Abstract
Microglia play a significant role in the generation and propagation of oxidative/nitrosative stress, and are the basis of neuroinflammatory responses in the central nervous system. Upon stimulation by endotoxins such as lipopolysaccharides (LPS), these cells release pro-inflammatory factors which can exert harmful effects on surrounding neurons, leading to secondary neuronal damage and cell death. Our previous studies demonstrated the effects of botanical polyphenols to mitigate inflammatory responses induced by LPS, and highlighted an important role for cytosolic phospholipase A2 (cPLA2) upstream of the pro-inflammatory pathways (Chuang et al. in J Neuroinflammation 12(1):199, 2015. doi:10.1186/s12974-015-0419-0). In this study, we investigate the action of botanical compounds and assess whether suppression of cPLA2 in microglia is involved in the neurotoxic effects on neurons. Differentiated SH-SY5Y neuroblastoma cells were used to test the neurotoxicity of conditioned medium from stimulated microglial cells, and WST-1 assay was used to assess for the cell viability of SH-SY5Y cells. Botanicals such as quercetin and honokiol (but not cyanidin-3-O-glucoside, 3CG) were effective in inhibiting LPS-induced nitric oxide (NO) production and phosphorylation of cPLA2. Conditioned medium from BV-2 cells stimulated with LPS or IFNγ caused neurotoxicity to SH-SY5Y cells. Decrease in cell viability could be ameliorated by pharmacological inhibitors for cPLA2 as well as by down-regulating cPLA2 with siRNA. Botanicals effective in inhibition of LPS-induced NO and cPLA2 phosphorylation were also effective in ameliorating microglial-induced neurotoxicity. Results demonstrated cytotoxic factors from activated microglial cells to cause damaging effects to neurons and potential use of botanical polyphenols to ameliorate the neurotoxic effects.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Neuroinflammation underlies the pathophysiology of numerous neurological and psychiatric conditions, including Alzheimer’s disease, Parkinson’s disease, multiple sclerosis, stroke, and even schizophrenia (Glass et al. 2010; Gonzalez-Scarano and Martin-Garcia 2005). Microglia, the resident immune cells in the central nervous system, are recognized as an important cell type for propagation of inflammatory responses in the brain (Block and Hong 2005; Block et al. 2007; Aguzzi et al. 2013). Bacterial endotoxin LPS is traditionally established to activate the Toll-like receptor 2/4 and is coupled to the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) pathway to cause transcription/translation of a number of inflammatory genes, including inducible nitric oxide synthase (iNOS). In BV-2 microglial cells, interferon-γ (IFNγ) acting through the IFNγ receptor and coupled to the canonical Janus kinase–signal transducer and activator of transcription (JAK-STAT) pathway can also induce transcription pathways for the production of iNOS/nitric oxide (NO). Our study provided evidence for a cross-talk mechanism between the two pathways, with common activated components identified, such as extracellular signal-regulated kinases 1/2 (ERK1/2), cytosolic phospholipase A2 (cPLA2), and the nicotinamide adenine dinucleotide phosphate-oxidase (NADPH oxidase) (Chuang et al. 2013, 2015; Sheng et al. 2011).
Many botanical compounds, especially the polyphenols from fruits and vegetables, have been shown to exert beneficial effects against neurological conditions (Sun et al. 2011). While the mechanism of action of these botanicals may be multifaceted, many have pointed to the anti-inflammatory and antioxidative properties of these compounds (Galli et al. 2002; Sun et al. 2008). Previous studies from our laboratory as well as from others have demonstrated the effects of botanicals, such as quercetin from berries and honokiol from the magnolia bark, to mitigate microglial-induced production of NO and reactive oxygen species (ROS) upon stimulation by LPS or pro-inflammatory cytokines (Chuang et al. 2013; Jiang et al. 2014). These studies also unveil differences in potency in their ability to mitigate antioxidative and anti-inflammatory responses (Simonyi et al. 2015; Sun et al. 2015).
The phospholipases A2 (PLA2) family, which is comprised mainly of cytosolic phospholipase A2 (cPLA2), calcium-independent phospholipase A2 (iPLA2), and secretory phospholipase A2 (sPLA2), has the ability to hydrolyze fatty acids in the sn-2 position of membrane phospholipids and release polyunsaturated fatty acids, including arachidonic acid (AA) and docosahexaenoic acid (DHA) (Burke and Dennis 2009; Leslie 2015). While DHA is considered as an essential fatty acid with beneficial health effects, metabolism of AA by cyclooxygenases (COX-1 and COX-2) and lipoxygenases (LOX) to produce prostaglandins and leukotrienes is a well-established mechanism for inflammatory responses in the biological systems (Calder 2008). Works done in our laboratory, as well as others, have suggested cPLA2 to play an important role in the oxidative and nitrosative stress responses in activated microglia cells (Chuang et al. 2015; Ribeiro et al. 2013). Besides playing an integral part in the pathway involving ERK1/2 → cPLA2 → NADPH oxidase/ROS → NF-κB → iNOS/NO, our study also demonstrated possible link from cPLA2 to downstream components through the action of lipoxygenase-12/15 in BV-2 microglial cells (Chuang et al. 2015).
In this study, we test whether botanicals that are effective in mitigating LPS- or IFNγ-mediated inflammatory pathways also involve suppression of cPLA2 phosphorylation and activation, and whether suppression of LPS-induced inflammatory responses can mitigate microglia-induced toxicity to neurons. Human neuroblastoma cells (SH-SY5Y) that are differentiated into neuron-like phenotype by retinoic acid were exposed to conditioned media from microglia treated with LPS/IFNγ as well as botanicals and cPLA2 inhibitors. Our results demonstrated the role of cPLA2 in microglia inflammation responses and ability for botanicals to protect neurons from toxic effects induced by activated microglial cells.
