Abstract
Though previous studies have indicated a relationship between the proliferation of endothelial cells and vascular smooth muscle cells (VSMCs) in co-culture, the results have been contradictory and the signaling mechanism poorly understood. In this transmembrane co-culture study, VSMCs and endothelial cells were grown to confluence on opposite sides of a microporous membrane to mimic the intima/media border of vessels. The endothelial layer was injured, and then cultured for 3 days, resulting in partial re-endothelialization. VSMC proliferation across from the injured/partially recovered endothelial region was significantly higher than across from the de-endothelialized region (a sevenfold increase) and the uninjured region (a threefold increase). ELISA indicated that PDGF, which was undetectable in uninjured co-culture and homotypic controls, increased after injury and the addition of a piperazinyl-quinazoline carboxamide PDGF receptor inhibitor blocked VSMC proliferation across from the injured/partially recovered region. We conclude that co-culture signaling initiated by endothelial cell injury locally stimulates VSMC proliferation and that this signaling could be mediated by PDGF-BB.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Over 1.7 million angioplasty, stent, and bypass procedures are performed each year on occluded arteries and over 50% of these fail within 10 years through spontaneous re-occlusion [1]. Re-occlusion often occurs due to neointimal hyperplasia, when vascular smooth muscle cells (VSMCs) over-proliferate, migrate inward, and choke off the blood flow [2, 3]. In general, this process is initiated by endothelial injury and subsides as the endothelium heals [4]. In addition, stenosis sites are often very small and local [5], while the remaining length of vessel experiences little to no lumenal narrowing [6]. We hypothesized that injury to an endothelial layer in close contact with quiescent VSMCs will induce highly local proliferation in the VMSCs, and that this proliferation is mediated by growth factor release and gap-junction signaling. We tested this hypothesis using a transmembrane co-culture model of endothelial injury across from confluent VSMCs. We analyzed proliferation in local regions across from the injury front and in the uninjured and de-endothelialized regions under control conditions and with an inhibitor of the platelet-derived growth factor (PDGF) receptor and a disrupter of gap junctions. We concluded that an endothelial injury front does significantly induce VSMC proliferation in local regions and that the signal is mediated by PDGF. Results also suggest that this proliferation is partially mediated through gap-junction signaling.
Several current preventative drug-based therapies, such as drug-eluting stents that secrete paclitaxel, sirolimus, or rapomycin and inhibitors of E2F transcription factors [7], attempt to inhibit VSMC over-proliferation by targeting overall effectors of cell proliferation. Though such approaches lead to significantly lower re-occlusion rates over uncoated stents, re-occlusion is not completely eliminated and, furthermore, inhibition of proliferation is non-specific; therefore, healing of the endothelium is also compromised [8]. In contrast, the European Pharmacopoeia has recently approved stents coated with a specific inhibitor of the PDGF receptor, which is highly expressed in VSMCs and has extremely low expression in endothelial cells of large vessels [9], and expected by some scientists to have better long-term effectiveness though these inhibitors can reduce but not eliminate neointimal formation in porcine studies [10]. A clearer understanding of the signaling involved in occlusive arterial regions could lead to more specific therapeutic approaches that prevent VSMC over-proliferation and at the same time, promote healing, beneficial remodeling, and re-endothelialization in an adapted arterial vessel.
We hypothesize that injured endothelial regions of transmembrane endothelial/VSMC co-culture will induce highly localized VSMC proliferation. This effect could be dependent on gap-junction communication and extracellular PDGF. To test our hypothesis, we used a transmembrane co-culture model of endothelial injury across from confluent VSMCs, in a system very similar to ones used in other studies [11–15]. We used imaging techniques to determine localized proliferation levels of VSMCs based on the proximity to regions of an endothelial injury front. We then challenged this system with an inhibitor of PDGF receptor and an inhibitor of gap junctions and measured the effect on VSMC proliferation.
Materials and Methods
Cells and Culture Conditions
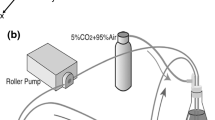
All experiments in this study used bovine arterial VSMCs (AG08504, Coriell Cell Repositories, Camden, NJ) at passage 7 or 8 and bovine arterial endothelial cells (AG08503, Coriell Cell Repositories) at passage 3 or 4. Endothelial cells or VSMCs (for controls) were plated on the lower, outside surface of inverted polyester terephthalate membrane inserts with 0.4 μm pores (BD Falcon, San Jose, CA) at 100,000 cells/cm2 in low-glucose DMEM supplemented with 50 μg/ml penicillin, 50 μg/ml streptomycin, and 200 mM l-glutamine (Invitrogen, Carlsbad, CA) plus 10% bovine calf serum (BCS) (Hyclone, Logan, UT) and incubated at 37°C and 5% CO2 until confluent (approximately 72 h). VSMCs were then plated on the opposite side of the membrane (i.e. the inside of the insert) also at 100,000 cells/cm2. A schematic of this experimental setup is shown in Fig. 1. After both sides reached confluence, media was exchanged for media containing 2% BCS. Previous experiments found that endothelial cells begin to detach when cultured in medium with less than 2% BCS. After 48 h of incubation, approximately half of the endothelial monolayer was scraped away with a polyethylene cell lifter, leaving a confluent endothelial layer over half the membrane and a linear injury front across the membrane. Membranes were then washed with sterile phosphate buffered saline (Invitrogen, Carlsbad, CA) and cultured for 72 h in 2% BCS at 37°C. In each experiment, control wells were run with VSMCs plated opposite uninjured endothelial cells and VSMCs plated on both sides of the membrane.
