients with acromegaly. All other laboratory parameters were similar across groups (Table 1) . It was found that LVEF was lower in patients with acromegaly than in the healthy control group (Table 1). Correlation analysis was performed between serum Elabela and other demographic, clinical, laboratory and echocardiographic parameters (Table 2). Linear regression analysis was performed for the parameters that were significantly associated with serum Elabela in the univariate analysis. As a result of this analysis; SBP, NT-proBNP, IGF-1, and GH values were found to be closely and positively related to serum Elabela level, whereas age and LVEF values were found to be closely and negatively related (Table 2).
Discussion
The most important finding of our study is that serum Elabela level was found to be significantly increased in patients with acromegaly compared to healthy controls. As far as we know, our study is the first study to show that serum Elabela levels are increased in patients with acromegaly. Another important finding of our study is determining that serum Elabela level in acromegaly patients is closely related to low LVEF and high NT-proBNP serum levels, which are among the findings of cardiovascular involvement. Many parameters have been investigated in the studies on the effects of apelin and Elabela on the cardiovascular system, and the findings show that they slow down the course of diseases that cause cardiac hypertrophic and fibrotic processes such as hypertension, heart failure, and MI [10]. Due to all these positive and cardiovascular protective effects, it was thought that they could be targets for possible treatment methods [12]. Although there are many studies on cardiovascular diseases and their relation to serum Elabela or Apelin level [7, 8], as far as we have investigated, there is no study on serum Apelin or Elabela level in patients with acromegaly. As it is known, HT is very common in acromegaly patients and is associated with left ventricular hypertrophy [2, 3. 13.]. Also, increased GH and IGF-1 levels in acromegaly patients are associated with decreased systolic functions in these patients [3, 13]. These peptides (Apelin and Elabela), which are generally cardiac protectors and whose synthesis is increased for secondary protection in various cardiovascular diseases, may also have protective effects in patients with acromegaly. In our study, it was found for the first time that serum Elabela level increases significantly in acromegaly patients and is very closely and negatively related to LV-EF and very closely and positively related to NT-proBNP level (Fig. 3). In the light of this important finding obtained in our study, serum Elabela level was thought to increase with the direct activation of the apelinergic system in response to the cardiac involvement findings in acromegaly patients. It was thought that the rise in level of Elabela, which increased significantly (approximately tenfold) in patients with acromegaly, may have been due to system activation for cardiovascular protection, similar to previous Apelin and Elabela studies. Since there is no similar study in the literature, the data in our study could not be compared with other studies. Another important result obtained in our study is to show that GH and IGF-1 levels, which are closely related with disease activity in acromegaly patients, are also closely related to serum Elabela level. In this case, the increased activity of apelinergic system may have an effect on slowing or regressing the negative cardiovascular effects of increased IGF-1 levels.
Limitations
Although biochemical measurement of serum Elabela level was performed in our study, no study on APJ receptor level was performed on the tissue samples. It could have been more meaningful to observe similar findings at the cardiac myocytes. In our study, there was a relationship between Elabela level, NT-proBNP level, and LV-EF; however, LV wall thickness and LV mass index measurements were not performed as echocardiographic parameters. If LVH was also evaluated, more meaningful results could be obtained. In our study, the level of Elabela was measured only before acromegaly treatment. Elabela level may have increased with the purpose of cardiac protection such as preventing cardiac hypertrophy and heart failure. Therefore, if the serum level was evaluated after acromegaly treatment, more meaningful results could be obtained. Our study should be supported by new studies with the same characteristics and which evaluates the presence of LVH, examines tissue samples, and measures Elabela level after acromegaly treatment.
Conclusion
According to the results of our study, patients with acromegaly have increased serum Elabela values compared to healthy controls. This finding could be due to an upregulation of the apelinergic system in order to exert cardiovascular positive effects in response to cardiovascular involvement in patients with acromegaly. High serum Elabela level may be a sign of cardiac involvement in patients with acromegaly. Therefore, Elabela level can also be used as a follow-up parameter in these patients. Patients with high serum Elabela level can be called for frequent follow-ups and examined closely in order to avoid cardiovascular events that may develop in the future. However, this finding still needs to be confirmed by further studies with more participants.