Materials and Methods
Materials
Culture medium includes the following: Dulbecco’s modified Eagle’s medium (DMEM), penicillin/streptomycin, and 0.25 % (w/v) trypsin/EDTA from GIBCO (Gaithersburg, MD), and endotoxin-free fetal bovine serum from Atlanta Biologicals (Lawrenceville, GA). LPS (rough strains) from Escherichia coli F583 (Rd mutant) was purchased from Sigma-Aldrich (St. Louis, MO). IFNγ was purchased from R&D Systems (Minneapolis, MN). Retinoic acid was purchased from Sigma-Aldrich (St. Louis, MO). Quercetin (Sigma-Aldrich, St. Louis, MO) and cyanidin 3-O-glucoside (Indofine Chemical Comp., Hillsborough, NJ), honokiol (lot # M8P0236), and magnolol (lot # M8F3374) (≧98 % pure based on HPLC) (Nacalai Tesque, Inc, Kyoto, Japan) were dissolved in DMSO as stock solution. Pharmacological inhibitors used in this study including arachidonyl trifluoromethyl ketone (AACOCF3) and pyrrophenone were purchased from Cayman Chemical (Ann Arbor, MI). siRNA against cPLA2 Mm_Pla2g4a_8 FlexiTube siRNA (NM_008869) and AllStars Negative Control siRNA were purchased from Qiagen (Hilden, Germany). RNA interference Lipofectamine RNAiMAX Transfection Reagent was from Life Technology (Carlsbad, CA). Antibodies used for Western blot studies include rabbit polyclonal anti-p-cPLA2 (Cell Signaling, Beverly, MA), anti-cPLA2 rabbit polyclonal, goat anti-mouse IgG-horseradish peroxidase, and goat anti-rabbit IgG-horseradish peroxidase (Santa Cruz Biotechnology, Santa Cruz, CA). WST-1 assay was performed using the kit provided by Clontech (Mountain View, CA).
Immortalized Microglial BV-2 Cell Culture
The murine BV-2 cell line, generated by infecting primary microglia cells with a v-raf/v-myc oncogene carrying retrovirus (J2), was obtained as a gift from Dr. R. Donato (University of Perugia, Italy) and prepared as previously described (Shen et al. 2005). Briefly, BV-2 cells were cultured in DMEM (high glucose) supplemented with 10 % FBS, containing 100 units/mL penicillin and 100 μg/mL streptomycin. Cultures are maintained in 5 % CO2 incubator at 37 °C. For subculture, cells were gently scraped off from the culture plates, re-suspended in pre-warmed culture medium, seeded in 6-/96-well plates, and incubated until 80–90 % confluence for experiments. For experiments, BV-2 cells were typically used between passages 7–12.
Human Neuroblastoma SH-SY5Y Cell Line
The immortalized human neuroblastoma cells, SH-SY5Y, were obtained from American Type Culture Collection (Manassas, VA). SH-SY5Y cells were subcultured by suspension in Trypsin–EDTA (0.25 %) at room temperature for 1 min to detach cells from plate. Fresh DMEM/F12 medium supplemented with 10 % FBS was added to deactivate trypsin, followed by centrifugation. Medium was aspirated, and cells were resuspended in 1:1 mix of DMEM/F12 supplemented with 10 % FBS containing 100 units/mL of penicillin and 100 μg/mL of streptomycin, seeded in 96-well plates, and maintained in 5 % CO2 at 37 °C. Two days after plating, culture medium was replaced with 1:1 mix of DMEM/F12 supplemented with 3 % of FBS, 10 µM of retinoic acid, and penicillin/streptomycin. Retinoic acid-supplemented culture medium was renewed every 3 days. Culture was allowed 5 days for differentiation prior to use. SH-SY5Y cells were used between passages 4–8 in this experiment. Cell condition and morphology were assessed by using a phase-contrast Nikon DIAPHOT 300 microscope equipped with a CCD cool camera. MagnaFire2.1C software was used for microscopic image capture and processing. Representative bright-field pictures were captured under a 20x objective lens.
cPLA2 RNA Interference Knockdown in BV-2 Cells
BV-2 cells were transfected following the same protocol described previously (Chuang et al. 2015). Briefly, BV-2 cells were seeded in 24-well plates with antibiotics-free DMEM containing 10 % of FBS for 24 h. When culture reached 70–80 % confluence, cells were transfected with either AllStars negative control siRNA (Qiagen), or cPLA2 siRNA (NM_008869, Qiagen) (final concentration of 40 nM) using the RNAiMAX transfection reagent (Invitrogen) in the mixture of Opti-MEM and DMEM mediums for 48 h prior to being used for experiments. This procedure resulted in a roughly 70 % down-regulation of cPLA2 expression as described in the previous study (Chuang et al. 2015).
NO Determination
NO released from BV-2 cells was measured using the Griess reagent protocol. In brief, BV-2 cells in 96-well plate were serum-starved in phenol red-free DMEM for 3 h, followed by incubation with varying concentrations of botanical compounds for 1 h. Cells were then incubated with LPS or IFNγ at 37 °C for 16 h. Aliquots of conditioned medium (50 μL) were incubated with 50 μL of the reagent A [1 % (w/v) sulfanilamide in 5 % phosphoric acid, Sigma-Aldrich] for 10 min at room temperature covered in dark. This was followed by the addition of 50 μL of reagent B [0.1 % (w/v) N-1-napthylethylenediamine dihydrochloride, Sigma-Aldrich] for 10 min at room temperature, protected from light, and absorbance at 543 nm was read using a microplate reader (Biotek Synergy 4, Winooski, VT). Serial dilutions of sodium nitrite (0–100 μM) were used to generate the nitrite standard curve.
Assessment of Microglial-Induced Neuronal Toxicity
BV-2 cells were plated in 24-well plates until confluence. Cells were serum-starved for 3 h, pretreated with or without botanicals for 1 h, and then stimulated with LPS or IFNγ for 24 h. The conditioned media was collected and centrifuged at 1500 rpm for 3 min to remove cell debris. Medium of differentiated SH-SY5Y culture in 96-well plates was then replaced with 100 µL of cell-free conditioned medium from treated BV-2 cells, and incubated for 48 h. Cell viability for SH-SY5Y cells was determined using the WST-1 assay protocol (Clontech). Briefly, 10 µL of the WST-1 reagent was added in each well. Cells were incubated for 1 h at 37 °C, and absorbance was read at 450 nm (with reference wavelength at 650 nm) with a microplate reader.