Bovine arterial VSMCs are grown to confluence in transmembrane culture in medium containing 2% bovine calf serum (BCS) with confluent endothelial cells in a model system similar to ones used in other studies [11–15]. An injury front is then created by removing half the endothelial cells with a cell lifter
Proliferation Measurement
After 72 h of incubation, 10 μM of brominated deoxyribo-uridine (BrdU) was added to each well and cells (in low-serum media with 2% BCS) were incubated at 37°C for 60 min. The BrdU is known to incorporate only into newly synthesized DNA, thereby identifying proliferating cells. Cultures were then fixed with methanol at 4°C for 30 min, DNA was denatured with 2 M HCl for 60 min, cell membranes were permeabilized with 0.1% Triton X100 for 5 min, and non-specific binding was blocked with 1% bovine serum albumin for 30 min. The top sides of the cultures (with VSMCs) were stained with a 1:20 dilution of FITC-conjugated anti-BrdU followed by a 1:1000 dilution of Hoechst 33342 (Molecular Probes, Eugene, OR) stain for DNA. Images were captured at 10× using a Axiovert S100 fluorescent microscope (Carl Zeiss North America, Thornwood, NY) equipped with a charge-coupled device (CCD) camera (Princeton Instruments, Princeton, NJ) using Metamorph Imaging Software (Universal Imaging Corporation, Downingtown, PA). After imaging the cell membranes in phase-contrast to visualize the injury regions, the cells on the lower side of the membrane were wiped off and the membrane was re-imaged at 20× with a FITC and DAPI filter (Chroma Technology Corporation, Rockingham, VT) to ensure that only the cells on the top layer were imaged. A total of 25 images were taken for each membrane, in a 5 × 5 grid, which was usually aligned such that the center column of five images covered the injured/recovered region.
Images were combined into a montage of the entire imaged membrane area and individual image segments were categorized as uninjured endothelial, injured/ partially recovered or injured non-recovered. Images were analyzed using a custom-written particle analysis script in ImageJ v.1.33u (National Institute of Health, Washington, DC) that counted total nuclei, identified by intensity and size, in each FITC and Hoechst image. Images in early experiments were also counted by hand to verify the accuracy of the automated counting software. Reported proliferation values were calculated as the average of the number of BrdU positive nuclei divided by the number of total nuclei in each image identified as a particular region of the culture.
ELISA Analysis
Enzyme-linked immunosorbent assay (ELISA) was used to measure the amount of soluble PDGF-BB in culture media. At 3 days after injury, media was removed and quickly frozen to −80°C. To concentrate the proteins present to detectable levels, 0.70 ml of media was first lyophilized for 8 h at −60°C and 150 mTorr. The remaining precipitate was again dissolved in 100 μl of distilled water. These samples were then analyzed with an ELISA kit for PDGF-BB (RayBiochem, Norcross, GA) according to the manufacturer’s instructions.
PDGF Receptor and Gap-Junction Inhibition
The PDGF receptor was inhibited with 4-(6,7-dimethoxy-4-quinazolinyl)-N-(4-phenoxyphenyl)-1-piperazine-carboxamide (PDGFR Inhibitor III, Calbiochem, San Diego, CA) at a concentration of 50 μM, which is well above the ID50 dose [16]. Gap junctions were disrupted using 50 μM palmitoleic acid (Sigma, St. Louis, MO) [17]. In inhibition experiments, the inhibitor was added to the media immediately after injury. The ability of the PDGFR inhibitor to inhibit VSMC growth was evaluated by growing VSMCs to confluence on tissue culture plastic in 10% BCS, then culturing them in 2% BCS for 48 h before addition of 10 ng/ml PDGF (R&D System, Minneapolis, MN) or 10 ng/ml PDGF plus 1 μM PDGFR inhibitor added concurrently. Cells were incubated overnight with BrdU and stained as detailed above.
Statistics
Differences between populations were analyzed with a Student t-test. For multiple comparisons, a Bonferroni correction was used. Results with corrected P < 0.05 were considered significant. Error bars are given as s.d. unless otherwise stated.
Results
Endothelial Cell Injury Zones
Bovine arterial endothelial and vascular smooth muscle cells were grown to confluence on opposite sides of a microporous membrane before the endothelial side was injured by scraping off approximately 60% of the cells. A schematic of the experimental setup is shown in Fig. 1. Within 3 days following endothelial cell injury, cells form three distinct regions (Fig. 2): an uninjured, endothelialized region which ends at the site of initial injury, an injured/recovered region, which extends from the initial injury site to the line of advancing re-endothelialization, and an injured/non-recovered region, which has no recovery of endothelial cells. The cells in the injured region are elongated and sub-confluent and have clear morphological differences from the cells in the uninjured, confluent region. The line of initial endothelial injury also appears denser compared to the uninjured endothelial region.
Regions of endothelial injury model. An injury front was created by scraping away half of a confluent layer of endothelial cells with a cell lifter, followed by incubation in 2% BCS. (a) After 3 days, three distinct regions of endothelial injury are apparent: the uninjured endothelial region that comprises the area of endothelial cells not removed by scraping; the injured/recovered region that comprises the portion of membrane where endothelial cells were removed but cells have proliferated and migrated to recover; and the injured/non-recovered region that comprise the area where cells were removed and no endothelial cells are present. (b) A higher resolution region of the same image shows changes in endothelial cell morphology across the injury front. Scale bar is 0.5 mm in both images. Magnification is 10×
Proliferation Rates of VSMCs are Localized and Depend on Contact with Specific Endothelial Injury Zones
Data presented are the average of percent proliferation in 75 images per condition. The percentage of proliferating VSMCs is not significantly different in any total culture (Fig. 3a, black bars). However, a region-by-region analysis of proliferation rates (Fig. 3a, gray bars) reveals a significant increase in VSMC proliferation directly opposite the injured/partially recovered region in an injury co-culture model compared to all other regions and cultures (P < .05). Furthermore, the percent proliferating VSMCs across from in the injured/unrecovered region was significantly lower than the uninjured region (P < .05).
