Abstract
The science of food allergy has been rapidly evolving before our eyes in the past half century. Like other allergic disorders, the prevalence of food allergies has dramatically increased, and coupled with the increased public awareness of anaphylaxis due to food allergy, this has driven an explosion in basic and clinical research in this extremely broad subject. Treatment of food allergies has evolved and practices such as food challenges have become an integral part of an allergy practice. The impact of the increase of food allergy has driven package labeling laws, legislation on emergency treatment availability in schools and other public places, and school policy. But to this day, our knowledge of the pathogenesis of food allergy is still incomplete. There are the most obvious IgE-mediated immediate hypersensitivity reactions, but then multiple previously unidentified conditions such as eosinophilic esophagitis, food protein-induced enterocolitis syndrome, milk protein allergy, food-induced atopic dermatitis, oral allergy syndrome, and others have complicated the diagnosis and management of many of our patients who are unable to tolerate certain foods. Many of these conditions are not IgE-mediated, but may be T cell-driven diseases. The role of T regulatory cells and immune tolerance and the newly discovered immunological role of vitamin D have shed light on the variable clinical presentation of food allergy and the development of new methods of immunotherapy in an example of bench-to-bedside research. Component-resolved diagnostic techniques have already begun to allow us to more precisely define the epitopes that are targeted in food allergic patients. The development of biological modulators, research on genomics and proteomics, and epigenetic techniques all offer promising avenues for new modes of therapy of food allergy in the twenty-first century.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Food allergies affect approximately 3.5–4.0 % of the world’s population [1]. Immediate-type food allergies are mediated by the production of IgE antibodies to specific proteins that occur naturally in allergenic foods. A distinction should be made between chemicals that are immunogenic and those that are allergenic. The diversity of the human immune response encompasses mechanisms that allow for the development of antibodies to any protein; thus, all proteins and many other molecules are potentially immunogenic. Not all proteins are allergenic. In a type 1 immediate hypersensitivity reaction, allergenicity is defined as the ability to stimulate the production of IgE antibodies that will provoke a clinical reaction [2, 3]. Because of the roles of genetics and a predominant host-related factor in the development of food allergies, one man’s food may truly be another man’s poison. Individuals with food allergies react adversely to the ingestion of foods and food ingredients that most consumers can safely ingest [4].
In addition to type 1 hypersensitivity reactions, there are other forms of immunological responses to foods. Immunological reactions to foods are classified into three main types based on the underlying immune mechanism and clinical manifestations. The first, already mentioned, are the immunoglobulin E (IgE)-mediated reactions, which can present with varying severity (e.g., from hives to anaphylaxis), but usually occur within 30–60 min after ingestion of the food. In addition, non-IgE-mediated reactions to food (cellular, delayed type IV hypersensitivity) occur in such diseases as celiac disease and food protein colitis or enterocolitis [5, 6]. Finally, there can be a mixture of the two aforementioned mechanisms, i.e., a mixed “IgE/non-IgE” immune-mediated mechanism. The pathogenic mechanisms behind this group of diseases are unclear. Examples of diseases with mixed mechanisms include eosinophilic esophagitis or gastroenteritis, atopic dermatitis, and food protein-induced enterocolitis syndrome (FPIES) [7, 8].
While new knowledge and new techniques have generated novel and promising methods for the diagnosis and management of food allergies, they have exposed new challenges as well. The challenges speak to determining the role of foods in non-IgE-mediated diseases, including eosinophilic esophagitis and gastroenteritis, atopic dermatitis, and food protein intolerances. The controversies and uncertainties that occur around these diseases illustrate our lack of a complete understanding of the biochemical and molecular basis for food “allergies.” Multiple studies have been performed over the last few years to elucidate the mechanisms for this group of non-IgE- or partially IgE-mediated diseases, and new techniques in molecular biology have helped drive research into new modes of therapy. The relationships between genetics and environment and the interaction between epigenetic regulation of genes has helped usher in a brave new world that offers not only new opportunities but also a completely new set of previously unrecognized risks.
Defining the Global Prevalence of Food Allergy—Challenges in the Definition of Food Allergy
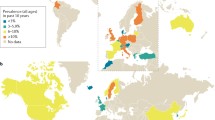
One of the most fundamental questions in food allergy is “How common are food allergies?” The answer to this question can vary extensively depending on whom you talk to or where and how the study was conducted [9]. Communities all over the world have devoted extensive resources to determine the prevalence of food allergies. However, the answers to these questions are derived from a diverse array of studies in different geographical settings and by non-standardized research designs and methodologies. These inconsistencies have made it difficult to compare the prevalence of food allergies between different communities. Different methodologies to evaluate the prevalence of food allergies include (1) collecting participants’ allergic or relative information by questionnaire, telephone survey, or clinical charts; (2) skin prick test; (3) allergen-specific IgE antibody determination; and (4) food challenge. The indiscriminant use of these technologies often yields wildly different prevalences. Well-defined guidelines and standardized protocols and algorithms must be developed in order to conduct meaningful epidemiological studies on food allergy. Once methods can be standardized, large population-based cohort studies can be conducted to provide additional insights and with reliable data for comparison between geographical regions.
Another common question is “Are food allergies on the rise?” As with all atopic diseases, it appears that this is indeed the case. However, the data on this are again difficult to analyze because of the differences in methodology and in study design. In spite of this, it is generally accepted that there is an increased prevalence, even though much of the recent attention to food allergies in the general public may be related to a heightened awareness and better patient advocacy.
The Changing Geoepidemiology of Food Allergies
The specific foods that trigger anaphylaxis include milk, egg, soy, wheat, peanut, tree nuts, fish, and shellfish. These foods account for over 90 % of cases of anaphylaxis to foods. However, the prevalence of each individual food allergy varies between children and adults. Milk, soy, and egg allergy is more common in children, while seafood allergy is more common in adults. Early studies revealed that in the Western world, the prevalence of adverse food reactions in children ranged from 6 to 8 % [10]. A study by Young et al. [11] performed in the UK revealed the prevalence in the general public to be about 1–2 %. These studies were at least partially done using double-blind placebo-controlled oral food challenges. In a meta-analysis by the RAND Corp., the prevalence of allergy to the most common foods including egg, milk, peanut, shellfish, and fish was conducted. While there were marked differences in the methodology used to conduct the 51 studies reviewed, the overall prevalence of an allergy to “any” food was 12 % when self-reported by children and 13 % in adults in a subset of studies, but the range of prevalence observed was widely variable—between 3 and 35 % [9]. It is thought that the reason for this is the widely differing methodologies used to define a food allergy.
Evidence exists for an increase in the prevalence of food allergies, but this evidence may not always be as universally convincing as one might believe. A study comparing peanut allergy in 3- to 4-year-old children during two time periods spaced 6 years apart (1989 versus 1994–1996) revealed a modest increase in peanut allergy which was not statistically significant (0.5 versus 1 %) [12].