Western Blot Analysis
Cells were lysed in RIPA buffer containing 50 mM Tris–HCl (pH 7.5), 150 mM NaCl, 1 % Nonidet P-40, and 0.1 % SDS. The cell lysate was centrifuged at 10,000 × g for 15 min at 4 °C and transferred to a clean tube to remove cell debris. After measuring protein concentration in each sample using the BCA protein assay kit (Pierce Biotechnology, Rockford, IL), 10 μg of protein from each sample was loaded in SDS-PAGE for electrophoresis. Proteins were then transferred from gel to 0.45-μm nitrocellulose membranes. Membranes were incubated in Tris-buffered saline containing 0.1 % Tween 20 (TBS-T) and 5 % nonfat milk for 1 h at room temperature. The membranes were then incubated at 4 °C overnight with antibodies against cPLA2 (1:1000) or phospho-cPLA2 (1:1000). After repeated washing with 1X TBS-T, blots were incubated with goat anti-rabbit IgG-HRP (1:4000) for 1 h at room temperature. Immunolabeling was detected by chemiluminescence WestPico/Femto and developed in X-ray film developer. Films were scanned, and the optical density of protein bands was quantified with the QuantityOne software (Bio-Rad, Hercules, CA).
Statistical Analysis
Data were presented as mean ± SEM. Results were analyzed by one-way ANOVA followed by Dunnett’s multiple comparison tests (V4.00; GraphPad Prism Software Inc., San Diego, CA). Statistical significance was considered for P < 0.05.
Results
Quercetin and Honokiol, but Not Cyanindin-3-O-glucoside, Significantly Suppress LPS-induced NO Production in BV-2 Microglial Cells
Our previous studies have demonstrated the ability for LPS and IFNγ to activate NF-κB and JAK-STAT pathways and induce oxidative and nitrosative products in BV-2 microglial cells (Sheng et al. 2011). In order to assess the anti-inflammatory properties of the botanical compounds of interest, we pretreat the BV-2 cells with quercetin, honokiol, or C3G (chemical structures are shown in Fig. 1) 1 h before LPS stimulation. C3G was used as a negative control because our previous study showed that this polyphenol derivative was not effective in inhibiting LPS-induced NO in BV-2 cells (Simonyi et al. 2015). At 16 h after LPS stimulation, we observed significant dose-dependent decrease in NO production after pretreatment with quercetin and honokiol, but not with C3G (Fig. 2a–c). The indicated concentrations of quercetin, honokiol, and C3G had been discussed to pose no toxicity to BV-2 cells in previous studies (Simonyi et al. 2015; Sun et al. 2015; Kaushik et al. 2012).
LPS-induced NO production in BV-2 cells was inhibited by quercetin and honokiol, but not C3G. BV-2 cells were serum-starved for 4 h in serum-free DMEM. One hour prior to stimulation, cells were pretreated with the indicated concentrations of a quercetin, b honokiol, or c C3G. Cells were then stimulated with 200 ng/mL of LPS. Conditioned media was collected 16 h post-stimulation, and NO concentrations were measured by Griess protocol as described in the text. Results were expressed as the mean ± SEM (n = 3), and significant difference between LPS and LPS + polyphenol groups was determined by one-way ANOVA followed by Dunnett’s posttests, *P < 0.05, **P < 0.01, ***P < 0.001
Quercetin and Honokiol, but Not C3G, Suppress cPLA2 Phosphorylation in BV-2 Cells after LPS Stimulation
In our recent study, we demonstrated cPLA2 to play a significant role in the oxidative/nitrosative response in microglial cells and regulation of cPLA2 phosphorylation by ERK1/2 (Chuang et al. 2015). In addition, we demonstrated that cPLA2 activation is important in regulating downstream ROS production by NADPH oxidase as well as the induction of iNOS and NO (Chuang et al. 2015). In this study, we tested whether botanicals that are active in inhibiting LPS-induced NO also suppress cPLA2 phosphorylation. After pretreating the BV-2 cells with the botanical compounds for 1 h followed by stimulation with LPS, cells were lysed 4 h post-stimulation and protein was analyzed for levels of total and phospho-cPLA2. As shown in Fig. 3, p-cPLA2 level was increased after LPS stimulation, and was suppressed dose dependently by quercetin (Fig. 3a, b) and honokiol (Fig. 3c, d). Consistent with previous results showing inability for C3G to inhibit LPS-induced NO production, this compound also did not inhibit LPS-induced phosphorylation of cPLA2 (Fig. 3e, f).
LPS-stimulated cPLA2 phosphorylation in BV-2 cells was inhibited by quercetin and honokiol, but not C3G. BV-2 cells were serum-starved for 4 h in serum-free DMEM. One hour prior to stimulation, cells were pretreated with the indicated concentrations of a, b quercetin c, d honokiol, or e, f C3G. Cells were then stimulated with 200 ng/mL LPS. Cells were lysed, and proteins were collected and processed at 4 h post-stimulation. Western blot was performed to determine protein expression. cPLA2 protein band appeared just below the 100-kDa molecular weight standard. Representative blots a, c, e from three independent experiments, and b, d, f quantification of protein expression. Results were expressed as the mean ± SEM (n = 3), and significant difference between LPS and LPS + polyphenol groups was determined by one-way ANOVA followed by Dunnett’s posttests, *P < 0.05, ***P < 0.001
Effects of LPS/IFNγ-stimulated Microglial Cells on Neurotoxicity in Differentiated SH-SY5Y Cells
Microglia-induced neurotoxicity is a well-established phenomenon seen in acute inflammation in the CNS (Block et al. 2007). Our recent study demonstrated pharmacologic and genetic ablation of cPLA2 not only mitigated LPS/IFNγ-induced oxidative/nitrosative responses but also protected microglial cells to express the inflammatory phenotype (Chuang et al. 2015). In this study, an in vitro system was used to assess whether conditioned media from activated microglial cells may offer deleterious effects on differentiated SH-SY5Y cells. Neuroblastoma SH-SY5Y cells were treated with 10 µM retinoic acid medium for 5 days. Under observation by light microscopy, undifferentiated SH-SY5Y cells appeared to be clumped with short neurites (Fig. 4a), whereas differentiated SH-SY5Y cells showed neuron-like morphology with extended neurites and dendrite formation (Fig. 4b). When the differentiated SH-SY5Y cells were treated with conditioned medium from BV-2 cells that were stimulated with LPS or IFNγ (24 h), significant toxicity was observed after 48 h of incubation (Fig. 4c). To verify that the microglial-mediated toxicity was due to released substances in the microglia cultured medium and not the effects of LPS or IFNγ in the conditioned medium alone, cell-free medium containing only LPS or IFNγ added to SH-SY5Y culture demonstrated no toxicity (Fig. 4c).