Proliferation rates of VSMCs in transmembrane co-culture with confluent VSMCs (VSMC control), endothelial cells (uninjured ECs), and an endothelial injury model. The lumped analysis of all regions of each culture (black bars) shows no significant difference in VSMC growth between any culture condition. Analysis of the separate regions of the injury model co-culture (gray bars) reveals significantly higher VSMC proliferation across from the injured/recovered region compared to all other regions and cultures (P < .05). The proliferation rate in the de-endothelialized region is also significantly lower than the rate in the uninjured region (P < .05). Presented values are averages of percent BrdU positive cells in image frames. Experiments were repeated three times. Error bars represent s.e.m. Total image frames in each region are n = 75, 75, 75, 12, 23, 40 for VSMC, endothelial, endothelial injury model, injured/non-recovered, injured/recovered, and uninjured endothelial regions, respectively. Representative images of (b) injured/non-recovered, (c) injured/recovered and (d) uninjured endothelial regions of an injury model membrane are shown with stains for all nuclei (DAPI, blue) and BrdU positive nuclei (green)
ELISA Analysis for Soluble PDGF-BB in Transmembrane Cultures
To measure the amount of PDGF in the culture medium, an ELISA was performed on culture media taken from cultures in the same conditions (low-serum media with 2% BCS) and at the same 72 h time point (n = 3). Though PDGF-BB was undetectable in media from the uninjured endothelial/VSMC coculture or VSMC mono-culture controls, media from co-cultures with endothelial injury showed a surge of PDGF-BB that peaked at 32.5 pg/ml at 3 days after injury (Fig. 4). This peak PDGF-BB was significantly greater than the concentration at 1 day, in VSMC mono-culture controls, in endothelial mono-culture controls, and media with 2% BCS (P < .05).
ELISA of media from the endothelial injury co-culture reveals a peak in the total concentration of PDGF-BB at 3 days after injury. In contrast, media from uninjured cultures and VSMC control cultures had no detectable PDGF-BB. This peak PDGF-BB was significantly greater than the concentration at 1 day in VSMC mono-culture controls, endothelial mono-culture controls, and media with 2% BCS (P > 0.05). Data points represent the average of three experiments
PDGF Receptor Inhibitor
Analysis of VSMC proliferation at confluent densities on tissue culture plastic was used to assess the ability of a PDGF receptor inhibitor to block PDGF-BB-induced proliferation of VSMCs in monoculture. When 10 ng/ml of PDGF-BB was added to the cells, proliferation significantly increased from 0.7% to 30% in the absence of PDGF-receptor inhibition (P < .05). The addition of 1 μM PDGF-receptor inhibitor resulted in a significant decrease in proliferation (P < .05). In contrast, there was no significant change in VSMC proliferation in control cultures upon addition of the inhibitor (Fig. 5).
The use of 1 μm PDGFR inhibitor (PDGFRi) was sufficient to significantly inhibit the stimulation of VSMC proliferation in confluent cultures plated on tissue-culture plastic by the addition of 10 ng/ml PDGF-BB (P < 0.01). Proliferation rates were not significantly changed upon addition of the inhibitor to cultures in 2% BCS without additional PDGF-BB. Note also that the addition of 10 ng/ml PDGF-BB increased proliferation rates by a factor of 40 in a confluent culture without the presence of inhibitor. Data points represent averages of five separate experiments of 450–850 cells each. Asterisks represent significant differences (P < 0.01 for all cases)
PDGF receptor inhibitor was added to transmembrane cultures to assess the effect of PDGF on endothelial injury-induced VSMC proliferation. VSMCs proliferated significantly less in the presence of 50 μM PDGF receptor inhibitor in the VSMC control cultures and the injured/recovered and uninjured regions of the injury model co-culture (P < .05, Fig. 6). Furthermore, PDGF receptor inhibitor reduced the proliferation level in the injured/recovered region of the injury model to a level where it was not significantly different from the injured/non-recovered region and the uninjured endothelial culture.
The addition of 50 μM PDGFR inhibitor to transmembrane cultures (white bars) results in a significant reduction in proliferation in VSMC control cultures and VSMCs across from the injured/partially recovered region and the uninjured region of co-cultures with the endothelial injury model (P < .05). The addition of palmitoleic acid (shaded bars), which disrupts gap-junction communication, significantly reduced VSMC proliferation across from the injured/recovered region of co-cultures with the endothelial injury model and significantly increased proliferation in VSMC control cultures. Data are taken at 72 h after injury, as in Fig. 3 and all other figures. Experiments were repeated three times. Error bars represent s.e.m. Total image frames in each region for control cultures are the same as in Fig. 3. Total image frames in PDGFR inhibitor treated culture are n = 75, 75, 25, 23, 27 for VSMC, endothelial, injured/non-recovered, injured/recovered, and uninjured endothelial regions, respectively. Total image frames in PDGFR inhibitor treated culture are n = 75, 75, 12, 27, 36 for VSMC, endothelial, injured/non-recovered, injured/recovered, and uninjured endothelial regions, respectively
Effect of Gap-Junction Inhibition in Transmembrane Cultures
The addition of 50 μM palmitoleic acid to disrupt gap-junction formation in transmembrane systems resulted in significantly higher proliferation in VSMC monocultures and significantly lower proliferation in the injured/recovered region of endothelial injury model co-cultures (P < .05) (Fig. 6). Note that palmitoleic acid is a non-specific gap-junction disruptor and this experimental system was unable to distinguish between the effects of disrupting VSMC–VSMC gap junctions, VSMC–EC gap junctions, or EC–EC gap junctions.
Discussion
In this study, we have demonstrated that endothelial injury significantly stimulates VSMC proliferation only in a highly localized region directly across a membrane from the injured/recovered portion of the endothelial injury front. The lack of overall global stimulation may explain contradictions between previously published studies of the effects of confluent and sub-confluent endothelial cells on VSMCs in transmembrane cultures [12, 18]. Highly local signaling effects could also help explain the formation of focused occlusive regions in vessels and arterial grafts that exhibit normal healing elsewhere.
