Abstract
The main aim of oncologists worldwide is to understand and then intervene in the primary tumor initiation and propagation mechanisms. This is essential to allow targeted elimination of cancer cells without altering normal mitotic cells. Currently, there are two main rival theories describing the process of tumorigenesis. According to the Stochastic Model, potentially any cell, once defunct, is capable of initiating carcinogenesis. Alternatively the Cancer Stem Cell (CSC) Model posits that only a small fraction of undifferentiated tumor cells are capable of triggering carcinogenesis. Like healthy stem cells, CSCs are also characterized by a capacity for self-renewal and the ability to generate differentiated progeny, possibly mediating treatment resistance, thus leading to tumor recurrence and metastasis. Moreover, molecular signaling profiles are similar between CSCs and normal stem cells, including Wnt, Notch and Hedgehog pathways. Therefore, development of novel chemotherapeutic agents and proteins (e.g., enzymes and antibodies) specifically targeting CSCs are attractive pharmaceutical candidates. This article describes small molecule inhibitors of stem cell pathways Wnt, Notch and Hedgehog, and their recent chemotherapy clinical trials.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
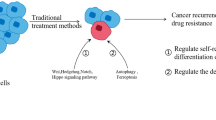
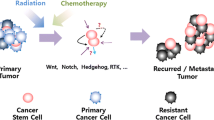
Tumors are considered as hierarchically organized systems with heterogeneous cell populations, including tumor-initiating, stromal, endothelial, hematopoietic and infiltrating cells with variable self-renewal capabilities, differentiation and tumor propagation potential [1–3]. There are two main rival theories explaining the process of tumorigenesis: the Cancer Stem Cell (CSC) and Stochastic Models (Fig. 1). The Stochastic Model (or clonal evolution) suggests that many tumor cells have the same potential to give a rise to cancer growth and metastasis [4, 5]. Many years later Canadian researchers found that only a small subset of acute myeloid leukemia (AML) cancer cells were capable of sustaining tumor growth after transplantation into non-obese diabetic mice with severe combined immunodeficiency disease (NOD/SCID) [6, 7]. This resulted in the development of the CSC model suggesting that some cancer cell populations are reminiscent of somatic stem cells. According to this model cancer develops from small populations of tumor initiating cells through the accumulation of genetic, epigenetic and somatic defects, and altered signaling within the cell’s micro-environment [8]. These altered cells are intermixed with the bulk of tumor cells, which may require a different treatment strategy for effective inhibition and suppression of tumor growth and relapse. However, probably both premises play a role in the cancer development and the predominance of a particular mechanism of cancerogenesis depends on micro-environmental cues, cancer types, tumor development stage and other factor types [2, 9, 10].
A schematic comparison between the CSC and Stochastic models. In the Stochastic model all cells within a tumor are biologically equivalent. The chances of cancer development are stochastically dependent on the environmental cues, accumulated mutations and epigenetic abnormalities. Any cell within the tumor bulk has an equal potential to initiate tumorigenesis and differentiate into different subsets of cancer cells. Meanwhile, the Cancer Stem Cell model assumes that cancer cells can be divided into distinct cell populations, where only cancer stem cells are capable to initiate tumorigenesis
There are several well characterized molecular signaling cascades in CSCs including Wnt, Notch and Hedgehog pathways [11]. These pathways are broadly involved in self-renewal, proliferation and differentiation mechanisms of CSC. The existence of different populations of heterogeneous cells with different molecular signaling profiles and cell surface phenotypes may be largely responsible for the frequent failure of conventional treatments, contributing to frequent recurrence and relapse. This article will discuss these three CSC signaling pathways and the novel chemotherapeutic candidate drugs that target crucial components of the cascades.
Wnt Pathway-Targeting Chemotherapeutic Drugs
Understanding normal cellular homeostasis is a prerequisite for understanding the molecular mechanisms underlying the origin and development of tumor cells. One of the key signaling cascades involved in cell proliferation and stem cell renewal processes is the Wnt pathway. This signaling cascade can be subdivided into either β-catenin dependent or independent pathways. Both pathways regulate determination of cell fate, proliferation and differentiation mechanisms [12], as well as cell polarity and motility processes [13]. Meanwhile, the dysregulation of these mechanisms has been reported to be the main factor causing development of various cancer types including colorectal cancer (CRC) [14], acute myeloid leukemia (AML) [15], chronic myeloid leukemia (CML) [16], gastric cancer [17] and many others.