SH-SY5Y differentiation and microglia-induced neurotoxicity. a, b Representative bright-field microscopic photography of SH-SY5Y cells prior to differentiation with and without 10 µM retinoic acid for 5 days. c Cell viability of SH-SY5Y cells after exposure to conditioned medium prepared from BV-2 cells treated with LPS or IFNγ for 24 h, or with LPS and IFNγ in fresh culture medium for 48 h. Results were expressed as the mean ± SEM (n = 3), and significant difference between the respective groups was determined by one-way ANOVA followed by Dunnett’s posttests, ***P < 0.001, ^^^P < 0.001
Inhibition of cPLA2 Activity/expression in BV-2 Cells Resulted in a Decrease in Microglia-Induced Neurotoxicity in SH-SY5Y Cells
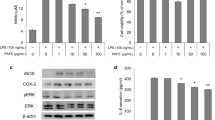
Using the above-mentioned protocol, SH-SY5Y cells that were incubated with conditioned medium from LPS/IFNγ-stimulated BV-2 cells pretreated with cPLA2 inhibitors (AACOCF3 and pyrrophenone) showed a significant decrease in cell viability (Fig. 5). The protective effect with AACOCF3 (Fig. 5a, b) appeared to be similar to the specific cPLA2 inhibitor, pyrrophenone (Fig. 5c, d). Similarly, SH-SY5Y cell toxicity was also reduced when SH-SY5Y cells were treated with conditioned medium prepared from stimulated BV-2 cells with cPLA2 knocked down by siRNA (Fig. 6a, b).
Microglia-induced neurotoxicity was attenuated by cPLA2 inhibitors. BV-2 cells were serum-starved for 4 h in serum-free DMEM. One hour prior to stimulation, cells were pretreated with the indicated concentrations of cPLA2 inhibitors: a, b AACOCF3 or c, d pyrrophenone. Cells were then stimulated with a, c 200 ng/mL LPS or b, d 20 ng/mL IFNγ for 24 h, and conditioned medium is then transferred to SH-SY5Y culture. a–d Cell viability of SH-SY5Y cells after exposure to conditioned medium for 48 h. Results were expressed as the mean ± SEM (n = 3) and analyzed by one-way ANOVA followed by Dunnett’s posttests, *P < 0.05, **P < 0.01, ***P < 0.001 as compared to LPS/IFNγ alone
Microglia-induced neurotoxicity was attenuated by down-regulation of cPLA2 with siRNA. BV-2 cells were transfected with siRNA against cPLA2 24 h before being stimulated with a LPS or b IFNγ. BV-2 cells were then stimulated with 200 ng/mL LPS or 20 ng/mL IFNγ for 24 h, and conditioned medium is then transferred to SH-SY5Y culture. a, b Cell viability of SH-SY5Y cells after exposure to conditioned medium for 48 h. Results were expressed as the mean ± SEM (n = 3), and significant difference between the respective groups was determined by one-way ANOVA followed by Dunnett’s posttests, **P < 0.01, ***P < 0.001, ^P < 0.05
Pretreatment of BV-2 Cells with Quercetin and Honokiol, but not C3G, Reduced Neurotoxicity in Differentiated SH-SY5Y Cells
As demonstrated above, botanicals such as quercetin and honokiol and not C3G inhibited LPS-induced NO production and cPLA2 phosphorylation, and it is reasonable to test whether these botanicals can also reduce microglial-induced neurotoxicity in SH-SY5Y cells. As shown in Fig. 7, conditioned media from microglia treated with quercetin or honokiol, but not C3G, was able to reduce LPS-induced toxicity in SH-SY5Y cells (Fig. 7).
Microglia-induced neurotoxicity was attenuated by quercetin and honokiol but not C3G. BV-2 cells were serum-starved for 4 h in serum-free DMEM. One hour prior to stimulation, cells were pretreated with the indicated concentrations of a quercetin, b honokiol, or c C3G. BV-2 cells were then stimulated with 200 ng/mL LPS for 24 h, and conditioned medium is then transferred to SH-SY5Y culture. a–c Cell viability of SH-SY5Y cells after exposure to conditioned medium for 48 h. Results were expressed as the mean ± SEM (n = 3) and analyzed by one-way ANOVA followed by Dunnett’s posttests, *P < 0.05, **P < 0.01, ***P < 0.001 as compared to LPS alone
Discussion
Studies from our laboratory as well as from others have demonstrated different botanical polyphenols (including honokiol and quercetin) to mitigate LPS- and IFNγ-stimulated inflammatory responses in microglial cells (Chuang et al. 2013; Simonyi et al. 2015; Sun et al. 2015). These studies also unveiled the involvement of mitogen-activated protein kinases (MAPKs), e.g., ERK1/2, in regulating cross talk between LPS-stimulated NF-κB and IFNγ-stimulated JAK-STAT transcription pathways in mediating the inflammatory responses (Chuang et al. 2013; Jiang et al. 2014). Along this line, ERK1/2 activation was also linked to phosphorylation and activation of cPLA2, an important enzyme for regulating membrane phospholipids and release of AA for mediating the synthesis of eicosanoids and leukotrienoids (Chuang et al. 2015). Interestingly, despite of polyphenols showing similar structure, not all showed the same responses (Simonyi et al. 2015; Sun et al. 2015). For example, quercetin can inhibit LPS-induced NO more potently than cyanidin in BV-2 microglial cells (Sun et al. 2015). Furthermore, most polyphenols with glycosylated linkages were not effective in suppressing the inflammatory responses (Simonyi et al. 2015).