Previous studies have implemented co-culture models to investigate endothelial-VSMC interactions, but to our knowledge, no one has yet investigated the localized response of VSMCs and endothelial cells in these systems. Specifically, four types of in vitro models have been used: (1) conditioned media models, where media from a culture of endothelial cells was added to a culture of VSMCs [19], (2) transmembrane cultures with one cell type plated on a membrane that was inserted into a well containing the other cell type [19, 20], (3) direct transmembrane co-culture using porous membranes that allowed signaling molecules to pass between the cell types but prohibited cell migration between layers [12–15, 18, 21, 22], and (4) direct contact co-culture where a layer of endothelial cells is grown directly on smooth muscle cells or a scaffold loaded with smooth muscle cells [23]. Using a direct transmembrane co-culture, Axel et al. [12] found that confluent cultures of endothelial cells inhibited both the proliferation and migration rates of VSMCs, while proliferating (sub confluent) cultures of ECs had a proliferative effect on VSMCs. However, other studies also using direct transmembrane co-cultures gave contradictory results, i.e. VSMCs in direct contact co-cultures proliferated significantly more when opposite confluent ECs [11, 18]. Because both these studies used different amounts of serum, which contains growth factors that stimulate both VSMC and endothelial proliferation, and examined proliferative effects over much different time scales, they cannot be easily compared. Another possible reason for the discrepancy in these studies is that bulk methods were used to quantify total cell proliferation in a culture. Because occlusion is the result of a highly localized response to an injured endothelium, a method of quantifying localized effects based on proximity to regions of differing endothelial cell confluence and proliferation might not only illuminate local signaling, but would also more closely approximate physiological areas of endothelial injury.
Previous studies have observed that the phenotypic state of VSMCs significantly affects the behavior of ECs in co-culture and that ECs could be seeded and maintained only on quiescent VSMC cultures [23]. Specifically, quiescent human umbilical vein SMCs cultured in serum-free conditions prior to co-culture with human umbilical vein ECs significantly enhanced EC proliferation, junction formation, and NO secretion when compared to proliferative SMCs cultured in 10% serum [24]. In addition, secreting VSMCs cultured in serum-containing media have been shown to promote inflammatory and non-proliferative phenotypes in transmembrane co-culture of ECs, compared to co-culture with serum-starved VSMCs [21]. In this study, VSMCs had been cultured in 10% serum prior to, and during cell plating opposite confluent EC cultures. The VSMCs quickly proliferated and could reasonably be expected to be in a proliferative and secreting phenotype. However, once the VSMC layer reached confluence, the media was replaced with low-serum media and the proliferation of VSMC dropped to low levels, with around 2% of cells proliferating. The specific phenotypic state of the VSMCs, and any phenotypic state accordingly induced in ECs, is unknown and warrants further investigation in future studies.
Others have demonstrated a significant change in the rate of proliferation, with a 40-fold increase in [3H]thymidine incorporation for endothelial cells that were sub-confluent compared to cells that were inhibited by cell contact [25]. Studies have also found that by 48 h after endothelial injury, cells up to 10 rows deep exhibited proliferation, with 80% of cells proliferating in this region compared to less than 10% in all deeper regions [26]. In our cultures, this proliferative region of 10 cells deep covered approximately half to two-thirds of the defined injured/recovered region. As seen in Fig. 2b, endothelial cells in these first 10 layers appeared much more elongated and spread and made less contact with other endothelial cells than regions further from the injury front. Furthermore, one recent study found that endothelial cells just a few hundreds of microns behind the injury front exhibited active migration by extending lamellipodia underneath the cells in front of them [27]. Based on our findings that the proliferative and migratory behavior is altered only in a region within a few hundred microns of the injury front, we hypothesized that the production and secretion of stimulatory proteins observed in models of endothelial injury are also due to cells in this specific region.
Endothelial cells produce several secreted growth factors that influence VSMC proliferation, including platelet-derived growth factor (PDGF) [13], basic fibroblast growth factor (bFGF) [28], transforming growth factor beta (TGF-β) [13], and prostacyclin [29] as well as the membrane-soluble factor nitric oxide (NO) [29–31], and a yet-unidentified endothelium-derived hyperpolarizing factor (EDHF) [32]. Of these different factors, PDGF has been identified as a major effector of VSMC proliferation. Studies using in situ preparations of rat arteries found that endothelial injury results in increased PDGF secretion [33, 34]. Furthermore, Axel et al. [13] showed that sub-confluent endothelial cells have increased PDGF secretion compared to confluent endothelial layers and that PDGF significantly stimulates VSMC growth. Further evidence of the importance of PDGF in studies in rats showed that a polyclonal antibody to PDGF inhibits neointimal formation after de-endothelialization by balloon angioplasty [35]. Secreted PDGF exists as a dimer of either or both of the two isoforms, PDGF-A and PDGF-B. The dimer PDGF-BB is the most physiologically important in intercellular crosstalk between cell types and, in general, developmental signaling occurs unidirectionally with endothelial cells secreting PDGF-BB and VSMCs expressing PDGF-BB receptor, though both VSMCs and endothelial cells secrete PDGF-BB [36, 37]. Thus, all experiments in this study use antibodies specific for the homodimer PDGF-BB to specifically investigate signaling from endothelial cells that affects VSMCs.
We found that the levels of PDGF in the overall media increased after endothelial injury in these transmembrane co-cultures, and appears to peak at 3 days after injury. It should be noted that PDGF levels in control cultures were not measured at 3 days after injury so a significant increase in PDGF release at that time over uninjured cells or VSMC monocultures is unknown. However, PDGF levels were still significantly higher than in control cultures at 4 days after injury. Furthermore, the VSMC proliferative response across from the injured/recovered region of endothelial injury was nearly eliminated by the addition of the PDGF receptor inhibitor. We also observed that the PDGFR inhibitor significantly inhibited proliferation in control VSMC monocultures, which had undetectable levels of PDGF-BB (as measured by ELISA). Thus, the inhibitor was likely non-specific and may have been inhibiting signaling from other growth factors which may have been present in the media or secreted by the proliferating VSMCs. We suggest that future studies use PDGFR inhibitor at a much lower dilution that used in this study (50 μM), especially considering that we found in this study that a concentration of 1 μM PDGFR inhibitor was sufficient to completely inhibit the effects of PDGF (Fig. 4). We therefore consider the PDGF results to be preliminary and suggest further studies into specific growth factor inhibition, possibly through the use of other inhibitors, inhibition of PDGF directly, or downregulation of PDGF with RNAi.