With regard to specific allergens, differences abound between different cultures, which generate different diet, exposures, and lifestyles. In most studies from different global regions, the incidence of a strictly defined peanut allergy in pediatric studies usually ranges between 1 and 2 %, but it does appear that rates are generally lower in people who are from Singapore or Israel. In spite of the different methodologies, the prevalence of food allergy is similar between geographic regions. Some exceptions include a lower incidence of tree nut allergy and a higher incidence of seafood allergy compared to the rest of the countries surveyed [13, 14].
Overall, seafood allergy is one of the most common food allergies, affecting more than 2 % of the US population [15], and is associated with the majority of emergency department visits related to severe food allergy in adults [9, 16]. Peanut allergy is also rising in prevalence and, along with tree nuts, is considered a high-risk food for anaphylaxis. Peanut allergy has been the target of two recent developments in food allergy science, diagnostic component testing and oral immunotherapy. Some of the allergens in peanuts are lipid transfer proteins. Lipid transfer proteins (LTPs) are ubiquitous proteins that are found in many different plant species. These plants may represent some of the more common and also less common food allergies. The homozygous nature of LTPs may be partly the reason for the extensive cross-reactivity among various plant allergens [17–19]. Allergies to various foods have been reviewed extensively [20–23].
While it is common knowledge that food allergies are on the rise in westernized countries, it has also been perceived that food allergies, like other atopic diseases, are relatively uncommon in the underdeveloped or developing world. In fact, this may not be true [24]. Considering Africa, for example, recent evidence suggests that the estimated prevalence of allergic disorders in general in African countries range from 20 to 30 % [25]. This is accompanied by increasing rates over the past decade in the higher-income African countries. Notably, several food allergens exist in sub-Saharan Africa that do not present in Western or industrialized countries, including pineapple (Ghana), okra (Nigeria), and mopane worm (Botswana). Thus, Africa, while largely still underdeveloped, is already experiencing a rise in allergic diseases.
“Unusual” food allergies are also found among different cultures that do not consume a Western diet. It is known that sesame allergy is on the rise, and in various cultures, chickpea (India), buckwheat (China and Japan), and chestnut (Korea) may be more common among the native population in other parts of the world.
Food Allergy and Disease Associations
The approach to the diagnosis of food allergy is fraught with difficulties that stem from the heterogeneity of food sensitivity as a disease. In fact, most of the patients that we see with a suspicion of a food allergy turn out to not have an allergy at all. Many of the adverse reactions that people suffer from as a result of suspected food ingestion are not immunologic or allergic reactions, but are instead a form of food intolerance or toxin exposure. We alluded to the difficulties these present with when trying to determine epidemiologic data using surveys and patient questionnaires. The difficulties do not end with allergy testing to foods as all forms of allergy testing for foods carry some degree of uncertainty. The sensitivity and specificity of both skin testing and allergen-specific IgE testing of sera are too low to allow for inclusion or exclusion of the diagnosis based on these tests alone. As a result, the gold standard for the diagnosis, or what is required to clear a patient of a food allergy once the diagnosis is made, is a double-blinded placebo-controlled food challenge. But these challenges are time-consuming and labor-intensive and are inadequately compensated for by the insurance industry and government payers, so these are usually only available on a widespread basis in major academic centers or hospital-based practices [26].
Besides the issues of acquisition of epidemiological data and testing for diagnostic purposes, the concern about relevance extends from skin testing to challenge tests in that food allergies are remarkably heterogeneous and, as mentioned above, including but not limited to type 1 hypersensitivity reactions, atopic dermatitis, FPIES, and eosinophilic esophagitis. The precise or predominant pathophysiology may vary depending on the condition. The test used to delineate the existence or absence of a food allergy should be aligned with the precise disease being tested. For example, if we are attempting to delineate whether or not a patient has anaphylaxis to egg, performing a food challenge may be an appropriate method to establish the diagnosis.
On the other hand, if the patient has atopic dermatitis, which is most likely a T cell-mediated event, then perhaps testing that involves IgE or food challenge is not the best method to arrive at a definitive diagnosis. Another example relates to eosinophilic esophagitis, where food is often believed to be a trigger for the inflammation seen in the tissue. What test should be performed to identify whether the trigger is a true culprit? Over the years, investigators have used skin testing, in vitro testing, and even patch testing to attempt to identify triggers. However, the relevance of each of these tests has yet to be completely understood. This has led to confusion regarding the treatment of EoE with regard to food avoidance and reintroduction.
Other mechanisms of food sensitivities lead to rare associations. In 1962, Douglas Heiner reported a case of a cow’s milk sensitivity in which the formation of milk protein–IgG complexes (milk precipitins) leads to pulmonary hemorrhage and pulmonary hemosiderosis. Other manifestations include chronic lung disease, wheezing, anemia, eosinophilia, iron deficiency, and failure to thrive [27–30]. Other immunological diseases result from aberrant IgA to gluten-containing foods or self-tissues, as in the case of celiac disease, resulting in an autoimmune phenomenon that involves intraepithelial lymphocytes that secrete a cytokine profile that will lead to chronic inflammation and the clinical features of abdominal pain and diarrhea. The tissue damage associated with the gluten-induced enteropathy is reversible with a gluten-free diet [31]. A strong relationship between celiac disease and the HLA-DQ2 and HLA-DQ8 haplotypes has been reported [32].
Foods have also been associated with some of the classical autoimmune diseases. A literature review of the relationship between lupus and nutrition was performed to identify foods which may either ameliorate or aggravate disease activity of systemic lupus erythematosus. In this review, the authors found that a diet high in protein, fat, or calories led to increased disease activity, as well as consumption of zinc, iron, and l-canavanine, which is found in alfalfa tablets. Foods or supplements with beneficial effects included antioxidants such as vitamins A and E, fish oil, selenium, calcium along with vitamin D, as well as a number of herbal products. The limitation of this review is that there were relatively few studies, a lack of human studies, and, in many cases, the study design was often poor, with missing control groups [33].
The Hygiene Hypothesis
The hygiene hypothesis originated out of the Eastern European farm studies of the 1980s in an attempt to explain why allergies in general are more common in developed countries when compared with underdeveloped countries. The hypothesis is that cleaner living shifts the T helper cell (Th) paradigm to a predominant Th2 skewing, leading to an increased incidence of allergy. In the farm studies, those patients exposed to farm animals during early childhood appeared to have a decreased incidence of allergic diseases. It was postulated at the time that it was exposure to endotoxin that appeared to protect infants from asthma [34]. But there was conflicting evidence against this, and those patients exposed to higher doses of endotoxin in urban housing have an increased prevalence of asthma. The hygiene hypothesis has also been attributed to food allergy [35]. More evidence then surfaced that it was particular infections, such as hepatitis A or helminthic infections, that led to a decreased incidence of atopy [36, 37]. Clearly, there is more to be learned about the relationship between infection and atopy. One should also not forget that some of the observations between atopy and infection can also be extended to autoimmune diseases [38], and it also may be possible that certain infections may protect against the development of diseases such as type 1 diabetes or multiple sclerosis.