In this study, results showed the ability for quercetin and honokiol, but not C3G, to mitigate LPS-induced cPLA2 phosphorylation in microglial cells. These results confirm our earlier study demonstrating the role of cPLA2 in mediating microglial oxidative/nitrosative responses (Chuang et al. 2015). Since Toll-like receptors are known to mediate the induction of ERK-cPLA2-NF-κB pathway, effects of botanicals possibly target the receptors, ERK1/2, or other targets upstream of cPLA2 in this oxidative/nitrosative pathway in microglia.
SH-SY5Y is a neuroblastoma cell line originally cloned from SK-N-SH cell line derived from the bone marrow biopsy of a 4-year-old patient. In contrast to the S-type SH-EP subcloned cell line from the SK-N-SH cells, SH-SY5Y cells are N-type (neuronal) and possess the ability to form neurites, as well as the capability to differentiate into cells along the neuronal lineage (La Quaglia and Manchester 1996). Despite the fact that SH-SY5Y cells typically grow in clusters due to its cancerous nature, treatment of retinoic acid can cause differentiation and dendrite formation, as well as limitation of unopposed cell growth. The differentiated SH-SY5Y cells exhibit a number of neuronal properties with active neurotransmitter synthetizing enzymes reflecting the dopaminergic phenotype (La Quaglia and Manchester 1996). Therefore, these cells are well suited as a model to investigate neuron–glial cell interaction (Biedler et al. 1978). In a study by Yang et al. (2015), immortalized rat astrocytes (DITNC) stimulated with tumor necrosis factor-α (TNFα) and IL-1β released sPLA2-IIA (an inflammatory enzyme) into the culture medium. When the cytokine-conditioned media containing sPLA2-IIA was applied to SH-SY5Y cells, there was alteration in membrane fluidity and increased secretion of amyloid precursor protein (APP) products (Yang et al. 2015). However, since murine microglial cells lack the ability to induce sPLA2-IIA, this enzyme cannot be included as the cause of neurotoxicity in this experimental paradigm.
In this study, we demonstrated that neuronal toxicity in differentiated SH-SY5Y cells occurred as a result of exposure to cytotoxic products from microglia activation, as incubation with LPS or IFNγ alone did not affect neuronal survival. This is in agreement with previous studies demonstrating that activated microglia induced cytotoxic effects to SH-SY5Y neurons (Pan et al. 2008; Wang et al. 2011). Although specific microglial products were not identified in this study, others have suggested that neurotoxic factors, such as superoxide, myeloperoxidase, prostaglandins, NO, glutamate, IL-1β, and TNFα, play a role in causing toxicity to neurons under in vitro and in vivo models (Lull and Block 2010; Liu et al. 2003; Colton and Gilbert 1987; Liu et al. 2002; Lee et al. 1993; Wang et al. 2005). Our studies underline the important role of cPLA2 in mediating the neuronal cytotoxic effects. Activation of cPLA2 is not only important for the synthesis of eicosanoids and leukotrienoids by COXs and LOXs, but due to its link to phosphorylation by pERK1/2, this enzyme also plays a role upstream of NF-kB pathway for the synthesis of inflammatory products (Chuang et al. 2015). In our previous study, cPLA2 inhibitor AACOCF3 was shown to protect BV-2 cells from LPS- and IFNγ-induced morphological changes (Chuang et al. 2015). Therefore, it is reasonable to consider that cPLA2 inhibition plays an important role in preventing neurotoxicity to SH-SY5Y cells by activated microglia. These results are in agreement with other studies demonstrating the protective effects of AACOCF3 in animal models of neurological diseases, including experimental stroke (Zhang et al. 2012), experimental autoimmune encephalitis for multiple sclerosis (Vana et al. 2011), as well as spinal cord injury (Huang et al. 2009; Liu et al. 2014).
In this study, we selected two botanical polyphenols, honokiol and quercetin, known for their antioxidative and anti-inflammatory properties. Consistent with the results with cPLA2 inhibitors, quercetin and honokiol not only are effective in inhibition of oxidative and nitrosative products, but can also suppress cPLA2-mediated pathways for the production of eicosanoids and leukotrienoids. Honokiol is a polyphenolic compound extracted from the bark of Magnolia officinalis, a common herbal plant in traditional Chinese and Korean medicine ( Lee et al. 2011). It has been shown to exert beneficial effects against neurological disorders, including Alzheimer’s disease, Parkinson’s disease, stroke, depression, and anxiety (Watanabe et al. 1983; Maruyama et al. 1998; Liu et al. 2005; Chen et al. 2011; Xu et al. 2008; Chang-Mu et al. 2010). Previous studies performed in our laboratory, as well as by others, had demonstrated anti-inflammatory effects of these compounds to mitigate production of ROS, iNOS/NO, and prostaglandins/leukotrienes in BV-2 microglial cells stimulated with LPS/IFNγ (Chuang et al. 2015; Kuo et al. 2010; Oh et al. 2009; Wu et al. 2011). The mechanism of action was suggested to involve inhibition of ERK1/2 phosphorylation, NADPH oxidase activation/translocation, and NF-κB activation. Indeed, our recent studies demonstrated the important role of ERK1/2 in mediating a number of signaling pathways, including cross talk between the NF-κB and JAK-STAT pathways and activation of cPLA2 in microglial cells (Chuang et al. 2015; Jiang et al. 2014). Quercetin is abundant in a variety of fruits, such as berries, apple, and even onion, and its ability to inhibit LPS-induced NF-κB inflammatory pathway and NO production in microglial cells has been well recognized (Chen et al. 2005; Kang et al. 2013; Kao et al. 2010). More recent studies provided evidence that quercetin not only is effective in suppressing LPS-induced inflammatory responses, but also capable of stimulating antioxidant pathway and synthesis of phase II enzymes and proteins (Sun et al. 2015; Kang et al. 2013). Studies in vivo indicated ability for quercetin to ameliorate damage against neurodegenerative diseases including cerebral ischemia (Annapurna et al. 2013; Hwang et al. 2009). These pleiotropic properties of quercetin qualify this to become an adaptogen with potential as a nutraceutical for intervention in neurodegenerative disorders (Calabrese et al. 2010; Lee et al. 2014).