Still, these results do not explain the localized response, as PDGF levels were increased throughout the media. Furthermore, even with the inhibition of PDGF receptor, VSMC proliferation across from the injured/recovered region was still significantly greater than proliferation in the uninjured region. This result suggests that some other signaling mechanism, with more localized signaling ability than secreted factors, also contributes to the observed VSMC proliferation response.
The majority of studies investigating VSMC proliferation have focused on effects of secreted molecules. However, it is known that endothelial cells and VSMCs can form direct gap junctions [32]. In explanted arterioles, researchers have demonstrated the functionality of myo-endothelial gap junctions to pass small molecules from the endothelium to VSMCs [38]. These gap junctions appear to be formed predominantly by connexin 43 and connexin 40 to a lesser extent [11, 39]. Studies have shown that connexin 43 expression is increased in VSMCs during hyperplasia [40]. Gap junctions have also been observed in in vitro co-cultures. For example, Fillinger et al. [11] noted that VSMCs projections through 0.4 μm membranes come into close contact with ECs, suggesting gap-junction formation, and Isakson et al. [41] showed that ECs and VSMCs across a transwell membrane form functional gap junctions that appear to be capable of transporting inositol triphosphate (IP3) and Ca2+ between the two cell types. Furthermore, an analysis of connexin 43 knockout mice suggests that the formation of gap junctions in early development inhibits the proliferation of proepicardial cells, which eventually differentiate into VSMCs [42]. We know of no previous studies that specifically investigated a link between myo-endothelial gap-junction signaling and proliferation. In this study, we use palmitoleic acid, which has been shown to be an effective, reversible disruptor of gap-junction communication [17], to examine the effects of gap junctions on VSMC proliferation.
The disruption of gap-junction communication significantly reduced the level of proliferation of VSMCs across from the injured/recovered region of endothelial injury, suggesting that gap junction-mediated signaling influences the VSMC response to endothelial injury. The inhibitor used was non-specific and disrupted all gap-junction communication between all of the cell types. We thus have no indication whether the reduction in proliferation was due to VSMC–VMSC gap junctions or VSMC-endothelial gap junctions. However, the gap-junction inhibitor caused a significant increase in proliferation in VSMC control cultures, suggesting that the disruption of VSMC–VSMC gap-junction communication may have an overall proliferative effect, while disruption of VSMC-EC gap-junction communication may inhibit proliferation. The enhancement of proliferation by palmitoleic acid in mono-culture is consistent with in vitro studies of similar fatty acid-based gap-junction disruptors such as oleic acid [43]. Further studies are warranted to investigate the specific nature of gap junctions involved in signaling in this system. Specifically, dye loading studies of each cell type using a confocal microscope to detect dye transfer differentially between cell types could verify whether myoendothelial gap junctions are present in this experimental system. Other studies using specific connexin inhibitors and dye indicators to report small molecule concentrations, such as experiments with the calcium reporter Fluo 4 by Isakson et al. [41], could identify specific mechanisms of communication.
We further observed that the variance of VSMC proliferation in an uninjured endothelium co-culture greatly increased when gap-junction signaling was disrupted. VSMCs appeared to have local regions of high proliferation and random, individual cell behaviors. This is in direct contrast with control cells that were more likely to exhibit coordinated, organ-level, behavior as opposed to individual behavior. Interestingly, an increase in the variance of proliferation was not observed in VSMCs opposite the uninjured region of injured co-cultures. These results indicate complex signaling between these cells with possible conditioning and cross talk between growth factor and gap-junction signaling pathways. For example, other studies have found that PDGF can disrupt gap-junction communication through multiple, complex pathways in an epithelial cell line [44].
Other studies have observed that the presence or absence of shear stress can alter the paracrine effects of endothelial cells on smooth muscle cells in co-culture. Shear stress over ECs has been show to inhibit proliferation [45], inhibit EC-induced migration [46, 47], and enhance expression of contractile proteins [14] in co-cultured transmembrane VSMCs, and the presence of co-cultured ECs inhibits SMC migration in response to flow [48]. In addition, shear stress has been shown to induce PDGF release by endothelial cells [49–51]. Furthermore, shear stress has been shown to upregulate connexin-43 expression in ECs [52, 53] and VSMCs [54], though decrease gap-junction communication in ECs [52, 55]. Co-culture of VSMCs with ECs in a “tissue engineered wall model” resulted in increased protein expression of connexin-43 and connexin-37 and connexin-40 as well as alterations in response of connexin mRNA expressions in response to flow [55]. Studies have also observed that VSMC transmembrane co-culture alters shear-stress-induced changes in endothelial adhesion molecule expression [22, 56], chemokines, and leukocyte adhesion factor expression [57], inflammatory gene expression, and NF-κB activation [58]. These studies suggest that the application of shear stress may enhance the vasoprotective effects of EC-VSMC co-culture and could increase the regional differences seen in VSMC proliferation in the injury model presented here. We therefore suggest further investigation into local signaling in EC-VSMC injury models under flow conditions as future work.
Myoendothelial contact has been observed in the coronary arteries of the dog and rabbit [59], in the thoracic aorta of fetal rats [60] and humans [61], in small arterioles in the human kidney [62, 63], and in hamster and rat arterioles [64]. Functional gap-junction communication has been observed in rat mesenteric arteries [65] and in rabbit iliac arteries [66]. Though, close contact of VSMCs and ECs in the adult aorta has not been observed and myoendothelial gap-junction communication has not been observed in large conduit vessels, such as the aorta, studies have previously demonstrated the ability of aortic endothelial and smooth muscle cells to form heterocellular gap junctions in vitro [67]. Therefore, the model presented in this study does not mimic the structure of the aorta, and the high passage cell lines used may not be fully representative of aortal cells in vivo, though these cells can be expected to form structural connections, and possibly gap junctions, as might occur in coronary arteries or other resistance vessels.