The signaling cascade begins when Wnt ligands bind to the Frizzled (Fz) transmembrane receptor. The complex cooperates with another transmembrane lipoprotein receptor related protein (LRP). Formation of this triple complex inhibits glycogen synthase kinase-3β (GSK-3β) activities, resulting in non-phosphorylated β-catenin cytoplasmic accumulation [18]. Non-phosphorylated β-catenin translocates into the nucleus, where it regulates gene transcription by binding to other transcription cofactors including lymphoid enhancer-binding factors (LEF), T-cell transcription factor (TCF), cAMP response element-binding protein (CREB) [19, 20]. Formation of the machinery triggers expression of downstream targets of the Wnt signaling pathway such as c-MYC, Axin-2 and ASCL2 [21]. Whereas the absence of Wnt ligand results in the formation of the β-catenin destruction complex, which is comprised of GSK-3β, casein kinase 1-alpha (CK1α), axin and adenomatous polyposis coli (APC), causing phosphorylation of β-catenin and degradation in endosomes.
Given the importance of the Wnt pathway in cancer progression, there are a number of chemotherapeutic agents in pre-clinical and clinical trial stages (Table 1). In a series of in vitro cytotoxicity tests and in vivo activity experiments conducted in zebrafish, Chen and colleagues [22] discovered two major types of Wnt pathway antagonists: Wnt ligand production inhibitors (IWPs) and Wnt response inhibitors (IWR). IWP-1 and IWP-2 molecules inhibit the membrane-bound acyltransferase Porcupine, which modifies Wnt ligands through palmitoylation and preventing accumulation of β-catenin molecules in the cytoplasm. This modification seems to be essential for the ligand’s signaling activity, such as IWR induced stabilization of Axin molecules, which in turn leads to the suppression of Wnt dependent signaling cascades [22]. Moreover, IWR suppressed Wnt-dependent regeneration processes in resected caudal fin of zebrafish can be reversed by washing out the drug [22]. Recently, Waaler and his colleagues have synthetized JW74 and JW67, two novel Wnt pathway antagonists [23]. Both molecules decreased the level of active β-catenin and increased expression levels of Axin2 molecules. Several molecules like 2,4-diamino-quinazoline and PNU74654 [24, 25] can also act as antagonists of β-catenin and TCF4. A similar effect was achieved by XAV939, which inhibits β-catenin signaling via interactions with the type 1 and 2 tankyrase-binding domain (TBD) of the Axin molecule [26]. However, these drugs have shown low selectivity, therefore further development and optimization is required. Other potential drugs from this series of molecules are ICG-001 analogues, which have shown some promising effects against various cancer stem cell types [27].
Several molecules have been reported to prevent activation of Wnt signaling by specifically targeting the PDZ domain of Dvl. This domain is a protein-protein interaction motif that directly binds to the Fz receptor, thereby activating the signaling cascade [28]. Three potential drugs NSC668036, FJ9 and 3289–8625 have shown to inhibit Dvl and Fz protein-protein interactions. Efficacies of these drugs are due to further validation on cancer stem cell lines [28].
Non-steroidal anti-inflammatory drugs (NSAIDs) have also proven to be effective against various cancer types [29] (Table 1). The majority of NSAIDs target cyclooxygenase type 1 and 2 with consequent prevention of nuclear β-catenin accumulation. For instance, sulindac molecules have demonstrated significantly decreasing nuclear localization of β-catenin in patients with familial adenomatous polyposis (FAP) [30].
Chemotherapeutic Agents Targeting Hedgehog Signaling
The Hedgehog (Hh) signaling pathway is an essential signaling cascade mediating cell polarity and migration mechanisms, regulating maintenance of stemness properties and guiding SC differentiation [31]. There are three homologues of the Hh ligand: Sonic Hedgehog (Shh), Indian Hedgehog (Ihh) and Desert Hedgehog (Dhh) [32]. The pathway initiates upon binding of Hh to the Patched 1 (Ptch1) receptor. Formation of the complex triggers release of Smoothened (Smo), which drives activation of Gli zinc-finger transcription factor family members (Gli1, Gli2, Gli3). It results in their nuclear translocation and expression of target genes [33].
