Abstract
Objective
Acromegaly is associated with increased cardiovascular morbidity and mortality. The data about the evaluation of coagulation and fibrinolysis in acromegalic patients are very limited and to our knowledge, platelet function analysis has never been investigated. So, we aimed to investigate the levels of protein C, protein S, fibrinogen, antithrombin 3 and platelet function analysis in patients with acromegaly.
Methods
Thirty-nine patients with active acromegaly and 35 healthy subjects were included in the study. Plasma glucose and lipid profile, fibrinogen levels, GH and IGF-1 levels and protein C, protein S and antithrombin III activities were measured in all study subjects. Also, platelet function analysis was evaluated with collagen/ADP and collagen–epinephrine-closure times.
Results
Demographic characteristics of the patient and the control were similar. As expected, fasting blood glucose levels and serum GH and IGF-1 levels were significantly higher in the patient group compared with the control group (pglc: 0.002, pGH: 0.006, pIGF-1: 0.001, respectively). But lipid parameters were similar between the two groups. While serum fibrinogen and antithrombin III levels were found to be significantly higher in acromegaly group (p fibrinogen: 0.005 and pantithrombin III: 0.001), protein S and protein C activity values were significantly lower in the patient group (p protein S: 0.001, p protein C: 0.001). Also significantly enhanced platelet function (measured by collagen/ADP- and collagen/epinephrine-closure times) was demonstrated in acromegaly (p col-ADP: 0.002, p col-epinephrine: 0.002). The results did not change, when we excluded six patients with type 2 diabetes in the acromegaly group. There was a negative correlation between serum GH levels and protein S (r: –0.25, p: 0.04)) and protein C (r: –0.26, p: 0.04) values. Likewise, there was a negative correlation between IGF-1 levels and protein C values (r: –0.39, p: 0.002), protein S values (r: –0.39, p: 0.001), collagen/ADP-closure times (r: –0.28, p: 0.02) and collagen/epinephrine-closure times (r:-0.26, p: 0.04). Also, we observed a positive correlation between IGF-1 levels and fibrinogen levels (r: 0.31, p: 0.01).
Conclusion
Acromegaly was found to be associated with increased tendency to coagulation and enhanced platelet activity. This hypercoagulable state might increase the risk for cardiovascular and cerebrovascular events in acromegaly.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Acromegaly is the clinical syndrome that results from excessive secretion of growth hormone. It is associated with increased cardiovascular risk, whose pathogenesis is not completely identified [1]. Cardiovascular abnormalities include hypertension, left ventricular hypertrophy and cardiomyopathy. The cardiomyopathy is characterized by diastolic dysfunction and arrhythmias [2]. Left ventricular concentric hypertrophy and left ventricular systolic and diastolic dysfunction was reported even in controlled disease [3]. Other cardiovascular risk factors include abnormal glucose metabolism (hyperinsulinism, impaired glucose tolerance or overt diabetes) and lipid profile (low levels of high-density lipoprotein (HDL)-cholesterol, high levels of small dense low-density lipoprotein (LDL)-cholesterol, triglyceride (TG), lipoprotein (Lp) (a)) [1, 4].
The role of atherosclerosis in the pathogenesis of cardiovascular complications of acromegaly is not clearly defined. The reported prevalence of coronary artery disease in acromegalic patients ranges from 3 to 37 % in different studies [1, 5]. There are several studies about coagulation and fibrinolysis in acromegalic patients as possible risk factors for cardiovascular disease [6–9]. Also, the effect of growth hormone (GH) on coagulation parameters has been previously studied [1, 7–9] but there are only two studies which evaluate protein C and S activities in patients with acromegaly [1, 6].
In addition to coagulation and fibrinolysis parameters, recently platelet hyperfunction was reported to be elevated in patients with myocardial infarction (MI) and proposed to be a risk factor for coronary atherosclerosis [10, 11]. To our knowledge, platelet function analysis has not been studied before in acromegalic patients. Therefore, in this study, we aimed to investigate the levels of protein C, protein S, fibrinogen, antithrombin 3 and platelet function analysis (performed with collagen/ADP and collagen–epinephrine) in patients with acromegaly. We also investigated the correlation between GH and insulin-like growth factor (IGF)-1 levels and these hemostatic parameters in acromegalic patients.