Future work is needed to verify the gap-junction coupling of VSMCs and endothelial cells in this type of transmembrane co-culture, as well as to identify the signaling molecules passed between the cell types and the directionality of the signaling. Ideally, a system that could specifically disrupt communication selectively between each cell type could help elucidate the specific effects of gap junctions on VSMC proliferation initiated by endothelial injury.
In conclusion, we have developed an in vitro model system for studying the localized effects of endothelial injury on VSMCs across a thin, porous membrane. An endothelial injury front stimulates VSMC proliferation in a highly localized region of cells directly opposite to the injury, without significantly affecting cells sharing the same media a short distance away. Though soluble PDGF appears to be the major signaling pathway, localized differences are apparent even when PDGF receptor signaling is downregulated with an inhibitor. Evidence suggests that gap-junction coupling may also influence this VSMC response and further research is needed into the role of gap-junction signaling in local vascular remodeling.
References
Belkin, M., & Whittemore, A. D. (2000). Infrainguinal bypass. In R. B. Rutherford (Ed.), Vascular surgery (5th ed., pp. 998–1018). Philadelphia: W.B. Saunders Company.
Thyberg, J., Blomgren, K., Roy, J., Tran, P. K., & Hedin, U. (1997). Phenotypic modulation of smooth muscle cells after arterial injury is associated with changes in the distribution of laminin and fibronectin. The Journal of Histochemistry and Cytochemistry, 45, 837–846.
Dilley, R. J., McGeachie, J. K., & Prendergast, F. J. (1987). A review of the proliferative behaviour, morphology and phenotypes of vascular smooth muscle. Atherosclerosis, 63, 99–107.
Painter, T. A. (1991). Myointimal hyperplasia: pathogenesis and implications. 2. Animal injury models and mechanical factors. Artificial Organs, 15, 103–118.
Conte, M. S. (1998). The ideal small arterial substitute: a search for the Holy Grail? The FASEB Journal, 12, 43–45.
Jacot, J. G., Abdullah, I., Belkin, M., Gerhard-Herman, M., Gaccione, P., Polak, J., et al. (2004). Early adaptation of human lower extremity vein grafts: Wall stiffness changes accompany geometric remodeling. Journal of Vascular Surgery, 39, 547–555.
Mann, M., Whittemore, A. D., Donaldson, M., Belkin, M., Conte, M. S., Polak, J., et al. (1999). Ex-vivo gene therapy of human vascular bypass grafts with E2F decoy: the PREVENT single-centre, randomised, controlled trial. Lancet, 354, 1493–1498.
Smith, E. J., & Rothman, M. T. (2003). Antiproliferative coatings for the treatment of coronary heart disease: what are the targets and which are the tools? Journal of Interventional Cardiology, 16, 475–483.
Marx, M., Perlmutter, R. A., & Madri, J. A. (1994). Modulation of platelet-derived growth factor receptor expression in microvascular endothelial cells during in vitro angiogenesis. The Journal of Clinical Investigation, 93, 131–139.
Levitzki, A. (2005). PDGF receptor kinase inhibitors for the treatment of restenosis. Cardiovascular Research, 65, 581–586.
Fillinger, M. F., Sampson, L. N., Cronenwett, J. L., Powell, R. J., & Wagner, R. J. (1997). Coculture of endothelial cells and smooth muscle cells in bilayer and conditioned media models. The Journal of Surgical Research, 67, 169–178.
Axel, D. I., Brehm, B., Wolburg-Buchholz, K., Betz, E., Koveker, G., & Karsch, K. R. (1996). Induction of cell-rich and lipid-rich plaques in a transfilter coculture system with human vascular cells. Journal of Vascular Research, 33, 327–339.
Axel, D. I., Riessen, R., Athanasiadis, A., Runge, H., Koveker, G., & Karsch, K. R. (1997). Growth factor expression of human arterial smooth muscle cells and endothelial cells in a transfilter coculture system. Journal of Molecular and Cellular Cardiology, 29, 2967–2978.
Hastings, N. E., Simmers, M. B., McDonald, O. G., Wamhoff, B. R., & Blackman, B. R. (2007). Atherosclerosis-prone hemodynamics differentially regulates endothelial and smooth muscle cell phenotypes and promotes pro-inflammatory priming. American Journal of Physiology Cell Physiology, 293, C1824–C1833.
Isakson, B. E., & Duling, B. R. (2005). Heterocellular contact at the myoendothelial junction influences gap junction organization. Circulation Research, 97, 44–51.
Matsuno, K., Ushiki, J., Seishi, T., Ichimura, M., Giese, N. A., Yu, J. C., et al. (2003). Potent and selective inhibitors of platelet-derived growth factor receptor phosphorylation. 3. Replacement of quinazoline moiety and improvement of metabolic polymorphism of 4-[4-(N-substituted (thio)carbamoyl)-1-piperazinyl]-6,7-dimethoxyquinazoline derivatives. Journal of Medicinal Chemistry, 46, 4910–4925.
Lavado, E., Sanchez-Abarca, L. I., Tabernero, A., Bolanos, J. P., & Medina, J. M. (1997). Oleic acid inhibits gap junction permeability and increases glucose uptake in cultured rat astrocytes. Journal of Neurochemistry, 69, 721–728.
Powell, R. J., Cronenwell, J., Fillinger, M., & Wagner, R. (1994). Effect of endothelial cells and transforming growth factor-beta1 on cultured vascular smooth muscle cell growth patterns. Journal of Vascular Surgery, 20, 787–794.
Nugent, M., Karnovsky, M., & Edelman, E. (1993). Vascular cell-derived heparin sulfate shows coupled inhibition of basic fibroblast growth factor binding and mitogenesis in vascular smooth muscle cells. Circulation Research, 73, 1051–1060.
Cucina, A., Borrelli, V., Randone, B., Coluccia, P., Sapienza, P., & Cavallaro, A. (2003). Vascular endothelial growth factor increases the migration and proliferation of smooth muscle cells through the mediation of growth factors released by endothelial cells. Journal of Surgical Research, 109, 16–23.