There are several drugs targeting Hh pathway components that are in preclinical and clinical trial stages (Table 2). The naturally occurring Hh-specific inhibitor, cyclopamine, inhibits Smo activities in Hh-pathway-related tumors such as medulloblastoma, basal cell carcinoma, rhabdomyosarcoma and others [34]. Moreover, in vitro experiments showed a significant decrease of insulin-like growth factor binding protein 6 (IGFBP6) and proliferating cell nuclear antigen (PCNA), simultaneously increasing BCL2-antagonist/killer 1 (Bak1) and BCL2-associated X (Bax) proteins in SW116 cells treated with cyclopamine, which leads to decreased proliferation and initiation of apoptotic mechanisms [35]. Other Smo-inhibiting synthetic molecules, such as GDC-449, IPI-926, Cur-61414 and BMS-833923 are also at pre-clinical and clinical stages. GDC-0449 has recently entered phase II clinical trials on recurrent medulloblastoma, glioma, gastric carcinoma, breast cancer, prostate carcinoma, lung carcinoma and others [36] (Table 2).
Apart from trials with advanced stage patients, the drugs have also been tested on several types of cancer stem cells. The drug has been administered to patients with metastatic cancer, who had previously been treated with FOLFOX (combination of folinic acid, fluorouracil and oxaliplatin) or FOLFIRI (combination of folinic acid, fluorouracil and irinotecan) [37]. LDE225 (Novartis) and PF-04449913 (Pfizer) have also demonstrated some promising results presented in preliminary data [38]. Phase I trials on patients with advanced solid tumors have shown no dose-limiting toxicities at all given dosages, including up to 800 mg daily [38]. Moreover, decreased expression levels of Gli-1 mRNA in skin and medulloblastomas provided evidence for in vivo Hh pathway inhibition [38]. Phase I trials of PF-04449913 in patients with hematologic malignancies were conducted to determine DLTs and phase II dose recommendations [39]; overall the drug was reported to be tolerated well and even indicated some efficacy against chronic myeloid leukemia patients.
Notch Signaling Pathway Inhibitors
The Notch cascade, may also contribute to dysregulation of homeostasis in cancer stem cells. Mammals possess four Notch receptor homologues (1, 2, 3 and 4) that interact with two different families of ligands: Delta-like ligands (DLLs) and Jagged ligands [11, 40]. Upon formation of the ligand-receptor complexes, conformational changes of the receptor lead to the exposure of cleavage sites to metalloprotease and γ-secretase, releasing the Notch intracellular domain (NICD). The NICD translocates to the nucleus and initiates transcription of target genes including HES (hairy enhancer of split), Myc and p21. Expression levels of Jagged ligands, Notch1 and HES1 were comparable to or slightly higher in cancer stem cells than that in normal intestinal crypt cells [11, 41, 42]. However, another study conducted by Meng and colleagues [42], revealed a positive correlation in HES1, Notch1 and NICD gene expression with the grade of colon cancer progression. Moreover, overexpression of these genes is hypothesized to be involved in chemo-resistance of colon cancer cells.
Notably, γ-secretase inhibition has become a common target for drug development. There are several gamma-secretase inhibitors currently being developed, which inhibit gamma-secretase mediated Notch cleavage in various types of tumors (Table 3). RO4929097 is a gamma-secretase inhibitor in phase I studies in neuroendocrine carcinoma and melanoma patients. The first phase II trial on refractory CRC patients showed a very good tolerability, with minor toxicity levels [43]. The drug showed tumor inhibiting properties in patients with melanomas [43]. The authors suggested that RO4929097 would be more effective in combination with other drugs. Initial experiments of another γ-secretase inhibitor, MK-0752, on T-cell acute lymphoblastic leukemia patients were unsuccessful due to the dose limited toxicity (DLT). Most of the patients had diarrhea and other gastrointestinal symptoms [44]. However, in another phase I trial of MK-0752 in children with refractory malignant CNS tumors, DLT was not observed [45]. Therefore these facts require further investigations and clarification.
Another essential element of the Notch pathway is the DLL4 ligand. It has been demonstrated to play a crucial role in vascularization of tumors [46]. It is believed that the blockade of the vascular endothelial growth factor (VEGF) receptor with monoclonal antibodies has a major therapeutic potential in inhibition of tumor driven angiogenesis [47]. However, severe forms proceed even when these receptors are blocked. Therefore additional angiogenesis-targeted treatments are required. Such therapy had been shown to be feasible by Noguera-Troise and colleagues who demonstrated the importance of DLL4 in the inhibition of tumor angiogenesis and growth in mice [48]. However, the blockade of the DLL4/Notch pathway resulted in enhanced formation of non-functional tumor vessels, thus the DLL4 antibody was concluded to be a negative regulator of tumor vascular growth.