Subjects and methods
Patients and study design
The study was a single-center, prospective, case–control study in patients with active acromegaly. Thirty-nine patients attending to our outpatient Endocrinology Clinic of Tepecik Research and Training Hospital with active acromegaly (age range 28−71 years) were recruited as acromegaly group between August 2013 and June 2014. The diagnosis of acromegaly was previously established by the typical clinical signs and symptoms (acral enlargement, soft tissue overgrowth, hyperhidrosis), by a failure of GH levels to suppress below 1 ng/ml during a standard (75 g) oral glucose tolerance test (OGTT) associated with increased IGF-1 values for age and sex. Pituitary imaging was obtained by pituitary magnetic resonance imaging (MRI) and 28 (71.8 %) patients had macroadenomas and 11 (28.2 %) had microadenomas. All patients were untreated and had active acromegaly at the time of the study. Thirty-two patients had undergone selective resection of pituitary adenomas by the transsphenoidal approach and 7 patients had been administered primary medical therapy as somatostatin receptor ligands (octreotide-LAR), since the patients had refused to undergo surgery. Also, somatostatin receptor ligands (octreotide-LAR or lanreotide) were given to patients who had undergone surgery, since they had still elevated GH and IFG-1 levels after surgery. The patients who had hypopituitarism were taking adequate replacement treatment.
A total of 35 healthy persons (15 men, 20 women), mean age 47.3 ± 4.7 years, attending to our Family Practice outpatient clinic just for check-up were included as the control group. None of the study participants in the control group were taking any medical treatments (estrogen, anti-hyperlipidemic therapy or treatment that affects hemostatic parameters) or had diseases (diabetes mellitus, dyslipidemia, thyroid dysfunction, atrial fibrillation, renal disease, liver cirrhosis) that might affect blood coagulation and fibrinolysis, and lipid profile.
Also, all participants were non-smokers or none of them had family history of clotting disorders and none of them were receiving any medication that might affect blood coagulation and fibrinolysis.
The study was approved by the medical ethics committee of the Tepecik Research and Training Hospital and all participants provided written informed consent.
Body mass index (BMI) was measured in all study subjects. BMI was calculated by the ratio between weight and height squared in kg/m2. Blood pressure was measured with the person in a seated position after a 5-min rest with an Omron M3 HEM-7131 electronic, auscultatory blood pressure reading machine. The first reading was discarded, and the mean of the next three consecutive readings was used.
After an overnight fast of 12 h, venous blood was collected from the antecubital vein for the evaluation of following biochemical parameters: plasma glucose and lipid profile (total cholesterol, HDL-cholesterol, LDL-cholesterol and triglycerides), fibrinogen, protein C and S, antithrombin III, platelet function analysis performed with collagen/ADP and collagen–epinephrine and GH and IGF-1 levels. Blood for haemostatic assays was collected in 3.2 % sodium citrate in a 9 to 1 blood to anticoagulant ratio and processed within 1 h of venipuncture. Also, ABO blood group was analyzed in all study participants.
Laboratory assessments
Glucose concentrations were measured by a hexokinase method with the Olympus AU-2700 analyzer. Triglycerides, total cholesterol and HDL-cholesterol were measured by an enzymatic method with Olympus AU-2700 analyzer using reagents from Olympus Diagnostics (Gmbh, Hamburg, Germany). LDL-cholesterol was calculated by the Friedewald’s equation method. Serum GH and IGF-1 levels were measured by a chemiluminescent immunometric assay (Immulite XPi, Siemens, Germany). The normal range for IGF-1 was age dependent (21–25 years; 116–358, 26–30 years; 117–329, 31–35 years; 115–307, 36–40 years; 109–284, 41–45 years; 101–267, 46–50 years; 94–252, 51–55 years; 87–238, 56–60 years; 81–225, 61–65 years; 75–212, 66–70 years; 69–200, 71–75 years; 64–188, 76–80 years; 59–177 ng/mL). Plasma fibrinogen levels (reference range 200– 400 mg/dL), protein S activity (reference range 76–135 %), protein C activity (reference range 70–140 %) and antithrombin III activity (reference range 83–128 %) were determined using ACL coagulation analyzer system (Instrumentation Laboratories, Lexington). Protein C and antithrombin activity was determined using the chromogenic method. Protein S activity was measured using a clotting assay for the measurement of a functional protein S level. The antithrombin, protein C and protein S inter-assay coefficients of variation (CVs) were 2.1, 2.6 and 3.1 % for the normal level, respectively.