Rose, S. L., & Babensee, J. E. (2008). Smooth muscle cell phenotype alters cocultured endothelial cell response to biomaterial-pretreated leukocytes. Journal of Biomedical Materials Research Part A, 84, 661–671.
Chiu, J. J., Chen, L. J., Lee, C. I., Lee, P. L., Lee, D. Y., Tsai, M. C., et al. (2007). Mechanisms of induction of endothelial cell E-selectin expression by smooth muscle cells and its inhibition by shear stress. Blood, 110, 519–528.
Lavender, M. D., Pang, Z., Wallace, C. S., Niklason, L. E., & Truskey, G. A. (2005). A system for the direct co-culture of endothelium on smooth muscle cells. Biomaterials, 26, 4642–4653.
Leung, B. M., & Sefton, M. V. (2007). A modular tissue engineering construct containing smooth muscle cells and endothelial cells. Annals of Biomedical Engineering, 35, 2039–2049.
Chen, D., Walsh, K., & Wang, J. (2000). Regulation of cdk2 activity in endothelial cells that are inhibited from growth by cell contact. Arteriosclerosis, Thrombosis, and Vascular Biology, 20, 629–635.
Ettenson, D. S., & Gotlieb, A. I. (1994). Endothelial wounds with disruption in cell migration repair primarily by cell proliferation. Microvascular Research, 48, 328–337.
Farooqui, R., & Fenteany, G. (2005). Multiple rows of cells behind an epithelial wound edge extend cryptic lamellipodia to collectively drive cell-sheet movement. Journal of Cell Science, 118, 51–63.
Stavri, G. T., Zachary, I., Baskerville, P. A., Martin, J. F., & Erusalimsky, J. D. (1995). Basic fibroblast growth factor upregulates the expression of vascular endothelial growth factor in vascular smooth muscle cells: Synergistic interaction with hypoxia. Circulation, 92, 11–14.
Zachary, I. (2001). Signaling mechanisms mediating vascular protective actions of vascular endothelial growth factor. American Journal of Physiology Cell Physiology, 280, C1375–C1386.
Tanner, F. C., Meier, P., Greutert, H., Champion, C., Nabel, E. G., & Luscher, T. F. (2000). Nitric oxide modulates expression of cell cycle regulatory proteins: A cytostatic strategy for inhibition of human vascular smooth muscle cell proliferation. Circulation, 101, 1982–1989.
Napoli, C., & Ignarro, L. J. (2001). Nitric oxide and atherosclerosis. Nitric Oxide, 5, 88–97.
Dora, K. A. (2001). Cell-cell communication in the vessel wall. Vascular Medicine, 6, 43–50.
Lindner, V., & Reidy, M. A. (1995). Platelet-derived growth factor ligand and receptor expression by large vessel endothelium in vivo. American Journal of Pathology, 146, 1488–1497.
Lindner, V., Giachelli, C. M., Schwartz, S. M., & Reidy, M. A. (1995). A subpopulation of smooth muscle cells in injured rat arteries expresses platelet-derived growth factor-B chain mRNA. Circulation Research, 76, 951–957.
Ferns, G. A., Raines, E. W., Sprugel, K. H., Motani, A. S., Reidy, M. A., & Ross, R. (1991). Inhibition of neointimal smooth muscle accumulation after angioplasty by an antibody to PDGF. Science, 253, 1129–1132.
Hellstrom, M., Kalen, M., Lindahl, P., Abramsson, A., & Betsholtz, C. (1999). Role of PDGF-B and PDGFR-beta in recruitment of vascular smooth muscle cells and pericytes during embryonic blood vessel formation in the mouse. Development, 126, 3047–3055.
Fredriksson, L., Li, H., & Eriksson, U. (2004). The PDGF family: four gene products form five dimeric isoforms. Cytokine & Growth Factor Reviews, 15, 197–204.
Little, T. L., Xia, J., & Duling, B. R. (1995). Dye tracers define differential endothelial and smooth muscle coupling patterns within the arteriolar wall. Circulation Research, 76, 498–504.
Christ, G. J., Spray, D. C., el-Sabban, M., Moore, L. K., & Brink, P. R. (1996). Gap junctions in vascular tissues. Evaluating the role of intercellular communication in the modulation of vasomotor tone. Circulation Research, 79, 631–646.
Deglise, S., Martin, D., Probst, H., Saucy, F., Hayoz, D., Waeber, G., et al. (2005). Increased connexin43 expression in human saphenous veins in culture is associated with intimal hyperplasia. Journal of Vascular Surgery, 41, 1043–1052.
Isakson, B. E., Ramos, S. I., & Duling, B. R. (2007). Ca2+ and inositol 1, 4, 5-trisphosphate-mediated signaling across the myoendothelial junction. Circulation Research, 100, 246–254.
Li, W. E., Waldo, K., Linask, K. L., Chen, T., Wessels, A., Parmacek, M. S., et al. (2002). An essential role for connexin43 gap junctions in mouse coronary artery development. Development, 129, 2031–2042.
Fisher, W. E., Boros, L. G., & Schirmer, W. J. (1995). Reversal of enhanced pancreatic cancer growth in diabetes by insulin. Surgery, 118, 453–457.
Bornfeldt, K. E., Raines, E. W., Graves, L. M., Skinner, M. P., Krebs, E. G., & Ross, R. (1995). Platelet-derived growth factor. Distinct signal transduction pathways associated with migration versus proliferation. Annals of the New York Academy of Sciences, 766, 416–430.
Nackman, G. B., Fillinger, M. F., Shafritz, R., Wei, T., & Graham, A. M. (1998). Flow modulates endothelial regulation of smooth muscle cell proliferation: A new model. Surgery, 124, 353–360. discussion 360–1.
Sakamoto, N., Ohashi, T., & Sato, M. (2006). Effect of fluid shear stress on migration of vascular smooth muscle cells in cocultured model. Annals of Biomedical Engineering, 34, 408–415.