The effect of the anti-DLL4 antibody on xenograft models of CRC patients with oncogenic KRAS mutations has been tested as a combination drug therapy [49]. KRAS mutations in CRC patients are common and are associated with treatment resistance to anti-EGFR therapy. Anti-DLL4 alone, as well as in combination with widely used chemotherapeutic drugs (such as irinotecan), has decreased tumor cell proliferation and angiogenesis in both wild type and KRAS mutant mouse tissue. These results indicate the utility of DLL4 inhibition on treating CRC xenograft tumors. Despite quite a number of experiments and positive effects on cancer patients with this agent there are no reports on dosage and administration regimes for clinical development.
Conclusions and Further Perspectives
The CSC model provides a potential explanation of tumor initiation, progression and metastasis mechanisms in many cancer types. The logic is that failure to eliminate the subset of CSCs within the tumor bulk leads to cancer recurrence, chemotherapy resistance and metastasis. Recent investigations have provided insights into the role and mechanisms of Wnt, Hh and Notch pathways in the development of a number of cancer types. Higher activity of these pathways in CSCs compared to normal somatic cells, as well as the interplay between the molecular pathway components, may contribute to the cellular diversity and complexity of the problem. It is important to emphasize that a number of signaling cascades highly expressed in normal stem cells may also be upregulated in CSCs [50], therefore contributing to CSC driven tumor development and recurrence mechanisms. This fact has hindered the development of effective cancer stem cell-specific therapies. The development of these novel therapeutic approaches may be complicated by significant issues. Despite pioneering works in the field, when scientists used the cell surface phenotype for enrichment and investigations of CSCs, some recent investigations suggest that CSCs undergo dynamic changes at the level of biomarker expression during tumorigenesis [51, 52]. Interestingly, the accumulation of genetic aberrations and instabilities, a common feature of cancer cells, recently has also been extrapolated to cancer progenitor cells [53] suggesting novel mechanisms of cancer transformation and development. For example, chromosomal instabilities and increased expression of c-Myc have been detected in in vivo experiments on fibrosarcoma [54] and non-tumorigenic neural cells [55] leading to the acquaintance of CD133+ phenotype and increased ability to develop cranial malignancies. This makes it difficult to monitor the effects of biologic and therapeutic effects of current drugs on CSCs. Nonetheless, exploring and understanding pathway cross-talk mechanisms may also provide a platform for designing and developing new experimental drugs. It will widen the perspectives of therapeutic options and treatment regimens for effective inhibition of tumor development and relapse.
References
Reya, T., Morrison, S. J., Clarke, M. F., & Weissman, I. L. (2001). Stem cells, cancer, and cancer stem cells. Nature, 414, 105–11.
Shackleton, M., Quintana, E., Fearon, E. R., & Morrison, S. J. (2009). Heterogeneity in cancer: cancer stem cells versus clonal evolution. Cell, 138, 822–9.
Dick, J. E. (2008). Stem cell concepts renew cancer research. Blood, 112, 4793–807.
Iyer, K. S., & Saksena, V. N. (1970). A stochastic model for the growth of cells in cancer. Biometrics, 26, 401–10.
Odoux, C., Fohrer, H., Hoppo, T., et al. (2008). A stochastic model for cancer stem cell origin in metastatic colon cancer. Cancer Research, 68, 6932–41.
Lapidot, T., Sirard, C., Vormoor, J., et al. (1994). A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature, 367, 645–8.
Bonnet, D., & Dick, J. E. (1997). Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nature Medicine, 3, 730–7.
Al-Hajj, M., & Clarke, M. F. (2004). Self-renewal and solid tumor stem cells. Oncogene, 23, 7274–82.
Takebe, N., & Ivy, S. P. (2010). Controversies in cancer stem cells: targeting embryonic signaling pathways. Clinical Cancer Research, 16, 3106–12.
Wang, W. K., Quan, Y., Fu, Q. B., et al. (2014). Dynamics between Cancer Cell Subpopulations Reveals a Model Coordinating with Both Hierarchical and Stochastic Concepts. PLoS One, 9, 1.
Takebe, N., Harris, P. J., Warren, R. Q., & Ivy, S. P. (2011). Targeting cancer stem cells by inhibiting Wnt, Notch, and Hedgehog pathways. Nature Reviews. Clinical Oncology, 8, 97–106.
Klaus, A., & Birchmeier, W. (2008). Wnt signalling and its impact on development and cancer. Nature Reviews Cancer, 8, 387–98.