Platelet function analysis was evaluated by the platelet function analyzer (PFA-100) [12, 13]; a US Food and Drug Administration-approved device (Dade Behring) was used for measuring platelet function. It aspirates blood in vitro from a blood specimen into disposable test cartridges through a microscopic aperture cut into a biologically active membrane at the end of the capillary. The membrane of the cartridges is coated with collagen and adenosine diphosphate (ADP) or collagen and epinephrine inducing a platelet plug to form which closes the aperture. The time passed between the aspiration of the blood to the closure of the aperture and termination of the flow of blood is called closure time (CT). Normal collagen/epinephrine CT is 85–165 s and collagen/ADP-CT 71–118 s [14].
Statistical analysis
Results are expressed as mean ± SD. The patient and the control group were compared using Student t test. The Chi-square test was used for non-parametric variables. Pearson correlation analysis was carried out to evaluate correlation between GH/IGF-1 levels and biochemical and coagulation parameters. p < 0.05 was considered statistically significant. Statistical analysis was performed with SPSS 15.0 statistical software.
Results
Clinical characteristics of the patient and the control groups are described in Table 1. There were no significant differences between the two groups according to age, gender, BMI and systolic and diastolic blood pressure values. Fasting blood glucose levels and serum GH and IGF-1 levels were significantly higher in the patient group compared with the control group (p Glc: 0.002, p GH: 0.006, p IGF-1: 0.001, respectively). In the acromegaly group, 6 patients had type 2 diabetes and in the control group none of the subjects had diabetes. But lipid parameters were similar between the two groups (Table 2).
ABO blood groups which interfere in von Willebrand factor (vWF) and affect platelet activity were similar between the two groups (p: 0.168).
While serum fibrinogen and antithrombin III levels were found to be significantly higher in acromegaly group (p fibrinogen: 0.005 and p antithrombin III: 0.001) with respect to control group, protein S and protein C activity values were significantly lower in the patient group (p protein S: 0.001, p protein C: 0.001). We still observed similar findings when we excluded the diabetic patients in the acromegaly group (p fibrinogen: 0.005 and p antithrombin III: 0.001, p protein S: 0.001, p protein C: 0.001).
Acromegalic patients were found to have significantly enhanced platelet function as measured by collagen/ADP- and collagen/epinephrine-closure times. Collagen/ADP- and collagen/epinephrine-closure times were significantly decreased in patients with acromegaly (p col-ADP: 0.002, p col-epinephrine: 0.002). Also the results were not changed significantly, when we excluded the diabetic patients in the acromegaly group (p col-ADP: 0.01, p col-epinephrine: 0.011).
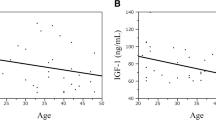
In patients with acromegaly, there was a weak negative correlation between serum GH levels and collagen/ADP-closure time (r –0.35, p: 0.05). There was a negative correlation between serum GH levels and protein S (r –0.25, p: 0.04)) and protein C (r –0.26, p: 0.04) values. As IGF-1 levels are concerned, we found a negative correlation between IGF-1 levels and protein C values (r –0.39, p: 0.002), protein S values (r –0.39, p: 0.001), collagen/ADP-closure times (r –0.28, p: 0.02) and collagen/epinephrine-closure times (r –0.26, p: 0.04). Also, we observed a positive correlation between IGF-1 levels and fibrinogen levels (r 0.31, p: 0.01).
Discussion
Cardiovascular and cerebrovascular events are the primary cause of death in acromegaly [6, 15]. The increase in mortality may be attributed to the increased prevalence of hypertension, insulin resistance, dyslipidemia, hypertrophic cardiomyopathy, and endothelial dysfunction in patients with acromegaly. GH hypersecretion increases insulin resistance, producing impaired glucose tolerance and diabetes mellitus [15–17]. Hypertension occurs in 33−46 % of acromegalic patients, with a predominance of diastolic blood pressure elevation that increases in prevalence with age [15–17]. Dyslipidemia observed in acromegaly include elevated triglyceride, lipoprotein (a) and small dense, LDL-cholesterol levels [15, 18]. In addition to cardiomyopathy, valvular heart disease, arrhythmias and conduction disorders are frequent in acromegaly [15, 18]. Considering the aforementioned risk factors, in our study, fasting blood glucose levels were significantly higher in patients with acromegaly compared with the control group, but lipid profile and blood pressure values were similar between the two groups.