Eosinophilic Esophagitis
Before the early 1990s, this disorder was virtually nonexistent. It was originally described in patients who had recalcitrant gastroesophageal reflux disease, who underwent endoscopy and biopsy findings showed eosinophils which do not normally occur in the esophagus. The incidence of this disease has increased markedly since its first description, but the role that awareness plays in this trend is unknown. Although it was thought that this was a disease that could be attributable to a “food allergy,” it is not entirely clear what the pathogenesis may be. It is clearly more than an IgE-mediated disease, and skin testing and in vitro allergen-specific IgE testing often fail to correlate with the clinical course. Testing for delayed hypersensitivity using patch testing to foods has also not yielded promising results in determining the correct trigger.
Another unknown is the natural course of this disease as we have, as of yet, few longitudinal studies. Does chronic eosinophilia in the esophagus always lead to strictures and other structural problems in the future? This is not entirely clear. The manipulation of diet to improve symptoms and histological findings has included several different approaches, varying from extreme measures such as amino acid diets to six or seven food eliminations diets to targeting or single food elimination. None of the measures is universally successful, but the more drastic measures seem to work better, albeit with significant consequences of their own, including malnutrition and failure to thrive. More recently, genomic studies have shown that a 96-gene transcriptome may be useful as a biomarker for the disease [39]. Other therapeutic measures include maintenance on high-dose proton pump inhibitors, swallowing topical steroids and biological modulators that target IL-5 [40].
Galactose Alpha-1,3-Galactose and Allergy to Meat
The occurrence of urticaria in otherwise healthy individuals after consumption of meat has been one of the more interesting and esoteric food-associated allergic diseases in recent years. This was first reported in the mid-2000s, when a series of patients were being treated with the biological modulator cetuximab, a monoclonal antibody against epidermal growth factor used for the treatment of head and neck cancers and colorectal cancers. The report by Chung et al. [41] in the New England Journal of Medicine identified the galactose-alpha-1,3-galatose moiety in the Fc portion of the monoclonal antibody as the oligosaccharide that triggered the cross-reacting allergic reaction. It was shown that antibodies to galactose-alpha-1,3-galactose existed prior to exposure to cetuximab. Cetuximab is a monoclonal antibody that is produced in the mouse cell line SP2/0, and the cell line expresses the gene for alpha-1,3-galatosyltranferase. Subsequently, it was observed that people who were bitten by a tick known as the “lone star tick” also developed a hypersensitivity reaction upon exposure to meat [42]. The rash was atypical of an IgE reaction because it often was delayed. It is believed that the delay is due to the time it takes to absorb and process the food. It was noted that these patients also had antibodies to galactose-alpha-1,3-galactose. The significance of the tick bites is also not entirely clear at this time.
Component-Resolved Diagnosis in Food Allergy
Component-resolved diagnosis (CRD) or diagnostics is a technique adapted from traditional skin or in vitro-specific IgE testing, in which the specific allergenic proteins are used for detection of sensitivity. Formerly used crude or partially purified extracts have been successfully and widely used in the diagnosis of food allergy. While there are limitations, these techniques, in combination with food challenges, have been effective in establishing the existence of a food allergy and providing relevant clinical advice to patients. So what is the benefit of testing to single allergenic proteins? In vitro CRD testing is at present still significantly more expensive than traditional testing with extracts. Current research has suggested that the presence of antibodies to different specific proteins within a particular food may provide insight into the severity of an allergy or whether or not a patient would be able to pass a food challenge. For example, in the case of peanut allergy, it has been demonstrated that different populations may have sensitivities to different peanut allergens. For example, differences in sensitivity patterns in American, Swedish, and Spanish patients to Ara h1, h3, and h9 may be associated with earlier presentation of allergy to peanut (1 year versus 2 years of age). It is also now known that Bet v1 cross-reacts with Ara h9, and in these cases, the allergy to peanut may have resulted from a sensitization to another food. Correspondingly, there has been a higher incidence of severe allergic reactions reported in patients with sensitization to Ara h1, h2, and h3 compared to those sensitized to Ara h8 and Bet v1 [43–47]. Other significant component-resolved diagnostic allergenic proteins are shown in Table 1.
T Cells in Food Allergy
The Biology of Treg Cells
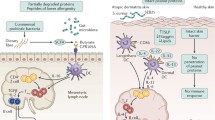
Regulatory T cells (Treg) are naturally produced in the thymus (nTreg) or induced in the peripheral tissues (iTreg). Phenotypically, both nTreg and iTreg express CD4+CD25+ and the transcription factor forkhead box p3 (Foxp3) and are actively involved in the maintenance of self-tolerance and immune homeostasis via suppressive mechanisms. Generally, Treg exerts its suppressive function on antigen-presenting cells, involving lymphocyte function-associated antigen-1 and CTLA4. Auxiliary suppressive mechanisms of Treg have been shown to involve IL-10, TGFβ, IL-35, and/or other mediators depending on the environment and the type of immune response [48, 49]. Secretion of retinoic acid by CD103+ DC in the lamina propria of the small intestine facilitates the differentiation of naive T cells to Foxp3+ cells [50]. The establishment of iTreg cells, specific to an orally administered antigen, accounts for induction of oral tolerance. Food allergy, which is an increasingly prevalent disease with potential life-threatening clinical manifestations, is considered to result from an impaired development of oral tolerance or a breakdown in existing oral tolerance [51].
Murine models indicate that “high-dose” tolerance involves deletion of effector T cells, whereas repeated exposures to “low doses” of antigen are thought to be the optimal stimulus for the development of Treg cells, which suppress immune responses through regulatory cytokines such as IL-10 and TGF-β [52]. Recently, various types of Treg cells, including TGF-β-producing Th3 cells, IL-10-producing Tr1 cells, CD4+CD25+ T cells, CD8+ suppressor T cells, and γδ T cells, have been shown to have essential roles in peripheral immune tolerance. Treg cells and IL-10 have been shown to regulate mast cell activation by inhibiting Fcε RI expression [53, 54]. Their ability to control hypersensitivity reactions involved in food allergy and their potential use as immunotherapy for food allergy have become a major focus in the field.
What Is the Role of T Regulatory Cells in Food Allergies?
Tregs play a leading role in the development of homeostasis in the immune system. The transcription factor Foxp3 is required for CD4+CD25+ Treg cells functions, and mutations in the Foxp3 gene are associated with severe food allergies and atopic dermatitis [55] in patients with immunodysregulation polyendocrinopathy enteropathy X-linked syndrome. In these patients, the numbers of CD4+CD25+FOXP3+ regulatory T cells are extremely low in the peripheral blood, and the CD4+CD25+ T cells that are present exhibit little regulatory function [55].
A recent study was conducted to determine the role of Tregs in the pathogenesis of atopic diseases by comparing the frequency and activation markers of Tregs in peripheral blood mononuclear cells between 20 children with atopic diseases and 50 healthy children. They found that the percentage of Tregs in allergic patients was significantly decreased in comparison to healthy controls. The frequency of Tregs in patients with symptoms of atopic dermatitis and/or food allergy was significantly lower than in patients without these symptoms. Furthermore, there is an association between Tregs and the IgE serum concentration [56]. Studies on the role of Tregs in asthma have not reached much consensus. Some studies have reported decreased Treg activity in patients with active allergic airway disease [57, 58], whereas others have reported no differences between asthmatics and controls [59]. It should be noted that most human studies on Tregs have relied on peripheral blood mononuclear cells, which do not necessarily reflect the immunological microenvironment of the target tissues.