Seifert, J. R. K., & Mlodzik, M. (2007). Frizzled/PCP signalling: a conserved mechanism regulating cell polarity and directed motility. Nature Reviews Genetics, 8, 126–38.
Vermeulen, L., De Sousa, E. M. F., van der Heijden, M., et al. (2010). Wnt activity defines colon cancer stem cells and is regulated by the microenvironment. Nature Cell Biology, 12, 468–76.
Simon, M., Grandage, V. L., Linch, D. C., & Khwaja, A. (2005). Constitutive activation of the Wnt/beta-catenin signalling pathway in acute myeloid leukaemia. Oncogene, 24, 2410–20.
Zhao, C., Blum, J., Chen, A., Kwon, H. Y., Jung, S. H., Cook, J. M., Lagoo, A., & Reya, T. (2007). Loss of beta-catenin impairs the renewal of normal and CML stem cells in vivo. Cancer Cell, 12, 528–41.
Ooi, C. H., Ivanova, T., Wu, J., et al. (2009). Oncogenic pathway combinations predict clinical prognosis in gastric cancer. PLoS Genetics, 5, e1000676.
MacDonald, B. T., Tamai, K., & He, X. (2009). Wnt/beta-catenin signaling: components, mechanisms, and diseases. Developmental Cell, 17, 9–26.
Takemaru, K. I., & Moon, R. T. (2000). The transcriptional coactivator CBP interacts with beta-catenin to activate gene expression. Journal of Cell Biology, 149, 249–54.
Hecht, A., Vleminckx, K., Stemmler, M. P., van Roy, F., & Kemler, R. (2000). The p300/CBP acetyltransferases function as transcriptional coactivators of beta-catenin in vertebrates. EMBO Journal, 19, 1839–50.
de Sousa, E. M. F., Vermeulen, L., Richel, D., & Medema, J. P. (2011). Targeting Wnt signaling in colon cancer stem cells. Clinical Cancer Research, 17, 647–53.
Chen, B., Dodge, M. E., Tang, W., et al. (2009). Small molecule-mediated disruption of Wnt-dependent signaling in tissue regeneration and cancer. Nature Chemical Biology, 5, 100–7.
Waaler, J., Machon, O., von Kries, J. P., et al. (2011). Novel synthetic antagonists of canonical Wnt signaling inhibit colorectal cancer cell growth. Cancer Research, 71, 197–205.
Chen, Z., Venkatesan, A. M., Dehnhardt, C. M., et al. (2009). 2,4-Diamino-quinazolines as inhibitors of beta-catenin/Tcf-4 pathway: potential treatment for colorectal cancer. Bioorganic & Medicinal Chemistry Letters, 19, 4980–3.
Trosset, J. Y., Dalvit, C., Knapp, S., et al. (2006). Inhibition of protein-protein interactions: the discovery of druglike beta-catenin inhibitors by combining virtual and biophysical screening. Proteins, 64, 60–7.
Huang, S. M., Mishina, Y. M., Liu, S., et al. (2009). Tankyrase inhibition stabilizes axin and antagonizes Wnt signalling. Nature, 461, 614–20.
Emami, K. H., Nguyen, C., Ma, H., et al. (2004). A small molecule inhibitor of beta-catenin/CREB-binding protein transcription [corrected]. Proceedings of the National Academy of Sciences of the United States of America, 101, 12682–7.
Shan, J., Shi, D. L., Wang, J., & Zheng, J. (2005). Identification of a specific inhibitor of the dishevelled PDZ domain. Biochemistry, 44, 15495–503.
Takahashi-Yanaga, F., & Kahn, M. (2010). Targeting Wnt signaling: can we safely eradicate cancer stem cells? Clinical Cancer Research : an Official Journal of the American Association for Cancer Research, 16, 3153–62.
Boon, E. M., Keller, J. J., Wormhoudt, T. A., Giardiello, F. M., Offerhaus, G. J., van der Neut, R., & Pals, S. T. (2004). Sulindac targets nuclear beta-catenin accumulation and Wnt signalling in adenomas of patients with familial adenomatous polyposis and in human colorectal cancer cell lines. British Journal of Cancer, 90, 224–9.
Ingham, P. W., & McMahon, A. P. (2001). Hedgehog signaling in animal development: paradigms and principles. Genes & Development, 15, 3059–87.
Varjosalo, M., & Taipale, J. (2008). Hedgehog: functions and mechanisms. Genes & Development, 22, 2454–72.