In addition to these risk factors, alterations in hemostatic parameters might also contribute to the increased cardiovascular and cerebrovascular mortality in acromegaly patients. Fibrinogen is a coagulation factor involved in thrombus formation. Several prospective studies have consistently shown that a direct, independent, and statistically significant association exists between fibrinogen levels and subsequent incidence of heart disease [6, 8, 19]. Increased fibrinogen levels have been reported in patients with acromegaly [6–8, 20]. Similarly, in our study we observed increased fibrinogen levels in acromegalic patients. The increased fibrinogen levels may cause a tendency toward coagulation in patients with acromegaly.
Antithrombin III is a natural anticoagulant that inhibits the activated coagulation factors thrombin (factor IIa), factor Xa, and, to a lesser extent, factor XIa and factor IXa. Antithrombin III deficiency leads to increased risk of venous and arterial thrombosis. There are only three studies that evaluate the antithrombin III levels in acromegalic patients [1, 6, 9].
Sartorio et al. [9] and Vilar et al. [1] reported similar antithrombin III levels between acromegalic patients and healthy controls. In concordance with our results, Erem et al. found a significant increase in AT III levels in patients with acromegaly. The increase in antithrombin III levels was suggested as a protective mechanism and/or compensatory response against hypercoagulable state [6].
Activated protein C cleaves and inhibits coagulation factors FVIIIa and FVa. As a cofactor, protein S enhances the anticoagulant activity of activated protein C [6, 21]. Only Vilar et al. [1] and Erem et al. [6] examined protein C and protein S values before, in patients with acromegaly. Both of them found significantly decreased protein S values in patients with acromegaly. Likewise, in the present study, we observed significantly decreased protein S and protein C activities in acromegalic patients with respect to healthy controls.
Platelet activation is a hallmark of acute coronary syndrome. Increased platelet activation that was evaluated by PFA-100 (platelet function analyser) which measured decreased collagen/ADP and collagen–epinephrine-closure times, was previously reported in patients with acute coronary syndrome and myocardial infarction [14, 15]. But to our knowledge, platelet function analysis has not been investigated before in patients with acromegaly. In our study, we observed significantly decreased collagen/ADP and collagen–epinephrine-closure times, indicating the platelet hyperfunction, in acromegalic patients with respect to controls. Apart from the alterations in proteins or co-factors functioning in the coagulation pathway, platelet hyperactivity may also contribute to hypercoagulability that was reported in patients with acromegaly.
Vilar et al. found a significant positive correlation between IGF-1 and antithrombin III levels and significant positive correlation between GH and fibrinogen and antithrombin III levels, whereas Sartorio et al. found no correlations between hemostatic variables and GH or IGF-1 [1, 9]. In our study, we observed a negative correlation between serum GH levels and collagen/ADP-closure time, protein S and protein C activities. Also, we found a negative correlation between IGF-1 levels and protein C and protein S activities, collagen/ADP- and collagen/epinephrine-closure times. These findings may reflect the effect of GH or IGF-1 on hemostatic parameters and may explain the tendency toward hypercoagulability.
In conclusion, our findings have shown that patients with acromegaly had higher fibrinogen levels, lower protein C and S activity values and enhanced platelet function with respect to controls. In addition, we found correlation between IGF-1 levels and these hemostatic parameters. Therefore, it may be suggested that, aforementioned changes in hemostatic parameters may lead to a hypercoagulable state which might increase the risk for cardiovascular and cerebrovascular events in acromegaly.