Wang, H. Q., Huang, L. X., Qu, M. J., Yan, Z. Q., Liu, B., Shen, B. R., et al. (2006). Shear stress protects against endothelial regulation of vascular smooth muscle cell migration in a coculture system. Endothelium, 13, 171–180.
Redmond, E. M., Cullen, J. P., Cahill, P. A., Sitzmann, J. V., Stefansson, S., Lawrence, D. A., et al. (2001). Endothelial cells inhibit flow-induced smooth muscle cell migration: Role of plasminogen activator inhibitor-1. Circulation, 103, 597–603.
Palumbo, R., Gaetano, C., Antonini, A., Pompilio, G., Bracco, E., Ronnstrand, L., et al. (2002). Different effects of high and low shear stress on platelet-derived growth factor isoform release by endothelial cells: Consequences for smooth muscle cell migration. Arteriosclerosis, Thrombosis, and Vascular Biology, 22, 405–411.
Aromatario, C., Sterpetti, A. V., Palumbo, R., Patrizi, A. L., Di Carlo, A., Proietti, P., et al. (1997). Fluid shear stress increases the release of platelet derived growth factor BB (PDGF BB) by aortic endothelial cells. Minerva Cardioangiologica, 45, 1–7.
Dardik, A., Yamashita, A., Aziz, F., Asada, H., & Sumpio, B. E. (2005). Shear stress-stimulated endothelial cells induce smooth muscle cell chemotaxis via platelet-derived growth factor-BB and interleukin-1alpha. Journal of Vascular Surgery, 41, 321–331.
DePaola, N., Davies, P. F., Pritchard, W. F., Jr., Florez, L., Harbeck, N., & Polacek, D. C. (1999). Spatial and temporal regulation of gap junction connexin43 in vascular endothelial cells exposed to controlled disturbed flows in vitro. Proceedings of the National Academy of Sciences of the United States of America, 96, 3154–3159.
Inai, T., Mancuso, M. R., McDonald, D. M., Kobayashi, J., Nakamura, K., & Shibata, Y. (2004). Shear stress-induced upregulation of connexin 43 expression in endothelial cells on upstream surfaces of rat cardiac valves. Histochemistry and Cell Biology, 122, 477–483.
Cowan, D. B., Lye, S. J., & Langille, B. L. (1998). Regulation of vascular connexin43 gene expression by mechanical loads. Circulation Research, 82, 786–793.
Johnson, T. L., & Nerem, R. M. (2007). Endothelial connexin 37, connexin 40, and connexin 43 respond uniquely to substrate and shear stress. Endothelium, 14, 215–226.
Chiu, J. J., Chen, L. J., Lee, P. L., Lee, C. I., Lo, L. W., Usami, S., et al. (2003). Shear stress inhibits adhesion molecule expression in vascular endothelial cells induced by coculture with smooth muscle cells. Blood, 101, 2667–2674.
Chiu, J. J., Chen, L. J., Chen, C. N., Lee, P. L., & Lee, C. I. (2004). A model for studying the effect of shear stress on interactions between vascular endothelial cells and smooth muscle cells. Journal of Biomechanics, 37, 531–539.
Chiu, J. J., Chen, L. J., Chang, S. F., Lee, P. L., Lee, C. I., Tsai, M. C., et al. (2005). Shear stress inhibits smooth muscle cell-induced inflammatory gene expression in endothelial cells: Role of NF-kappaB. Arteriosclerosis, Thrombosis, and Vascular Biology, 25, 963–969.
Kristek, F., & Gerova, M. (1992). Myoendothelial relations in the conduit coronary artery of the dog and rabbit. Journal of Vascular Research, 29, 29–32.
Sosa-Melgarejo, J. A., & Berry, C. L. (1992). Myoendothelial contacts in the thoracic aorta of rat fetuses. Journal of Pathology, 166, 311–316.
Sosa-Melgarejo, J. A., & Berry, C. L. (1995). Myoendothelial contacts in the human fetal aorta. Archives of Medical Research, 26, 431–435.
Sosa-Melgarejo, J. A., Berry, C. L., & Dodd, S. (1988). Myoendothelial contacts in the small arterioles of human kidney. Virchows Archiv. A, Pathological Anatomy and Histopathology, 413, 183–187.
Sosa-Melgarejo, J. A., & Berry, C. L. (1992). Myoendothelial contacts in arteriolosclerosis. Journal of Pathology, 167, 235–239.
Haas, T. L., & Duling, B. R. (1997). Morphology favors an endothelial cell pathway for longitudinal conduction within arterioles. Microvascular Research, 53, 113–120.
Sandow, S. L., & Hill, C. E. (2000). Incidence of myoendothelial gap junctions in the proximal and distal mesenteric arteries of the rat is suggestive of a role in endothelium-derived hyperpolarizing factor-mediated responses. Circulation Research, 86, 341–346.
Griffith, T. M., Chaytor, A. T., Bakker, L. M., & Edwards, D. H. (2005). 5-Methyltetrahydrofolate and tetrahydrobiopterin can modulate electrotonically mediated endothelium-dependent vascular relaxation. Proceedings of the National Academy of Sciences of the United States of America, 102, 7008–7013.
Martin, P. E., Wall, C., & Griffith, T. M. (2005). Effects of connexin-mimetic peptides on gap junction functionality and connexin expression in cultured vascular cells. British Journal of Pharmacology, 144, 617–627.
Acknowledgments
We thank P. Cook and M. Nugent for helpful discussions, E. Bartolak-Suki for critical reading of the manuscript, and C. Piron and E. Cosgrove for technical assistance with image analysis. This work was supported by the National Institute of Health (R01 HL72900-01), NASA (NAG9-1558) and a National Science Foundation CAREER Award (BES-9985338) to J.Y.W.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Jacot, J.G., Wong, J.Y. Endothelial Injury Induces Vascular Smooth Muscle Cell Proliferation in Highly Localized Regions of a Direct Contact Co-culture System. Cell Biochem Biophys 52, 37–46 (2008). https://doi.org/10.1007/s12013-008-9023-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12013-008-9023-6