Amakye, D., Jagani, Z., & Dorsch, M. (2013). Unraveling the therapeutic potential of the Hedgehog pathway in cancer. Nature Medicine, 19, 1410–22.
Taipale, J., Chen, J. K., Cooper, M. K., Wang, B., Mann, R. K., Milenkovic, L., Scott, M. P., & Beachy, P. A. (2000). Effects of oncogenic mutations in Smoothened and Patched can be reversed by cyclopamine. Nature, 406, 1005–9.
Wu, J. Y., Xu, X. F., Xu, L., Niu, P. Q., Wang, F., Hu, G. Y., Wang, X. P., & Guo, C. Y. (2011). Cyclopamine blocked the growth of colorectal cancer SW116 cells by modulating some target genes of Gli1 in vitro. Hepato-Gastroenterology, 58, 1511–8.
LoRusso, P. M., Rudin, C. M., Borad, M. J., et al. (2008). A first-in-human, first-in-class, phase (ph) I study of systemic Hedgehog (Hh) pathway antagonist, GDC-0449, in patients (pts) with advanced solid tumors. Journal of Clinical Oncology, 26, 15.
Stein, A., & Bokemeyer, C. (2014). How to select the optimal treatment for first line metastatic colorectal cancer. World J Gastroenterol, 20(4), 899–907.
Lin, T. L., & Matsui, W. (2012). Hedgehog pathway as a drug target: smoothened inhibitors in development. OncoTargets and Therapy, 5, 47–58.
Jamieson, C., Cortes, J. E., Oehler, V., et al. (2011). Phase 1 dose-escalation study of PF-04449913, an oral hedgehog (Hh) inhibitor, in patients with select hematologic malignancies. Blood, 118, 195–6.
Artavanis-Tsakonas, S., Rand, M., & Lake, R. (1999). Notch signaling: cell fate control and signal integration in development. Science, 284, 770–6.
Reedijk, M., Odorcic, S., Zhang, H., et al. (2008). Activation of Notch signaling in human colon adenocarcinoma. International Journal of Oncology, 33, 1223–9.
Meng, R. D., Shelton, C. C., Li, Y. M., Qin, L. X., Notterman, D., Paty, P. B., & Schwartz, G. K. (2009). gamma-Secretase inhibitors abrogate oxaliplatin-induced activation of the Notch-1 signaling pathway in colon cancer cells resulting in enhanced chemosensitivity. Cancer Research, 69, 573–82.
Huynh, C., Poliseno, L., Segura, M. F., et al. (2011). The Novel Gamma Secretase Inhibitor RO4929097 Reduces the Tumor Initiating Potential of Melanoma. PLoS One, 6(9), e25264.
Deangelo, D. J., Stone, R. M., Silverman, L. B., et al. (2006). A phase I clinical trial of the notch inhibitor MK-0752 in patients with T-cell acute lymphoblastic leukemia/lymphoma (T-ALL) and other leukemias. Journal of Clinical Oncology, 24, 357s-s.
Fouladi, M., Stewart, C. F., Olson, J., et al. (2011). Phase I trial of MK-0752 in children with refractory CNS malignancies: a pediatric brain tumor consortium study. Journal of Clinical Oncology : Official Journal of the American Society of Clinical Oncology, 29, 3529–34.
Ridgway, J., Zhang, G., Wu, Y., et al. (2006). Inhibition of Dll4 signalling inhibits tumour growth by deregulating angiogenesis. Nature, 444, 1083–7.
Rudge, J. S., Thurston, G., Davis, S., Papadopoulos, N., Gale, N., Wiegand, S. J., & Yancopoulos, G. D. (2005). VEGF trap as a novel antiangiogenic treatment currently in clinical trials for cancer and eye diseases, and VelociGene- based discovery of the next generation of angiogenesis targets. Cold Spring Harbor Symposia on Quantitative Biology, 70, 411–8.
Noguera-Troise, I., Daly, C., Papadopoulos, N. J., et al. (2006). Blockade of Dll4 inhibits tumour growth by promoting non-productive angiogenesis. Nature, 444, 1032–7.
Fischer, M., Yen, W. C., Kapoun, A. M., Wang, M., O’Young, G., Lewicki, J., Gurney, A., & Hoey, T. (2011). Anti-DLL4 inhibits growth and reduces tumor-initiating cell frequency in colorectal tumors with oncogenic KRAS mutations. Cancer Research, 71, 1520–5.