Immunologically, food allergy is the result of impaired development or a breakdown of oral tolerance. Fortunately, not all food allergies persist for life. Some outgrow their food allergies or regain their tolerance, whereas others remain susceptible. About 85 % of children allergic to foods such as cow’s milk, egg, wheat and other cereal grains, and soy “outgrow” (develop tolerance) their allergy, whereas only 15–20 % of children allergic to peanut, tree nuts, fish, and shellfish will show spontaneous tolerance. The reason why some food allergies can be outgrown whereas others are not is unclear, but may involve characteristics of linear versus conformational epitopes.
Several studies have specifically addressed the role of regulatory T cells in the resolution of milk allergy. For example, Karlssoon et al. [60] showed that in vivo oral milk challenges increased the number of CD4+CD25+ putative regulatory cells in milk-tolerant but not milk-reactive children. In another study, Shreffler et al. [61] investigated various groups of patients with IgE sensitization to milk who were either reactive to all forms of milk, tolerant to extensively heated milk, or tolerant to all forms of milk. The frequency of casein-specific CD4+CD25+CD27+ cells was significantly higher in those who were heated-milk-tolerant than other groups. The casein-specific T cells were of the CD4+CD25+CD27+FOXP3+ phenotype. Furthermore, depletion of CD25+ cells before in vitro proliferation stimulation significantly enhanced casein-specific effector T cell expansion, implicating the presence of T regulatory cell activity. Using Treg depletion in a T cell proliferation study, Sletten et al. [62] reported that casein-induced Treg are involved in the development of tolerance in cow’s milk allergy. Although these data suggest of a role for regulatory T cells in the natural history of milk allergy, understanding the mechanisms of the natural resolution of other food allergies will provide an understanding of common immune pathways that could be targeted therapeutically in those with persistent food allergy.
In an animal study, Balb/c mice were given high-dose beta-lactoglobulin by gavage feeding to induce tolerance [63]. Systemic sensitization and elicitation of the allergic reaction were totally inhibited in the beta-lactoglobulin-tolerant mice. Moreover, a high percentage of CD4+CD25+Foxp3+ was detected in the lavage of these mice, and in vivo depletion of CD25+ cells greatly increased the levels of beta-lactoglobulin-specific IgG1, IgG2, and IgE [63]. These data highlighted the contribution of Treg cells in inhibiting the elicitation of food allergic reactions to beta-lactoglobulin [63].
In another model of bovine beta-lactoglobulin allergy in C3H/HeOuJ mice, Kanjarawi et al. [64] reported that constitutive CD4+CD25+ regulatory T cells are important in alleviating clinical signs of immediate hypersensitivity to beta-lactogobulin. Furthermore, depletion of Treg cells resulted in more severe symptoms of systemic anaphylaxis together with enhanced levels of the mucosal mast cellprotease-1 upon oral β-lactoglobulin challenge. On the other hand, CD4+CD25+ regulatory T cells are required in maintaining tolerance and regulating the intensity of an IgE response, but are not critical in preventing sensitization in a model of peanut allergy [65].
Alternation in TGFbeta signaling pathway can affect T regulatory cell maturation and immune homeostasis. Patients with Loeys–Dietz syndrome are strongly predisposed to develop allergic disease, including asthma, food allergy, eczema, allergic rhinitis, and eosinophilic gastrointestinal disease, due to an autosomal dominant disorder caused by mutations in TGFbetaR1 and TGFbetaR2 [66]. Thus, maintenance of Tregs via the TGFbeta signaling pathway is a reasonable target for food allergy research. Based on its immunomodulatory properties, probiotics have been sought as a candidate in therapeutic approach in food allergies. Dietary supplementation of a probiotic, VSL#3, to peanut-sensitized mice was effective in ameliorating anaphylaxis and Th2-mediated inflammation via TGFbeta-induced FoxP3 Treg cells in a C3H/HeOuJ mouse model of allergic sensitization to peanut [67].
The Role of T Reg Cells in the Treatment of Food Allergies
Recently, the mechanism and significance of Treg cells to install oral tolerance was elegantly demonstrated in an ovalbumin model of food allergy. It was found that Treg cells, after their generation in lymph nodes, is necessary for Treg cells to home to the gut and to undergo local expansion to establish oral tolerance [68]. Yamashita et al. [69] demonstrated that oral tolerance to ovalbumin can be induced by the transfer of Tregs. Specifically, they showed that in the transfer model, anaphylaxis secondary to ovalbumin intake can be suppressed by transferring either whole mesenteric lymph node cells or Tregs from ovalbumin-treated mice. Furthermore, the levels of IL-4 and IL-10 mRNA expression were decreased, but IL-9 mRNA expression increased in the Treg-recipient mice [69]. With these exciting discoveries on the significance of Treg in modulation hypersensitivity reactions to food allergens and promising data in animal studies, translation of our understanding of Treg cell-induced tolerance to clinical management of food allergy should be on the foreseeable horizon.
A number of studies on the effects of dietary supplements on food allergy have implicated the contribution of Treg in modulating the food allergy response. In a randomized, double-blind, placebo-controlled study of the sublingual immunotherapy in patients with hazelnut allergy, an increase in specific IgG4 and IL-10 levels was present only in the experimental group, but not the placebo-treated group [70], hence suggesting the possibility of immunoregulation through Treg cells producing IL-10. This principle has also been demonstrated to be successful in studies using food allergy animal models. For example, mice fed a fish oil diet were reported to have reduced acute allergic symptoms in a murine model for cow’s milk allergy by suppressing the humoral response and enhancing local intestinal and systemic Treg responses when compared with mice fed with a control diet [71]. Oral feeding of Bifidobacteria as a probiotic suppressed the skewed Th2 response and increased the number of Treg in both spleen and intestines and improved intestinal barrier function in an ovalbumin mouse model of food allergy [72]. Schiavi et al. [73] successfully shifted the pathogenic Th2 response to a Th1/Treg response by using a mixture cocktail of eight probiotic strains in a murine model induced by oral sensitization with shrimp tropomyosin. Co-culturing of the probiotic mixture with spleen cells from mice sensitized with shrimp tropomysin led to a significant reduction of the Th2 cytokine profile upon allergen re-stimulation through suppression of IL-5 and IL-13 production and promoted the secretion of Treg/Th1 cytokines such as IL-10 and IFN-γ [73]. These data suggest that modifying the commensal gut flora via a Treg-mediated mechanism is capable of inhibiting a Th2 cytokine response.
To examine the role of B lymphocytes in the regulation of food allergy, Liu et al. [74] investigated the role of a subpopulation of tolerogenic B cells (CD5+CD19+CX3CR1+) in the generation of Treg cells and in the suppression of food allergy-induced intestinal inflammation in mice. Co-culturing of tolerogenic B cells with Th0 cells generated CD4+CD25+Foxp3+ Tregs. Furthermore, adoptive transfer with the tolerogenic B cells suppressed the food allergy-induced intestinal Th2 pattern inflammation in mice. Their data highlighted the potential of CD5+CD19+CX3CR1+ tolerogenic B cells to induce Tregs in the intestine and suppress food allergy-related Th2 pattern inflammation in mice.