Conclusions
This study demonstrated the activation of cPLA2, and increased oxidative and nitrosative products contribute to neurotoxic effects of microglial cells on SH-SY5Y neurons. Botanicals that are active in suppressing LPS-induced oxidative and inflammatory responses in microglial cells are also effective in protecting the cytotoxic effects on SH-SY5Y neurons.
References
Aguzzi, A., Barres, B. A., & Bennett, M. L. (2013). Microglia: Scapegoat, saboteur, or something else? [Research Support, Non-US. Gov’t Review]. Science, 339(6116), 156–161. doi:10.1126/science.1227901.
Annapurna, A., Ansari, M. A., & Manjunath, P. M. (2013). Partial role of multiple pathways in infarct size limiting effect of quercetin and rutin against cerebral ischemia–reperfusion injury in rats. European Review for Medical and Pharmacological Sciences, 17(4), 491–500.
Biedler, J. L., Roffler-Tarlov, S., Schachner, M., & Freedman, L. S. (1978). Multiple neurotransmitter synthesis by human neuroblastoma cell lines and clones. Cancer Research, 38(11 Pt 1), 3751–3757.
Block, M. L., & Hong, J. S. (2005). Microglia and inflammation-mediated neurodegeneration: Multiple triggers with a common mechanism. Progress in Neurobiology, 76(2), 77–98. doi:10.1016/j.pneurobio.2005.06.004.
Block, M. L., Zecca, L., & Hong, J. S. (2007). Microglia-mediated neurotoxicity: Uncovering the molecular mechanisms. Nature Reviews Neuroscience, 8(1), 57–69. doi:10.1038/nrn2038.
Burke, J. E., & Dennis, E. A. (2009). Phospholipase A2 structure/function, mechanism, and signaling [Research Support, N.I.H., Extramural Review]. Journal of Lipid Research, 50(Suppl), S237–S242. doi:10.1194/jlr.R800033-JLR200.
Calabrese, V., Cornelius, C., Dinkova-Kostova, A. T., Calabrese, E. J., & Mattson, M. P. (2010). Cellular stress responses, the hormesis paradigm, and vitagenes: Novel targets for therapeutic intervention in neurodegenerative disorders. Antioxidants & Redox Signaling, 13(11), 1763–1811. doi:10.1089/ars.2009.3074.
Calder, P. C. (2008). The relationship between the fatty acid composition of immune cells and their function. Prostaglandins Leukotrienes and Essential Fatty Acids, 79(3–5), 101–108. doi:10.1016/j.plefa.2008.09.016.
Chang-Mu, C., Jen-Kun, L., Shing-Hwa, L., & Shoei-Yn, L. S. (2010). Characterization of neurotoxic effects of NMDA and the novel neuroprotection by phytopolyphenols in mice [Research Support, Non-U.S. Gov’t]. Behavioral Neuroscience, 124(4), 541–553. doi:10.1037/a0020050.
Chen, J. C., Ho, F. M., Pei-Dawn Lee, C., Chen, C. P., Jeng, K. C., Hsu, H. B., et al. (2005). Inhibition of iNOS gene expression by quercetin is mediated by the inhibition of IkappaB kinase, nuclear factor-kappa B and STAT1, and depends on heme oxygenase-1 induction in mouse BV-2 microglia. European Journal of Pharmacology, 521(1–3), 9–20. doi:10.1016/j.ejphar.2005.08.005.
Chen, H. H., Lin, S. C., & Chan, M. H. (2011). Protective and restorative effects of magnolol on neurotoxicity in mice with 6-hydroxydopamine-induced hemiparkinsonism. Neurodegener Disease, 8(5), 364–374. doi:10.1159/000323872.
Chuang, D. Y., Chan, M. H., Zong, Y., Sheng, W., He, Y., Jiang, J. H., et al. (2013). Magnolia polyphenols attenuate oxidative and inflammatory responses in neurons and microglial cells [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t]. Journal of Neuroinflammation, 10, 15. doi:10.1186/1742-2094-10-15.
Chuang, D. Y., Simonyi, A., Kotzbauer, P. T., Gu, Z., & Sun, G. Y. (2015). Cytosolic phospholipase A2 plays a crucial role in ROS/NO signaling during microglial activation through the lipoxygenase pathway. Journal of Neuroinflammation, 12(1), 199. doi:10.1186/s12974-015-0419-0.
Colton, C. A., & Gilbert, D. L. (1987). Production of superoxide anions by a CNS macrophage, the microglia. FEBS Letters, 223(2), 284–288.
Galli, R. L., Shukitt-Hale, B., Youdim, K. A., & Joseph, J. A. (2002). Fruit polyphenolics and brain aging: Nutritional interventions targeting age-related neuronal and behavioral deficits [Review]. Annals of the New York Academy of Sciences, 959, 128–132.
Glass, C. K., Saijo, K., Winner, B., Marchetto, M. C., & Gage, F. H. (2010). Mechanisms underlying inflammation in neurodegeneration. Cell, 140(6), 918–934. doi:10.1016/j.cell.2010.02.016.
Gonzalez-Scarano, F., & Martin-Garcia, J. (2005). The neuropathogenesis of AIDS. Nature Reviews Immunology, 5(1), 69–81. doi:10.1038/nri1527.
