Abstract
The coculture of vascular endothelial cells (ECs) on collagen gels containing smooth muscle cells (SMCs) has been carried out to investigate cellular interactions associated with blood vessel pathophysiology under wall shear stress (WSS) conditions. However, due to a lack of gel stiffness, the previous collagen gel coculture constructs are difficult to use for pathologic higher WSS conditions. Here, we newly constructed a coculture model with centrifugally compressed cell–collagen combined construct (C6), which withstands higher WSS conditions. The elastic modulus of C6 was approximately 6 times higher than that of the uncompressed collagen construct. The level of α-smooth muscle actin, a contractile SMC phenotype marker observed in healthy arteries, was elevated in C6 compared with that of the uncompressed construct, and further increased by exposure to a physiological level WSS of 2 Pa, but not by a pathological level of 20 Pa. WSS conditions of 2 and 20 Pa also induced different expression ratios of matrix metalloproteinases and their inhibitors in the C6 coculture model but did not in monocultured ECs and SMCs. The C6 coculture model will be a powerful tool to investigate interactions between ECs and SMCs under pathologically high WSS conditions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Responses of vascular endothelial cells (ECs) and the interactions with smooth muscle cells (SMCs) under hemodynamic wall shear stress (WSS) conditions are known to contribute to physiological homeostasis and pathological processes of blood vessel walls. The response of these cells changes depending on the magnitude of WSS, and the effects of lower WSS conditions have been extensively investigated to date because of their pathological implications in atherosclerosis.4,10 Recently, pathologically higher WSS conditions of more than 10 Pa were suggested to be involved in aortic dilatation and dissection,11,12 but the details of cellular responses and interactions under high WSS conditions remain unknown.
Previous studies have revealed the importance of interactions between ECs and SMCs in vascular pathophysiology with in vitro coculture experiments.4,25,28 A gel of collagen type I, one of the primary components of vessel walls, is commonly employed as a scaffold for embedding SMCs in coculture models.9,10 Because the collagen gel itself does not have sufficient stiffness, in some cases, to withstand even physiological levels of WSS conditions (~ 2 Pa), a polymer porous membrane was also incorporated into the EC–SMC coculture models for increasing the stiffness of models in our previous studies.22,23,24 However, although ECs and SMCs in native arterial intima are separated by a thin basement membrane, which has many pores and enables direct interactions between ECs and SMCs, cellular interactions can be prevented or suppressed by the polymer porous membrane in these coculture models. Indeed, the membrane has been suggested to inhibit or attenuate the interaction between cells and adversely affect their responses.25,28 For a better understanding of vascular cell physiology under WSS conditions, a coculture model in which ECs are directly cultured on a collagen gel construct containing SMCs and withstand WSS without a membrane is required.
Although the coculture experiments applying higher WSS conditions have not been conducted, previous studies have shown to successfully increase the stiffness of collagen gel constructs by plastically compressing the gel.5,6 In the present study, we describe a new coculture model constructed with an alternative technique called a centrifugally compressed cell–collagen combined construct (C6),31,32 which provides enough stiffness to the model without a polymer membrane and enables performing not only physiological levels but also much higher WSS experiments. Firstly, we compared the mechanical properties and cellular conditions between uncompressed and compressed (C6) collagen constructs. Secondly, we assessed the effect of high WSS (applied to the EC–SMC coculture model composed of C6) on the expression of α-smooth muscle actin (α-SMA) as an SMC contractile phenotype marker and matrix metalloproteinase (MMP) and tissue inhibitor of metalloproteinase (TIMP), whose imbalanced expressions are known to cause aortic dilatation and dissection.15,33
Materials and Methods
Construction of the Coculture Model with a Compressed Collagen Construct
Primary human aortic ECs and SMCs were purchased from Lonza (USA). Cells were cultured with Medium 199 (Thermo Fisher Scientific, USA) supplemented with 20% heat-inactivated fetal bovine serum (Nichirei Biosciences Inc., Japan), 10 ng/mL human basic fibroblast growth factor (Wako, Japan), and 1 unit/mL penicillin–streptomycin (Wako, Japan). ECs and SMCs from the 4th to 8th passages were used for the experiments.