The Immunotherapy of Food Allergies
The current standard of care in food allergy is elimination of the triggering food from the diet and accessibility to epinephrine. Immunotherapy is a promising treatment approach. There is no accepted use of subcutaneous immunotherapy for food allergies, but recently, studies have indicated that oral immunotherapy to peanut or cow’s milk may be of benefit. On the other hand, no oral immunotherapy product for food allergy has been approved for clinical use in the USA [75]. While desensitization to most foods seems feasible, it remains unclear whether a permanent state of tolerance is achievable [76].
There have been many clinical trials on the use of oral immunotherapy to treat peanut allergy, but a 2012 Cochrane Database meta-analysis of oral immunotherapy for peanut allergy revealed only one randomized controlled trial [77]. A similar analysis of oral immunotherapy for milk allergy revealed that most trials were of small numbers and adverse events were common. Long-term tolerance was not demonstrable in most studies [78]. Oral immunotherapy for peanut allergy has been shown to provide prolonged unresponsiveness in children, but for only 1 month after withdrawal of the immunotherapy that had been administered for a 5-year period [79].
A more recent larger study of milk oral immunotherapy in 280 “low-risk” patients showed that a three-round oral induction therapy regimen every 4 weeks increased the patients’ tolerance for milk with minimal side effects [80]. An early study of seven patients with egg allergy was successful in desensitizing to egg protein [81, 82]. The concept was subjected to a larger trial published in 2012 on the successful desensitization to egg using oral immunotherapy in 40 children compared to 15 placebo subjects. There are ongoing trials on oral immunotherapy for egg allergy [83]. There are fewer data on oral immunotherapy to other foods. However, a study investigating the benefit of using anti-IgE therapy in conjunction with milk or peanut rapid oral immunotherapy suggests that anti-IgE may reduce the incidence of adverse events [84–86]. Another study found decreased specific T cell response to egg after oral desensitization when performed in conjunction with omalizumab. Other observations included a decrease in allergen-specific CD4+ T cells, an increase in specific IgG4, and a decrease in specific IgE levels [87].
Traditional Chinese Medicine in the Treatment of Food Allergies
The use of herbal medicines that are derived from traditional Chinese medicine and other cultures is being investigated for composition, efficacy, safety, and mechanism of action in many allergic or atopic diseases [88–91]. Chinese herbal medications have been used for centuries to treat various ailments. Perhaps the most well-known and well-studied herbal formulation used in the treatment of food allergies are food allergy herbal formula FAHF-1 and FAHF-2. In 2001, Li et al. [92] reported that FAHF-1 could block peanut anaphylaxis in a murine model of peanut allergy. The mice were treated beginning at 1 week following sensitization to peanut and the therapy continued for 7 weeks. Mice were then challenged to peanut. The authors found that FAHF-1 blocked peanut anaphylaxis, decreased Th2 cytokine synthesis, and abrogated histamine release after peanut challenge. A follow-up study using FAHF-2 (which has two fewer herbal components than FAHF-1) showed similar results, with elimination of peanut anaphylaxis in a mouse model following peanut challenge associated with a reduction in the synthesis of Th2 cytokines [93].
It should be mentioned that many of the individual components in FAHF-1 and FAHF-2 themselves have been shown to have immune effects. Most have not been studied in the clinical setting, and efficacy and safety profiles are still not well understood. Hopefully, further research will answer these questions and provide an additional mode of therapy for patients with food allergy.
Vitamin D and Food Allergy
Allen et al. [94] reported in 2013 that there is a higher incidence of food allergies the further removed the population is from the equator. They hypothesized that this was due to an insufficiency in vitamin D. Their study of a population sample of 5,276 one-year-old infants showed that there was a higher incidence of allergies to peanut and egg in patients with vitamin D levels ≤50 nmol/l (adjusted odds ratios of 11.51 and 3.79, respectively). These findings were independent of eczema status. The mechanism by which vitamin D affects food allergy is not completely clear, but it has been shown that vitamin D can induce T regulatory cells to secrete IL-10, thus reducing the activity of Th2 cells [95]. The effect of inhibition of 25-OH-vitamin D1-α-hydroxylase on skin barrier permeability leads to epicutaneous sensitization to food allergens [96], which can lead to the proliferation of intestinal mast cells, which then leads to an increased intestinal barrier permeability. This can then lead to food-induced anaphylaxis [97].
In another study from Germany, maternal and cord blood vitamin D levels were found to be positively associated with the risk of food allergies in the first 2 years of life. Maternal vitamin D levels were correlated with a higher incidence of sensitization to foods under 2 years of life. Higher cord blood levels also correlated with reduced cord blood Treg cell numbers [98]. This result is in direct contradiction to the implications of the previous study, further illustrating that the relationship between vitamin D and the immune system is more complex than we understand at present.
It has also been suggested that vitamin D deficiency correlates with an increased incidence of atopic dermatitis. In a case–control study of 29 children between 2 and 12 years old, vitamin D levels were found to be lower in patients with atopic dermatitis compared to controls [99]. It is also generally accepted that in about one third of cases of moderate to severe atopic dermatitis in children, a food allergy can lead to a flare-up in disease activity. It is still poorly understood how these two observations may be connected.
In addition to food allergies and atopic dermatitis, vitamin D has also been shown to play a role in the regulation of cytokine expression in many other diseases, including heart disease and autoimmune diseases [100–103].
Summary
Because of the complexities of food reactions, and because probably only 10 % of all reported food sensitivities are mediated through an immunological pathway, establishing the diagnosis and developing a management and treatment plan become extremely challenging. In some patients, lack of an understanding of the immunological or non-immunological basis of the diseases adds complexity to this task. Because of the heterogeneity of food allergy, and the challenges of finding a single, easily targetable mechanism in any particular patient, the diagnosis and treatment may be a matter of trial and error.
Until we learn more about the pathophysiology of food allergies and the mechanisms of action of the treatment modalities that we utilize, our strategies will continue to be more of an art than a science. Guidelines put forth by various reputable institutions from all continents seem to change from year to year according to the accumulation of new data. Perhaps this is to be expected as food allergies depend on host genetics, epigenetics, and environmental exposures. It is the balance between standardization of treatment and personal medicine that will drive future versions of these recommendations, but it is likely that the heterogeneity of the disease will preclude the development of a single unifying algorithm for the treatment of food allergies [104].