Huang, W., Bhavsar, A., Ward, R. E., Hall, J. C., Priestley, J. V., & Michael-Titus, A. T. (2009). Arachidonyl trifluoromethyl ketone is neuroprotective after spinal cord injury [Research Support, Non-U.S. Gov’t]. Journal of Neurotrauma, 26(8), 1429–1434. doi:10.1089/neu.2008-0835.
Hwang, I. K., Lee, C. H., Yoo, K. Y., Choi, J. H., Park, O. K., Lim, S. S., et al. (2009). Neuroprotective effects of onion extract and quercetin against ischemic neuronal damage in the gerbil hippocampus. Journal of Medicinal Food, 12(5), 990–995. doi:10.1089/jmf.2008.1400.
Jiang, J., Chuang, D. Y., Zong, Y., Patel, J., Brownstein, K., Lei, W., et al. (2014). Sutherlandia frutescens ethanol extracts inhibit oxidative stress and inflammatory responses in neurons and microglial cells [Research Support, N.I.H., Extramural]. PLoS ONE, 9(2), e89748. doi:10.1371/journal.pone.0089748.
Kang, C. H., Choi, Y. H., Moon, S. K., Kim, W. J., & Kim, G. Y. (2013). Quercetin inhibits lipopolysaccharide-induced nitric oxide production in BV2 microglial cells by suppressing the NF-kappaB pathway and activating the Nrf2-dependent HO-1 pathway. International Immunopharmacology, 17(3), 808–813. doi:10.1016/j.intimp.2013.09.009.
Kao, T. K., Ou, Y. C., Raung, S. L., Lai, C. Y., Liao, S. L., & Chen, C. J. (2010). Inhibition of nitric oxide production by quercetin in endotoxin/cytokine-stimulated microglia. Life Sciences, 86(9–10), 315–321. doi:10.1016/j.lfs.2009.12.014.
Kaushik, D. K., Mukhopadhyay, R., Kumawat, K. L., Gupta, M., & Basu, A. (2012). Therapeutic targeting of Kruppel-like factor 4 abrogates microglial activation. Journal of Neuroinflammation, 9, 57. doi:10.1186/1742-2094-9-57.
Kuo, D. H., Lai, Y. S., Lo, C. Y., Cheng, A. C., Wu, H., & Pan, M. H. (2010). Inhibitory effect of magnolol on TPA-induced skin inflammation and tumor promotion in mice. Journal of Agriculture and Food Chemistry, 58(9), 5777–5783. doi:10.1021/jf100601r.
La Quaglia, M. P., & Manchester, K. M. (1996). A comparative analysis of neuroblastic and substrate-adherent human neuroblastoma cell lines. Journal of Pediatric Surgery, 31(2), 315–318.
Lee, J., Jo, D. G., Park, D., Chung, H. Y., & Mattson, M. P. (2014). Adaptive cellular stress pathways as therapeutic targets of dietary phytochemicals: Focus on the nervous system. Pharmacological Reviews, 66(3), 815–868. doi:10.1124/pr.113.007757.
Lee, Y. J., Lee, Y. M., Lee, C. K., Jung, J. K., Han, S. B., & Hong, J. T. (2011). Therapeutic applications of compounds in the Magnolia family [Research Support, Non-U.S. Gov’t Review]. Pharmacology & Therapeutics, 130(2), 157–176. doi:10.1016/j.pharmthera.2011.01.010.
Lee, S. C., Liu, W., Dickson, D. W., Brosnan, C. F., & Berman, J. W. (1993). Cytokine production by human fetal microglia and astrocytes. Differential induction by lipopolysaccharide and IL-1 beta. Journal of Immunol, 150(7), 2659–2667.
Leslie, C. C. (2015). Cytosolic phospholipase A2: Physiological function and role in disease. Journal of Lipid Research,. doi:10.1194/jlr.R057588.
Liu, N. K., Deng, L. X., Zhang, Y. P., Lu, Q. B., Wang, X. F., Hu, J. G., et al. (2014). Cytosolic phospholipase A2 protein as a novel therapeutic target for spinal cord injury [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t]. Annals of Neurology, 75(5), 644–658. doi:10.1002/ana.24134.
Liu, B., Gao, H. M., & Hong, J. S. (2003). Parkinson’s disease and exposure to infectious agents and pesticides and the occurrence of brain injuries: Role of neuroinflammation. Environmental Health Perspectives, 111(8), 1065–1073.
Liu, B., Gao, H. M., Wang, J. Y., Jeohn, G. H., Cooper, C. L., & Hong, J. S. (2002). Role of nitric oxide in inflammation-mediated neurodegeneration. Annals of the New York Academy of Sciences, 962, 318–331.
Liu, B., Hattori, N., Zhang, N. Y., Wu, B., Yang, L., Kitagawa, K., et al. (2005). Anxiolytic agent, dihydrohonokiol-B, recovers amyloid beta protein-induced neurotoxicity in cultured rat hippocampal neurons. Neuroscience Letters, 384(1–2), 44–47.
Lull, M. E., & Block, M. L. (2010). Microglial activation and chronic neurodegeneration. Neurotherapeutics, 7(4), 354–365. doi:10.1016/j.nurt.2010.05.014.
Maruyama, Y., Kuribara, H., Morita, M., Yuzurihara, M., & Weintraub, S. T. (1998). Identification of magnolol and honokiol as anxiolytic agents in extracts of saiboku-to, an oriental herbal medicine. Journal of Natural Products, 61(1), 135–138.
Oh, J. H., Kang, L. L., Ban, J. O., Kim, Y. H., Kim, K. H., Han, S. B., et al. (2009). Anti-inflammatory effect of 4-O-methylhonokiol, compound isolated from magnolia officinalis through inhibition of NF-kappaB [corrected]. Chemico-Biological Interactions, 180(3), 506–514. doi:10.1016/j.cbi.2009.03.014.
Pan, X. D., Chen, X. C., Zhu, Y. G., Zhang, J., Huang, T. W., Chen, L. M., et al. (2008). Neuroprotective role of tripchlorolide on inflammatory neurotoxicity induced by lipopolysaccharide-activated microglia. Biochemical Pharmacology, 76(3), 362–372. doi:10.1016/j.bcp.2008.05.018.