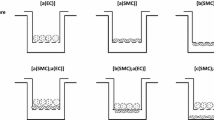
Solutions for the collagen gel layer, 0.5% type I collagen (Koken, Japan), 10 times the concentration of MEM-α (Thermo Fisher Scientific, USA), and the reconstruction buffer (0.05 mol/L NaOH, 200 mmol/L HEPES, and 0.26 mol/L NaHCO3) were mixed at a ratio of 8:1:1 at 4 °C, and SMCs were then suspended at densities ranging from 3 × 104 to 4 × 104 cells/mL. The mixed solution was poured into a 60-mm cell culture dish (Sumitomo Bakelite, Japan), in which a molding silicone ring (40 mm inner diameter, 52 mm outer diameter, and 10 mm thickness) was pre-installed, and incubated at 37 °C for 20 min (Fig. 1a). After being added to the culture medium, the collagen gel was incubated for 3 days. For constructing the centrifugally compressed cell–collagen combined construct (C6), a membrane filter (pore size 0.6 µm; GE Healthcare, Japan) and a silicone plate were placed on the molded collagen gel, and the gel was then set in a plate centrifuge (Kubota, Japan) at 3000 rpm (800×g) for 10 min (Figs. 1a, 1b). After the filter and the silicone plate were carefully removed, the culture medium was added and incubated for 7 days. ECs were then cultured directly on the C6 for 24 h. The models composed of only ECs (EC model) or SMCs (SMC model) were also constructed for comparison (Fig. 1c).
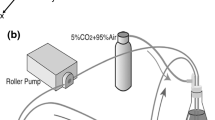
Diagrams of the constructed coculture models for flow-exposure experiments. (a) Schematic diagrams of the construction process of the centrifugally compressed cell–collagen combined construct (C6). (b) A photo-image of the compressed collagen construct (C6). (c) Schematic of the experimental models used in the present study: monoculture (EC and SMC models) and coculture (EC–SMC model). (d) A schematic diagram of the parallel-plate flow chamber, including the input/output unit, a silicone gasket, and the coculture model constructed in a cell culture dish.
Assessment of Collagen Construct
Collagen fibers in the collagen construct were observed by confocal reflection microscopy (Olympus, Japan). The elastic modulus of the collagen construct was also measured by atomic force microscope (AFM) indentation (JPK Instruments, Germany). We used a cantilever with pyramidal tips (2.9 µm in height and 15 nm in tip radius) and a spring constant of 0.08 N/m (OMCL-TR400PSA-1, Olympus). The indentations were performed at the displacement speed of 1.0 µm/s. We observed almost no hysteresis between the approach and retraction measurements, indicating that the deformation regime was largely elastic. The elastic modulus was calculated from the force curve according to the Sneddon model.26
Fluorescent Staining
The construct was washed three times with phosphate-buffered saline (PBS), stained with 1 mg/mL Calcein-AM (Invitrogen, USA) for 30 min, and fixed with 4% paraformaldehyde (Wako, Japan) for 30 min. Cell nuclei were also stained with 20 µg/mL Hoechest33342 (Invitrogen, USA). For staining of intercellular junction proteins of ECs, cells were incubated at room temperature (RT) with a monoclonal antibody against VE-cadherin (1:400, Santa Curz Biotechnology) for 1 h, followed by an Alexa Fluor 546-conjugated secondary antibody (1:400, Thermo Fisher Scientific) for 1 h at RT. Fluorescent images of the cells were obtained by confocal laser scanning microscopy (Olympus).
Flow-Exposure Experiment
A flow loop was constructed by connecting a pulse damper, a parallel-plate flow chamber, a reservoir, and a roller pump with silicone tubes, as described previously.23 The coculture model was incorporated into the parallel-plate flow chamber, in which an input/output unit and the coculture model was separated with a circular silicone gasket (0.3 or 0.5 mm in thickness) with a 24 × 35 mm rectangular hole at the center (Fig. 1d), forming the flow section over the EC monolayer of the model. The magnitude of WSS τ was calculated according to the following equation: τ = 6µQ/bh2, where Q = flow rate, µ = viscosity of culture media, b = width of the flow section, and h = height of the flow section. A spacer ring was placed to maintain the thickness of the model in the flow chamber. A steady WSS of 2 or 20 Pa, assumed to be the magnitude of peak WSS for physiological or pathological conditions in the aorta,12 was applied to the ECs of the coculture model in a parallel plate flow chamber for 24 h at 37 °C in a 95% air and 5% CO2 atmosphere.
Western Blotting
After two PBS washes, the ECs in the model were collected by trypsin treatment and lysed in ice-cold RIPA lysis buffer containing 1% protease inhibitor cocktail and 0.5% phenylmethylsulfonyl fluoride. After trypsinization, SMCs in the collagen gel were also lysed in the RIPA lysis buffer and underwent ultrasonic homogenization. The cell lysates were subjected to SDS-PAGE, transferred to PVDF membrane, and labeled with primary antibodies, followed by a horseradish peroxidase-conjugated secondary antibody (Cell Signaling Technology, USA). We used the following primary antibodies: rabbit monoclonal antibodies against α-SMA (1:1000, Abcam, UK), GAPDH (1:2000, Cell Signaling Technology), MMP-1 (1:1000), MMP-2 (1:1000), MMP-9 (1:1000), TIMP-1 (1:1000), and TIMP-2 (1:1000). The Western blotting bands were detected with ImageQuant LAS 4000 (GE Healthcare) and quantified with ImageJ software (National Institute of Health, USA).