References
Rona RJ, Keil T, Summers C, Gislason D, Zuidmeer L, Sodergren E et al (2007) The prevalence of food allergy: a meta-analysis. J Allergy Clin Immunol 120:638–646
Gultekin F, Doguc DK (2013) Allergic and immunologic reactions to food additives. Clin Rev Allergy Immunol 45:6–29
Bush RK, Hefle SL (1996) Food allergens. Crit Rev Food Sci Nutr 36(Suppl):S119–S163
Taylor SL, Hefle SL (2001) Ingredient and labeling issues associated with allergenic foods. Allergy 56(Suppl 67):64–69
Eigenmann PA (2009) Mechanisms of food allergy. Pediatr Allergy Immunol 20:5–11
Sabra A, Bellanti JA, Rais JM, Castro HJ, de Inocencio JM, Sabra S (2003) IgE and non-IgE food allergy. Ann Allergy Asthma Immunol 90:71–76
Mehr S, Kakakios A, Frith K, Kemp AS (2009) Food protein-induced enterocolitis syndrome: 16 year experience. Pediatrics 123:e459–e464
Sicherer SH (2000) Food protein-induced enterocolitis syndrome: clinical perspectives. J Pediatr Gastroenterol Nutr 30(Suppl):S45–S49
Sicherer SH (2011) Epidemiology of food allergy. J Allergy Clin Immunol 127:594–602
Bock SA (1987) Prospective appraisal of complaints of adverse reactions to foods in children during the first 3 years of life. Pediatrics 79:683–688
Young E, Stoneham MD, Petruckevitch A, Barton J, Rona R (1994) A population study of food intolerance. Lancet 343:1127–1130
Grundy J, Matthews S, Bateman B, Dean T, Arshad SH (2002) Rising prevalence of allergy to peanut in children: data from 2 sequential cohorts. J Allergy Clin Immunol 110:784–789
Sicherer SH, Munoz-Furlong A, Sampson HA (2004) Prevalence of seafood allergy in the United States determined by a random telephone survey. J Allergy Clin Immunol 114:159–165
Woods RK, Abramson M, Bailey M, Walters EH (2001) International prevalences of reported food allergies and intolerances. Comparisons arising from the European Community Respiratory Health Survey (ECRHS) 1991–1994. Eur J Clin Nutr 55:298–304
Leung NY, Wai CY, Shu S, Wang J, Kenny TP, Chu KH et al. (2012) Current immunological and molecular biological perspectives on seafood allergy: a comprehensive review. Clin Rev Allergy Immunol. doi:10.1007/s12016-012-8336-9
Lopata AL, O’Hehir RE, Lehrer SB (2010) Shellfish allergy. Clin Exp Allergy 40:850–858
Asero R, Pravettoni V (2013) Anaphylaxis to plant-foods and pollen allergens in patients with lipid transfer protein syndrome. Curr Opin Allergy Clin Immunol 13:379–385
Pascal M, Munoz-Cano R, Reina Z, Palacin A, Vilella R, Picado C et al (2012) Lipid transfer protein syndrome: clinical pattern, cofactor effect and profile of molecular sensitization to plant-foods and pollens. Clin Exp Allergy 42:1529–1539
Van Winkle RC, Chang C (2012) The biochemical basis and clinical evidence of food allergy due to lipid transfer proteins: a comprehensive review. Clin Rev Allergy Immunol. doi:10.1007/s12016-012-8338-7
Verma AK, Kumar S, Das M, Dwivedi PD (2013) A comprehensive review of legume allergy. Clin Rev Allergy Immunol 45:30–46
Sharp MF, Lopata AL (2013) Fish allergy: in review. Clin Rev Allergy Immunol. doi:10.1007/s12016-013-8363-1
Hajeb P, Selamat J (2012) A contemporary review of seafood allergy. Clin Rev Allergy Immunol 42:365–385
Ho MH, Wong WH, Chang C (2012) Clinical spectrum of food allergies: a comprehensive review. Clin Rev Allergy Immunol. doi:10.1007/s12016-012-8339-6
van Ree R, Yazdanbakhsh M (2007) Allergic disorders in African countries: linking immunology to accurate phenotype. Allergy 62:237–246
Kung SJ, Steenhoff AP, Gray C (2012) Food allergy in Africa: myth or reality? Clin Rev Allergy Immunol. doi:10.1007/s12016-012-8341-z
Shu SA, Chang C, Leung PS (2012) Common methodologies in the evaluation of food allergy: pitfalls and prospects of food allergy prevalence studies. Clin Rev Allergy Immunol. doi:10.1007/s12016-012-8337-8
Torres MJ, Giron MD, Corzo JL, Rodriguez F, Moreno F, Perez E et al (1996) Release of inflammatory mediators after cow’s milk intake in a newborn with idiopathic pulmonary hemosiderosis. J Allergy Clin Immunol 98:1120–1123
Williams S, Craver RD (1989) Cow’s milk-induced pulmonary hemosiderosis. J La State Med Soc 141:19–22
Stafford HA, Polmar SH, Boat TF (1977) Immunologic studies in cow’s milk-induced pulmonary hemosiderosis. Pediatr Res 11:898–903
Heiner DC, Sears JW, Kniker WT (1962) Multiple precipitins to cow’s milk in chronic respiratory disease. A syndrome including poor growth, gastrointestinal symptoms, evidence of allergy, iron deficiency anemia, and pulmonary hemosiderosis. Am J Dis Child 103:634–654
Rubio-Tapia A, Hill ID, Kelly CP, Calderwood AH, Murray JA (2013) ACG clinical guidelines: diagnosis and management of celiac disease. Am J Gastroenterol 108:656–676, quiz 77
Denham JM, Hill ID (2013) Celiac disease and autoimmunity: review and controversies. Curr Allergy Asthma Rep 13:347–353
Brown AC (2000) Lupus erythematosus and nutrition: a review of the literature. J Ren Nutr 10:170–183
Tantisira KG, Weiss ST (2001) Childhood infections and asthma: at the crossroads of the hygiene and Barker hypotheses. Respir Res 2:324–327
Helm RM, Burks AW (2000) Mechanisms of food allergy. Curr Opin Immunol 12:647–653
Matricardi PM, Bonini S (2000) High microbial turnover rate preventing atopy: a solution to inconsistencies impinging on the hygiene hypothesis? Clin Exp Allergy 30:1506–1510
von Hertzen LC (2000) Puzzling associations between childhood infections and the later occurrence of asthma and atopy. Ann Med 32:397–400
Rook GA (2012) Hygiene hypothesis and autoimmune diseases. Clin Rev Allergy Immunol 42:5–15
Wen T, Stucke EM, Grotjan TM, Kemme KA, Abonia JP, Putnam PE et al (2013) Molecular diagnosis of eosinophilic esophagitis by gene expression profiling. Gastroenterology 145:1289–1299
Papadopoulou A, Koletzko S, Heuschkel R, Dias JA, Allen KJ, Murch SH et al (2014) Management guidelines of eosinophilic esophagitis in childhood. J Pediatr Gastroenterol Nutr 58:107–118
Chung CH, Mirakhur B, Chan E, Le QT, Berlin J, Morse M et al (2008) Cetuximab-induced anaphylaxis and IgE specific for galactose-alpha-1,3-galactose. N Engl J Med 358:1109–1117