Ribeiro, R., Wen, J., Li, S., & Zhang, Y. (2013). Involvement of ERK1/2, cPLA2 and NF-kappaB in microglia suppression by cannabinoid receptor agonists and antagonists [Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, Non-P.H.S.]. Prostaglandins & Other Lipid Mediators, 100–101, 1–14. doi:10.1016/j.prostaglandins.2012.11.003.
Shen, S., Yu, S., Binek, J., Chalimoniuk, M., Zhang, X., Lo, S. C., et al. (2005). Distinct signaling pathways for induction of type II NOS by IFNgamma and LPS in BV-2 microglial cells. Neurochemistry International, 47(4), 298–307. doi:10.1016/j.neuint.2005.03.007.
Sheng, W., Zong, Y., Mohammad, A., Ajit, D., Cui, J., Han, D., et al. (2011). Pro-inflammatory cytokines and lipopolysaccharide induce changes in cell morphology, and upregulation of ERK1/2, iNOS and sPLA(2)-IIA expression in astrocytes and microglia [Research Support, N.I.H., Extramural Research Support, N.I.H., Intramural]. Journal of Neuroinflammation, 8, 121. doi:10.1186/1742-2094-8-121.
Simonyi, A., Chen, Z., Jiang, J., Zong, Y., Chuang, D. Y., Gu, Z., et al. (2015). Inhibition of microglial activation by elderberry extracts and its phenolic components. Life Sciences, 128, 30–38. doi:10.1016/j.lfs.2015.01.037.
Sun, G. Y., Chen, Z., Jasmer, K. J., Chuang, D. Y., Gu, Z., Hannink, M., et al. (2015). Quercetin attenuates inflammatory responses in BV-2 microglial cells: Role of MAPKs on the Nrf2 pathway and induction of heme oxygenase-1. PLoS ONE, 10(10), e0141509. doi:10.1371/journal.pone.0141509.
Sun, A. Y., Wang, Q., Simonyi, A., & Sun, G. Y. (2008). Botanical phenolics and brain health. Neuromolecular Medicine, 10(4), 259–274. doi:10.1007/s12017-008-8052-z.
Sun, A. Y., Wang, Q., Simonyi, A., & Sun, G. Y. (2011). Botanical phenolics and neurodegeneration. In I. F. F. Benzie & S. Wachtel-Galor (Eds.), Herbal medicine: Biomolecular and clinical aspects (2nd ed.). Boca Raton, FL: CRC Press.
Vana, A. C., Li, S., Ribeiro, R., Tchantchou, F., & Zhang, Y. (2011). Arachidonyl trifluoromethyl ketone ameliorates experimental autoimmune encephalomyelitis via blocking peroxynitrite formation in mouse spinal cord white matter [Research Support, Non-U.S. Gov’t]. Experimental Neurology, 231(1), 45–55. doi:10.1016/j.expneurol.2011.05.014.
Wang, T., Pei, Z., Zhang, W., Liu, B., Langenbach, R., Lee, C., et al. (2005). MPP+-induced COX-2 activation and subsequent dopaminergic neurodegeneration. FASEB J, 19(9), 1134–1136. doi:10.1096/fj.04-2457fje.
Wang, S., Wang, H., Guo, H., Kang, L., Gao, X., & Hu, L. (2011). Neuroprotection of Scutellarin is mediated by inhibition of microglial inflammatory activation. Neuroscience, 185, 150–160. doi:10.1016/j.neuroscience.2011.04.005.
Watanabe, K., Watanabe, H., Goto, Y., Yamaguchi, M., Yamamoto, N., & Hagino, K. (1983). Pharmacological properties of magnolol and honokiol extracted from Magnolia officinalis: Central depressant effects. Planta Medica, 49(2), 103–108.
Wu, F., Zhang, W., Li, L., Zheng, F., Shao, X., Zhou, J., et al. (2011). Inhibitory effects of honokiol on lipopolysaccharide-induced cellular responses and signaling events in human renal mesangial cells [Research Support, Non-U.S. Gov’t]. European Journal of Pharmacology, 654(1), 117–121. doi:10.1016/j.ejphar.2010.11.022.
Xu, Q., Yi, L. T., Pan, Y., Wang, X., Li, Y. C., Li, J. M., et al. (2008). Antidepressant-like effects of the mixture of honokiol and magnolol from the barks of Magnolia officinalis in stressed rodents [Research Support, Non-U.S. Gov’t]. Progress in Neuropsychopharmacology and Biological Psychiatry, 32(3), 715–725. doi:10.1016/j.pnpbp.2007.11.020.
Yang, X., Sheng, W., Ridgley, D. M., Haidekker, M. A., Sun, G. Y., & Lee, J. C. (2015). Astrocytes regulate alpha-secretase-cleaved soluble amyloid precursor protein secretion in neuronal cells: Involvement of group IIA secretory phospholipase A2. Neuroscience, 300, 508–517. doi:10.1016/j.neuroscience.2015.05.052.
Zhang, J., Barasch, N., Li, R. C., & Sapirstein, A. (2012). Inhibition of cytosolic phospholipase A(2) alpha protects against focal ischemic brain damage in mice [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t]. Brain Research, 1471, 129–137. doi:10.1016/j.brainres.2012.06.031.
Acknowledgments
This publication was made possible by NIH Grants 2P01 AG08357 from NIA and P50AT006273 from the National Center for Complementary and Alternative Medicines (NCCAM), the Office of Dietary Supplements (ODS), and the National Cancer Institute (NCI). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIA, NCCAM, ODS, NCI, or the National Institutes of Health.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have declared that there are no competing interests.
Rights and permissions
About this article
Cite this article
Chuang, D.Y., Simonyi, A., Cui, J. et al. Botanical Polyphenols Mitigate Microglial Activation and Microglia-Induced Neurotoxicity: Role of Cytosolic Phospholipase A2 . Neuromol Med 18, 415–425 (2016). https://doi.org/10.1007/s12017-016-8419-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12017-016-8419-5