Visvader, J. E., & Lindeman, G. J. (2008). Cancer stem cells in solid tumours: accumulating evidence and unresolved questions. Nature Reviews Cancer, 8, 755–68.
Harbinski, F., Craig, V. J., Sanghavi, S., et al. (2012). Rescue screens with secreted proteins reveal compensatory potential of receptor tyrosine kinases in driving cancer growth. Cancer Discovery, 2, 948–59.
Burrell, R. A., McGranahan, N., Bartek, J., & Swanton, C. (2013). The causes and consequences of genetic heterogeneity in cancer evolution. Nature, 501, 338–45.
Lagasse, E. (2008). Cancer stem cells with genetic instability: the best vehicle with the best engine for cancer. Gene Therapy, 15, 136–42.
Miura, M., Miura, Y., Padilla-Nash, H. M., et al. (2006). Accumulated chromosomal instability in murine bone marrow mesenchymal stem cells leads to malignant transformation. Stem Cells, 24, 1095–103.
Shiras, A., Chettiar, S. T., Shepal, V., Rajendran, G., Prasad, G. R., & Shastry, P. (2007). Spontaneous transformation of human adult nontumorigenic stem cells to cancer stem cells is driven by genomic instability in a human model of glioblastoma. Stem Cells, 25, 1478–89.
Lee, H. J., Wang, N. X., Shi, D. L., & Zheng, J. J. (2009). Sulindac inhibits canonical Wnt signaling by blocking the PDZ domain of the protein dishevelled. Angewandte Chemie-International Edition, 48, 6448–52.
Taipale, J., Chen, J. K., Cooper, M. K., Wang, B. L., Mann, R. K., Milenkovic, L., Scott, M. P., & Beachy, P. A. (2000). Effects of oncogenic mutations in smoothened and patched can be reversed by cyclopamine. Nature, 406, 1005–9.
Gajjar, A., Stewart, C. F., Ellison, D. W., et al. (2013). Phase I study of vismodegib in children with recurrent or refractory medulloblastoma: a pediatric brain tumor consortium study. Clinical Cancer Research : an Official Journal of the American Association for Cancer Research, 19, 6305–12.
LoRusso, P. (2009). Targeting the hedgehog pathway in medulloblastoma and advanced basal cell cancer therapy. Cancer Biology & Therapy, 8, v-vi.
Barginear, M., Clotfelter, A., & Van Poznak, C. (2009). Markers of bone metabolism in women receiving aromatase inhibitors for early-stage breast cancer. Clinical Breast Cancer, 9, 72–6.
Jimeno, A., Weiss, G. J., Miller, W. H., et al. (2013). Phase I study of the hedgehog pathway inhibitor IPI-926 in adult patients with solid tumors. Clinical Cancer Research, 19, 2766–74.
Williams, J. A. (2003). Hedgehog signaling pathway as a target for therapeutic intervention in basal cell carcinoma. Drug News & Perspectives, 16, 657–62.
Barginear, M. F., Leung, M., & Budman, D. R. (2009). The hedgehog pathway as a therapeutic target for treatment of breast cancer. Breast Cancer Research and Treatment, 116, 239–46.
Rodon, J., Tawbi, H. A., Thomas, A. L., et al. (2014). A phase I, multicenter, open-label, first-in-human, dose-escalation study of the oral smoothened inhibitor Sonidegib (LDE225) in patients with advanced solid tumors. Clinical Cancer Research : an Official Journal of the American Association for Cancer Research, 20, 1900–9.
Schott, A. F., Landis, M. D., Dontu, G., et al. (2013). Preclinical and clinical studies of gamma secretase inhibitors with docetaxel on human breast tumors. Clinical Cancer Research : an Official Journal of the American Association for Cancer Research, 19, 1512–24.
LoConte, N. K., Razak, A. R., Ivy, P., et al. (2015). A multicenter phase 1 study of gamma -secretase inhibitor RO4929097 in combination with capecitabine in refractory solid tumors. Investigational New Drugs, 33, 169–76.
Diaz-Padilla, I., Wilson, M. K., Clarke, B. A., et al. (2015). A phase II study of single-agent RO4929097, a gamma-secretase inhibitor of Notch signaling, in patients with recurrent platinum-resistant epithelial ovarian cancer: a study of the Princess Margaret, Chicago and California phase II consortia. Gynecologic oncology.