Kennedy JL, Stallings AP, Platts-Mills TA, Oliveira WM, Workman L, James HR et al (2013) Galactose-alpha-1,3-galactose and delayed anaphylaxis, angioedema, and urticaria in children. Pediatrics 131:e1545–e1552
Vereda A, van Hage M, Ahlstedt S, Ibanez MD, Cuesta-Herranz J, van Odijk J et al (2011) Peanut allergy: clinical and immunologic differences among patients from 3 different geographic regions. J Allergy Clin Immunol 127:603–607
Kim JS, Nowak-Wegrzyn A (2011) Component-resolved diagnostics: shedding light on the so-called ‘squishy science’ of food allergies? Int Arch Allergy Immunol 156:231–233
Dang TD, Tang M, Choo S, Licciardi PV, Koplin JJ, Martin PE et al (2012) Increasing the accuracy of peanut allergy diagnosis by using Ara h 2. J Allergy Clin Immunol 129:1056–1063
Nicolaou N, Murray C, Belgrave D, Poorafshar M, Simpson A, Custovic A (2011) Quantification of specific IgE to whole peanut extract and peanut components in prediction of peanut allergy. J Allergy Clin Immunol 127:684–685
Nicolaou N, Poorafshar M, Murray C, Simpson A, Winell H, Kerry G et al (2010) Allergy or tolerance in children sensitized to peanut: prevalence and differentiation using component-resolved diagnostics. J Allergy Clin Immunol 125:191–197, e1–13
Bilate AM, Lafaille JJ (2012) Induced CD4+Foxp3+ regulatory T cells in immune tolerance. Annu Rev Immunol 30:733–758
Josefowicz SZ, Lu LF, Rudensky AY (2012) Regulatory T cells: mechanisms of differentiation and function. Annu Rev Immunol 30:531–564
Coombes JL, Siddiqui KR, Arancibia-Carcamo CV, Hall J, Sun CM, Belkaid Y et al (2007) A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J Exp Med 204:1757–1764
Scurlock AM, Vickery BP, Hourihane JO, Burks AW (2010) Pediatric food allergy and mucosal tolerance. Mucosal Immunol 3:345–354
Vickery BP, Scurlock AM, Jones SM, Burks AW (2011) Mechanisms of immune tolerance relevant to food allergy. J Allergy Clin Immunol 127:576–584, quiz 85–86
Kashyap M, Thornton AM, Norton SK, Barnstein B, Macey M, Brenzovich J et al (2008) Cutting edge: CD4 T cell–mast cell interactions alter IgE receptor expression and signaling. J Immunol 180:2039–2043
Kennedy Norton S, Barnstein B, Brenzovich J, Bailey DP, Kashyap M, Speiran K et al (2008) IL-10 suppresses mast cell IgE receptor expression and signaling in vitro and in vivo. J Immunol 180:2848–2854
Torgerson TR, Ochs HD (2007) Immune dysregulation, polyendocrinopathy, enteropathy, X-linked: forkhead box protein 3 mutations and lack of regulatory T cells. J Allergy Clin Immunol 120:744–750, quiz 51–52
Stelmaszczyk-Emmel A, Zawadzka-Krajewska A, Szypowska A, Kulus M, Demkow U (2013) Frequency and Activation of CD4+CD25 FoxP3+ regulatory T cells in peripheral blood from children with atopic allergy. Int Arch Allergy Immunol 162:16–24
Hartl D, Koller B, Mehlhorn AT, Reinhardt D, Nicolai T, Schendel DJ et al (2007) Quantitative and functional impairment of pulmonary CD4+CD25hi regulatory T cells in pediatric asthma. J Allergy Clin Immunol 119:1258–1266
Lin YL, Shieh CC, Wang JY (2008) The functional insufficiency of human CD4+CD25high T-regulatory cells in allergic asthma is subjected to TNF-alpha modulation. Allergy 63:67–74
Lloyd CM, Hawrylowicz CM (2009) Regulatory T cells in asthma. Immunity 31:438–449
Karlsson MR, Rugtveit J, Brandtzaeg P (2004) Allergen-responsive CD4+CD25+ regulatory T cells in children who have outgrown cow’s milk allergy. J Exp Med 199:1679–1688
Shreffler WG, Wanich N, Moloney M, Nowak-Wegrzyn A, Sampson HA (2009) Association of allergen-specific regulatory T cells with the onset of clinical tolerance to milk protein. J Allergy Clin Immunol 123:43–52, e7
Sletten GB, Halvorsen R, Egaas E, Halstensen TS (2007) Memory T cell proliferation in cow’s milk allergy after CD25+ regulatory T cell removal suggests a role for casein-specific cellular immunity in IgE-mediated but not in non-IgE-mediated cow’s milk allergy. Int Arch Allergy Immunol 142:190–198
Adel-Patient K, Wavrin S, Bernard H, Meziti N, Ah-Leung S, Wal JM (2011) Oral tolerance and Treg cells are induced in BALB/c mice after gavage with bovine beta-lactoglobulin. Allergy 66:1312–1321
Kanjarawi R, Dercamp C, Etchart N, Adel-Patient K, Nicolas JF, Dubois B et al (2011) Regulatory T cells control type I food allergy to beta-lactoglobulin in mice. Int Arch Allergy Immunol 156:387–396
van Wijk F, Wehrens EJ, Nierkens S, Boon L, Kasran A, Pieters R et al (2007) CD4+CD25+ T cells regulate the intensity of hypersensitivity responses to peanut, but are not decisive in the induction of oral sensitization. Clin Exp Allergy 37:572–581
Frischmeyer-Guerrerio PA, Guerrerio AL, Oswald G, Chichester K, Myers L, Halushka MK et al (2013) TGFbeta receptor mutations impose a strong predisposition for human allergic disease. Sci Transl Med 5:195ra94
Barletta B, Rossi G, Schiavi E, Butteroni C, Corinti S, Boirivant M et al. (2013) Probiotic VSL#3-induced TGF-beta ameliorates food allergy inflammation in a mouse model of peanut sensitization through the induction of regulatory T cells in the gut mucosa. Mol Nutr Food Res 57:2233–2244
Hadis U, Wahl B, Schulz O, Hardtke-Wolenski M, Schippers A, Wagner N et al (2011) Intestinal tolerance requires gut homing and expansion of FoxP3+ regulatory T cells in the lamina propria. Immunity 34:237–246
Yamashita H, Takahashi K, Tanaka H, Nagai H, Inagaki N (2012) Overcoming food allergy through acquired tolerance conferred by transfer of Tregs in a murine model. Allergy 67:201–209
Enrique E, Pineda F, Malek T, Bartra J, Basagana M, Tella R et al (2005) Sublingual immunotherapy for hazelnut food allergy: a randomized, double-blind, placebo-controlled study with a standardized hazelnut extract. J Allergy Clin Immunol 116:1073–1079
van den Elsen LW, van Esch BC, Hofman GA, Kant J, van de Heijning BJ, Garssen J et al (2013) Dietary long chain n-3 polyunsaturated fatty acids prevent allergic sensitization to cow’s milk protein in mice. Clin Exp Allergy 43:798–810
Zhang LL, Chen X, Zheng PY, Luo Y, Lu GF, Liu ZQ et al (2010) Oral Bifidobacterium modulates intestinal immune inflammation in mice with food allergy. J Gastroenterol Hepatol 25:928–934