References
Katznelson L, Laws ER Jr, Melmed S et al (2014) Endocrine Society Acromegaly: an endocrine society clinical practice guideline. J Clin Endocrinol Metab 99(11):3933–3951. https://doi.org/10.1210/jc.2014-2700
Sumbul HE, Koc AS (2019) Hypertension is common in patients with newly diagnosed acromegaly and is ındependently associated with renal resistive ındex. High Blood Press Cardiovasc Prev 26(1):69–75. https://doi.org/10.1007/s40292-018-0293-9
Guo X, Fu H, Pang H, Xing B (2018) Risk of left ventricular hypertrophy and diastolic and systolic dysfunction in acromegaly: a meta-analysis. J Clin Neurosci 48:28–33. https://doi.org/10.1016/j.jocn.2017.10.067
Bankir M, Sumbul HE, Koc AS et al (2019) Elastography detected solid organ stiffness increased in patients with acromegaly. Medicine (Baltimore) 98:e14212
O’Dowd BF, Heiber M, Chan A et al (1993) A human gene that shows identity with the gene encoding the angiotensin receptor is located on chromosome 11. Gene 136:355–360
Perjés Á, Kilpiö T, Ulvila J et al (2016) Characterization of apela, a novel endogenous ligand of apelin receptor, in the adult heart. Basic Res Cardiol 111:2. https://doi.org/10.1007/s00395-015-0521-6
Simpkin JC, Yellon DM, Davidson SM et al (2007) Apelin-13 and apelin-36 exhibit direct cardioprotective activity against ischemia-reperfusion injury. Basic Res Cardiol 102:518–528
Dai T, Ramirez-Correa G, Gao WD (2006) Apelin increases contractility in failing cardiac muscle. Eur J Pharmacol 553:222–228
Chng SC, Ho L, Tian J, Reversade B (2013) ELABELA: a hormone essential for heart development signals via the apelin receptor. Dev Cell 27:672–680. https://doi.org/10.1016/j.devcel.2013.11.002
Zhang Y, Wang Y, Lou Y et al (2018) Elabela, a newly discovered APJ ligand: similarities and differences with Apelin. Peptides 109:23–32. https://doi.org/10.1016/j.peptides.2018.09.006
Schiller NB, Shah PM, Crawford M et al (1989) Recommendations for quantitation of the left ventricle by two-dimensional echocardiography. American Society of Echocardiography Committee on Standards, Subcommittee on Quantitation of Two-Dimensional Echocardiograms. J Am Soc Echocardiogr 2:358–367
Yang P, Maguire JJ, Davenport AP (2015) Apelin, Elabela/Toddler, and biased agonists as novel therapeutic agents in the cardiovascular system. Trends Pharmacol Sci 36:560–567. https://doi.org/10.1016/j.tips.2015.06.002
Nascimento GC, de Oliveira MT, Carvalho VC et al (2013) Acromegalic cardiomyopathy in an extensively admixed population: is there a role for GH/IGF-I axis? Clin Endocrinol (Oxf) 78(1):94–101. https://doi.org/10.1111/j.1365-2265.2012.04472.x
Author information
Authors and Affiliations
Contributions
Dr. Sumbul, Dr. Koc, Dr. Gulumsek, Dr. Ay and Dr. Avci Seyda: conceptualization, methodology, investigation, formal analysis, visualization, and writing – original draft. Dr. Sumbul, Dr. Gulumsek, Dr. Okyay and Dr. Sahin: conceptualization, methodology, resources, formal analysis, and writing – review and editing. Dr. Sumbul, Dr. Avci A, and Mr. Gold: conceptualization, methodology, and writing – review and editing. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was performed according to the recommendations set by the Declaration of Helsinki on Medical Research involving Human Subjects. The ethics committee of Cukurova University, Faculty of Medicine approved the study. Informed consent was obtained from all individual participants included in the study.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sumbul, H.E., Gulumsek, E., Avci, B.S. et al. Serum Elabela level is significantly increased in patients with acromegaly. Ir J Med Sci 192, 665–670 (2023). https://doi.org/10.1007/s11845-022-03042-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11845-022-03042-6