De Jesus-Acosta, A., Laheru, D., Maitra, A., et al. (2014). A phase II study of the gamma secretase inhibitor RO4929097 in patients with previously treated metastatic pancreatic adenocarcinoma. Investigational New Drugs, 32, 739–45.
Richter, S., Bedard, P. L., Chen, E. X., et al. (2014). A phase I study of the oral gamma secretase inhibitor R04929097 in combination with gemcitabine in patients with advanced solid tumors (PHL-078/CTEP 8575). Investigational New Drugs, 32, 243–9.
Tolcher, A. W., Messersmith, W. A., Mikulski, S. M., et al. (2012). Phase I study of RO4929097, a gamma secretase inhibitor of Notch signaling, in patients with refractory metastatic or locally advanced solid tumors. Journal of Clinical Oncology : Official Journal of the American Society of Clinical Oncology, 30, 2348–53.
Sahebjam, S., Bedard, P. L., Castonguay, V., et al. (2013). A phase I study of the combination of ro4929097 and cediranib in patients with advanced solid tumours (PJC-004/NCI 8503). British Journal of Cancer, 109, 943–9.
Olsauskas-Kuprys, R., Zlobin, A., & Osipo, C. (2013). Gamma secretase inhibitors of Notch signaling. OncoTargets and Therapy, 6, 943–55.
Egloff, A. M., & Grandis, J. R. (2012). Molecular pathways: context-dependent approaches to Notch targeting as cancer therapy. Clinical Cancer Research : an Official Journal of the American Association for Cancer Research, 18, 5188–95.
Tong, G., Wang, J. S., Sverdlov, O., et al. (2012). Multicenter, randomized, double-blind, placebo-controlled, single-ascending dose study of the oral gamma-secretase inhibitor BMS-708163 (Avagacestat): tolerability profile, pharmacokinetic parameters, and pharmacodynamic markers. Clinical Therapeutics, 34, 654–67.
Gurney, A., Axelrod, F., Bond, C. J., et al. (2012). Wnt pathway inhibition via the targeting of Frizzled receptors results in decreased growth and tumorigenicity of human tumors. Proceedings of the National Academy of Sciences of the United States of America, 109, 11717–22.
Yabuuchi, S., Pai, S. G., Campbell, N. R., et al. (2013). Notch signaling pathway targeted therapy suppresses tumor progression and metastatic spread in pancreatic cancer. Cancer Letters, 335, 41–51.
Messersmith, W. A., Shapiro, G. I., Cleary, J. M., et al. (2015). A Phase I, dose-finding study in patients with advanced solid malignancies of the oral gamma-secretase inhibitor PF-03084014. Clinical Cancer Research : an Official Journal of the American Association for Cancer Research, 21, 60–7.
Smith, D. C., Eisenberg, P. D., Manikhas, G., et al. (2014). A phase I dose escalation and expansion study of the anticancer stem cell agent demcizumab (anti-DLL4) in patients with previously treated solid tumors. Clinical Cancer Research : an Official Journal of the American Association for Cancer Research, 20, 6295–303.
Zemskova, M., Wechter, W., Bashkirova, S., Chen, C. S., Reiter, R., & Lilly, M. B. (2006). Gene expression profiling in R-flurbiprofen-treated prostate cancer: R-Flurbiprofen regulates prostate stem cell antigen through activation of AKT kinase. Biochemical Pharmacology, 72, 1257–67.
Le, P. N., McDermott, J. D., & Jimeno, A. (2015). Targeting the Wnt pathway in human cancers: therapeutic targeting with a focus on OMP-54 F28. Pharmacology & Therapeutics, 146, 1–11.
Previs, R. A., Coleman, R. L., Harris, A. L., & Sood, A. K. (2015). Molecular pathways: translational and therapeutic implications of the notch signaling pathway in cancer. Clinical Cancer Research, 21, 955–61.
Acknowledgments
Authors are thankful for financial support provided through the grant “Analysis of gene expression for different stages of colorectal cancer” (‘Programme-targeted funding 2014–2017’; Government of Republic of Kazakhstan).
Author Contributions
All authors equally contributed to the design, literature analysis and writing of the manuscript.
Disclosure of Interest
The authors have no commercial, proprietary, or financial interest in the products or companies described in this article.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Abetov, D., Mustapova, Z., Saliev, T. et al. Novel Small Molecule Inhibitors of Cancer Stem Cell Signaling Pathways. Stem Cell Rev and Rep 11, 909–918 (2015). https://doi.org/10.1007/s12015-015-9612-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12015-015-9612-x