Schiavi E, Barletta B, Butteroni C, Corinti S, Boirivant M, Di Felice G (2011) Oral therapeutic administration of a probiotic mixture suppresses established Th2 responses and systemic anaphylaxis in a murine model of food allergy. Allergy 66:499–508
Liu ZQ, Wu Y, Song JP, Liu X, Liu Z, Zheng PY et al. (2013) Tolerogenic CX3CR1 B cells suppress food allergy-induced intestinal inflammation in mice. Allergy 68:1241–1248
Fleischer DM, Burks AW, Vickery BP, Scurlock AM, Wood RA, Jones SM et al (2013) Sublingual immunotherapy for peanut allergy: a randomized, double-blind, placebo-controlled multicenter trial. J Allergy Clin Immunol 131:119–127, e1–7
Scurlock AM, Jones SM (2010) An update on immunotherapy for food allergy. Curr Opin Allergy Clin Immunol 10:587–593
Nurmatov U, Venderbosch I, Devereux G, Simons FE, Sheikh A (2012) Allergen-specific oral immunotherapy for peanut allergy. Cochrane Database Syst Rev 9:CD009014
Yeung JP, Kloda LA, McDevitt J, Ben-Shoshan M, Alizadehfar R (2012) Oral immunotherapy for milk allergy. Cochrane Database Syst Rev 11:CD009542
Vickery BP, Scurlock AM, Kulis M, Steele PH, Kamilaris J, Berglund JP et al. (2013) Sustained unresponsiveness to peanut in subjects who have completed peanut oral immunotherapy. J Allergy Clin Immunol. doi:10.1016/j.jaci.2013.11.007
Levy MB, Elizur A, Goldberg MR, Nachshon L, Katz Y (2014) Clinical predictors for favorable outcomes in an oral immunotherapy program for IgE-mediated cow’s milk allergy. Ann Allergy Asthma Immunol 112:58–63, e1
Burks AW, Jones SM (2008) Egg oral immunotherapy in non-anaphylactic children with egg allergy: follow-up. J Allergy Clin Immunol 121:270–271
Buchanan AD, Green TD, Jones SM, Scurlock AM, Christie L, Althage KA et al (2007) Egg oral immunotherapy in nonanaphylactic children with egg allergy. J Allergy Clin Immunol 119:199–205
Burks AW, Jones SM, Wood RA, Fleischer DM, Sicherer SH, Lindblad RW et al (2012) Oral immunotherapy for treatment of egg allergy in children. N Engl J Med 367:233–243
Schneider LC, Rachid R, Lebovidge J, Blood E, Mittal M, Umetsu DT (2013) A pilot study of omalizumab to facilitate rapid oral desensitization in high-risk peanut-allergic patients. J Allergy Clin Immunol 132:1368–1374
Nadeau KC, Kohli A, Iyengar S, DeKruyff RH, Umetsu DT (2012) Oral immunotherapy and anti-IgE antibody-adjunctive treatment for food allergy. Immunol Allergy Clin North Am 32:111–133
Nadeau KC, Schneider LC, Hoyte L, Borras I, Umetsu DT (2011) Rapid oral desensitization in combination with omalizumab therapy in patients with cow’s milk allergy. J Allergy Clin Immunol 127:1622–1624
Bedoret D, Singh AK, Shaw V, Hoyte EG, Hamilton R, DeKruyff RH et al (2012) Changes in antigen-specific T-cell number and function during oral desensitization in cow’s milk allergy enabled with omalizumab. Mucosal Immunol 5:267–276
Chang C (2013) Is integrative medicine the next new frontier in medicine? Clin Rev Allergy Immunol 44:205–207
Chang C, Gershwin ME (2013) Integrative medicine in allergy and immunology. Clin Rev Allergy Immunol 44:208–228
DiNicola C, Kekevian A, Chang C (2013) Integrative medicine as adjunct therapy in the treatment of atopic dermatitis—the role of traditional Chinese medicine, dietary supplements, and other modalities. Clin Rev Allergy Immunol 44:242–253
Ma HD, Deng YR, Tian Z, Lian ZX (2013) Traditional Chinese medicine and immune regulation. Clin Rev Allergy Immunol 44:229–241
Li XM, Zhang TF, Huang CK, Srivastava K, Teper AA, Zhang L et al (2001) Food allergy herbal formula-1 (FAHF-1) blocks peanut-induced anaphylaxis in a murine model. J Allergy Clin Immunol 108:639–646
Srivastava KD, Kattan JD, Zou ZM, Li JH, Zhang L, Wallenstein S et al (2005) The Chinese herbal medicine formula FAHF-2 completely blocks anaphylactic reactions in a murine model of peanut allergy. J Allergy Clin Immunol 115:171–178
Allen KJ, Koplin JJ, Ponsonby AL, Gurrin LC, Wake M, Vuillermin P et al (2013) Vitamin D insufficiency is associated with challenge-proven food allergy in infants. J Allergy Clin Immunol 131:1109–1116, e1–6
Xystrakis E, Kusumakar S, Boswell S, Peek E, Urry Z, Richards DF et al (2006) Reversing the defective induction of IL-10-secreting regulatory T cells in glucocorticoid-resistant asthma patients. J Clin Invest 116:146–155
Muehleisen B, Gallo RL (2013) Vitamin D in allergic disease: shedding light on a complex problem. J Allergy Clin Immunol 131:324–329
Bartnikas LM, Gurish MF, Burton OT, Leisten S, Janssen E, Oettgen HC et al (2013) Epicutaneous sensitization results in IgE-dependent intestinal mast cell expansion and food-induced anaphylaxis. J Allergy Clin Immunol 131:451–460, e1–6
Weisse K, Winkler S, Hirche F, Herberth G, Hinz D, Bauer M et al (2013) Maternal and newborn vitamin D status and its impact on food allergy development in the German LINA cohort study. Allergy 68:220–228
El Taieb MA, Fayed HM, Aly SS, Ibrahim AK (2013) Assessment of serum 25-hydroxyvitamin d levels in children with atopic dermatitis: correlation with SCORAD index. Dermatitis 24:296–301
Arnson Y, Itzhaky D, Mosseri M, Barak V, Tzur B, Agmon-Levin N et al (2013) Vitamin D inflammatory cytokines and coronary events: a comprehensive review. Clin Rev Allergy Immunol 45:236–247
Agmon-Levin N, Theodor E, Segal RM, Shoenfeld Y (2013) Vitamin D in systemic and organ-specific autoimmune diseases. Clin Rev Allergy Immunol 45:256–266
Yang CY, Leung PS, Adamopoulos IE, Gershwin ME (2013) The implication of vitamin D and autoimmunity: a comprehensive review. Clin Rev Allergy Immunol 45:217–226
Lerner A, Shapira Y, Agmon-Levin N, Pacht A, Ben-Ami Shor D, Lopez HM et al (2012) The clinical significance of 25OH-vitamin D status in celiac disease. Clin Rev Allergy Immunol 42:322–330
Yang YH, Chiang BL (2013) Novel approaches to food allergy. Clin Rev Allergy Immunol. doi:10.1007/s12016-013-8354-2
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Leung, P.S.C., Shu, SA. & Chang, C. The Changing Geoepidemiology of Food Allergies. Clinic Rev Allerg Immunol 46, 169–179 (2014). https://doi.org/10.1007/s12016-014-8411-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12016-014-8411-5