References
Vilar L, Naves LA, Costa SS, Abdalla LF, Coelho CE, Casulari LA (2007) Increase of classic and nonclassic cardiovascular risk factors in patients with acromegaly. Endocr Pract 13(4):363–372
Rajasoorya C, Holdaway IM, Wrightson P, Scott DJ, Ibbertson HK (1994) Determinants of clinical outcome and survival in acromegaly. Clin Endocrinol (Oxf) 41(1):95–102
Jurcut R, Găloiu S, Florian A, Vlădaia A, Ioniţă OR, Amzulescu MS, et al (2014) Quantifying subtle changes in cardiovascular mechanics in acromegaly: a Doppler myocardial imaging study. J Endocrinol Invest 37:1081–1090
Wildbrett J, Hanefeld M, Fücker K, Pinzer T, Bergmann S, Siegert G, Breidert M (1997) Anomalies of lipoprotein pattern and fibrinolysis in acromegalic patients: relation to growth hormone levels and insulin-like growth factor I. Exp Clin Endocrinol Diabetes 105(6):331–335
Colao A, Marzullo P, Di Somma C, Lombardi G (2001) Growth hormone and the heart. Clin Endocrinol (Oxf). 54(2):137–154
Erem C, Nuhoglu I, Kocak M, Yilmaz M, Sipahi ST, Ucuncu O, Ersoz HO (2008) Blood coagulation and fibrinolysis in patients with acromegaly: increased plasminogen activator inhibitor-1 (PAI-1), decreased tissue factor pathway inhibitor (TFPI), and an inverse correlation between growth hormone and TFPI. Endocrine 33(3):270–276. doi:10.1007/s12020-008-9088-4
Delaroudis SP, Efstathiadou ZA, Koukoulis GN, Kita MD, Farmakiotis D, Dara OG et al (2008) Amelioration of cardiovascular risk factors with partial biochemical control of acromegaly. Clin Endocrinol (Oxf) 69(2):279–284
Landin-Wilhelmsen K, Tengborn L, Wilhelmsen L, Bengtsson BA (1997) Elevated fibrinogen levels decrease following treatment of acromegaly. Clin Endocrinol (Oxf) 46(1):69–74
Sartorio A, Cattaneo M, Bucciarelli P, Bottasso B, Porretti S, Epaminonda P, Faglia G, Arosio M (2000) Alterations of haemostatic and fibrinolytic markers in adult patients with growth hormone deficiency and with acromegaly. Exp Clin Endocrinol Diabetes 108(7):486–492
Harrison P, Mackie I, Mathur A, Robinson MS, Hong Y, Erusalimsky JD, Machin SJ, Martin JF (2005) Platelet hyper-function in acute coronary syndromes. Blood Coagul Fibrinolysis 16(8):557–562
Frossard M, Fuchs I, Leitner JM, Hsieh K, Vlcek M, Losert H, Domanovits H et al (2004) Platelet function predicts myocardial damage in patients with acute myocardial infarction. Circulation 110(11):1392–1397 Epub 2004 Aug 16
Favaloro EJ (2002) Clinical application of the PFA-100. Curr Opin Hematol 9:407–415
Jilma B (2001) Platelet function analyzer (PFA-100): a tool to quantify congenital or acquired dysfunction. J Lab Clin Med 138:152–163
Renda G, Zurro M, Malatesta G, Ruggieri B, De Caterina R (2010) Inconsistency of different methods for assessing ex vivo platelet function: relevance for the detection of aspirin resistance. Haematol 95(12):2095–2101
Katznelson L, Laws ER Jr, Melmed S, Molitch ME, Murad MH, Utz A, Wass JA (2014) Endocrine Society. Acromegaly: an endocrine society clinical practice guideline. J Clin Endocrinol Metab 99(11):3933–3951
Mestron A, Webb SM, Astorga R, Benito P, Catala M, Gaztambide S et al (2004) Epidemiology, clinical characteristics, outcome, morbidity and mortality in acromegaly based on the Spanish Acromegaly Registry (Registro Espanol de Acromegalia, REA). Eur J Endocrinol 151(4):439–446
Arosio M, Reimondo G, Malchiodi E, Berchialla P, Borraccino A, De Marinis L, Italian Study Group of Acromegaly et al (2012) Predictors of morbidity and mortality in acromegaly: an Italian survey. Eur J Endocrinol 167(2):189–198
Colao A, Ferone D, Marzullo P, Lombardi G (2004) Systemic complications of acromegaly: epidemiology, pathogenesis, and management. Endocr Rev 25(1):102–152
Feinbloom D, Bauer KA (2005) Assessment of hemostatic risk factors in predicting arterial thrombotic events. Arterioscler Thromb Vasc Biol 25(10):2043–2053
Vitale G, Pivonello R, Galderisi M, D’Errico A, Spinelli L, Lupoli G, Lombardi G, Colao A (2001) Cardiovascular complications in acromegaly: methods of assessment. Pituit 4(4):251–257
Dahlbäck B, Villoutreix BO (2005) The anticoagulant protein C pathway. FEBS Lett 579(15):3310–3316
Conflict of interest
None.
Ethical approval
All procedures performed in this study was in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Colak, A., Yılmaz, H., Temel, Y. et al. Coagulation parameters and platelet function analysis in patients with acromegaly. J Endocrinol Invest 39, 97–101 (2016). https://doi.org/10.1007/s40618-015-0300-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40618-015-0300-0