Statistical Analysis
Results are expressed as mean ± standard error of the mean determined from at least three independent experiments. Student’s t-test was used for comparing differences between two groups, and the two-way analysis of variance (ANOVA) followed by Tukey test was used for comparing multiple groups. A p value of < 0.05 was considered statistically significant.
Results
Assessment of the Collagen Construct
Figure 2a shows the representative reflection microscopic images of collagen fibers. The fibers exhibited an evidently higher concentration of collagen and overlapping structures in the compressed collagen construct C6 than in the uncompressed construct. The gel thickness measured by confocal microscopy was 499.6 ± 18.2 µm for the uncompressed construct and 70.8 ± 5.9 µm for the C6 construct (mean ± SEM, n = 15), indicating that the concentration of the collagen gel was increased by approximately 7 times by the centrifugation. Typical force-indentation curves and elastic moduli for the uncompressed and C6 constructs obtained by the AFM indentation tests are shown in Fig. 2b. The elastic modulus of C6 was more than 6 times higher than that of the uncompressed construct (Uncompressed, 1.0 ± 0.2 kPa; C6, 6.9 ± 0.18 kPa, n = 3) (Fig. 2b).
Assessment of the compressed collagen construct. (a) Representative collagen fibers in the collagen constructs obtained by confocal reflection microscopy (left, uncompressed construct; right, compressed construct; bars = 10 µm). (b) Results of AFM indentation tests. A typical force-indentation curves for the uncompressed and C6 (compressed) constructs (left), and the elastic moduli of the uncompressed and compressed collagen constructs (right). *p < 0.05 (Student’s t-test, n = 3). (c) Representative confocal images of SMCs in the uncompressed (left) and compressed (right) collagen constructs (green, Calcein; blue, nucleus; bars = 100 µm).
Representative fluorescent images of SMCs in C6 and the uncompressed construct are shown in Fig. 2c. Almost all SMCs in both C6 and the uncompressed construct were stained with Calcein-AM, indicating that cells were alive after the 7-day culture in collagen constructs. The number of SMCs in the observed area was higher in C6 than in the uncompressed construct. SMCs exhibited a typical spindle shape in C6, whereas cells in the uncompressed construct were thinner and elongated. The α-SMA expression of SMCs in C6 was approximately 5 times higher than that of the cells in the uncompressed construct (Fig. 3). We also found decreasing tendencies of expression of MMPs and TIMPs in C6 compared with the uncompressed construct (Supplementary Fig. S1)
Expressions of α-smooth muscle actin (SMA) of SMCs. (a) Representative Western blot and (b) results of the densitometry of α-SMA of SMCs in uncompressed and compressed collagen constructs. The expression of α-SMA was normalized by glyceraldehyde-3-phosphate dehydrogenase (GAPDH). *p < 0.05 (Student’s t-test, n = 4).
Effect of WSS on the Expression of α-SMA in SMCs of the C6 Coculture Model
Figure 4a shows the fluorescence images of VE-cadherin expressed at intercellular junctions of ECs in the C6 coculture model exposed to 2 and 20 Pa WSS for 24 h. ECs maintained a confluent monolayer on the surface of C6 even after exposure to WSS.
Cell conditions after exposure to wall shear stress (WSS) conditions of 2 and 20 Pa for 24 h. (a) Fluorescence images of VE-cadherin in ECs on the surface of the compressed collagen construct (C6) coculture model, indicating an EC monolayer was maintained after exposure to 2 and 20 Pa WSS. The direction of flow was from left to right (green, VE-cadherin; blue, nucleus; bars = 50 µm). (b) Representative Western blots and (c) results of the densitometry of α-SMA of SMCs in the monoculture (n = 6) and the coculture models constructed with C6 (2 Pa, n = 5; 20 Pa, n =6). The “Coculture model (SMC)” means the sample of SMCs extracted from the C6 coculture model. The expressions of α-SMA are normalized by GAPDH and expressed as relative to statically cultured models. *p < 0.05 (Tukey’s post hoc test).
Results of Western blotting for α-SMA of SMCs are shown in Fig. 4b. The expression of α-SMA in SMCs was significantly increased in the C6 coculture model exposed to WSS of 2 Pa compared with that of cells in the SMC monoculture model at 2 Pa and those in the C6 coculture model exposed to the 20 Pa condition. Two-way ANOVA showed a significant interaction in α-SMA expression between WSS and coculture conditions (p < 0.05). Although the protein expression level was not adequate for Western blotting analysis, we also found by immunofluorescence microscopy that the number of cells expressed calponin-1, another contractile phenotype maker, tended to slightly increase in the compressed collagen gel compared with the uncompressed (Supplementary Fig. S2).
Effect of WSS on the Expression of MMP and TIMP in C6 Coculture Model Cells
Figures 5 and 6 show the MMPs and TIMPs expression of SMCs and ECs in C6 monoculture and coculture models after exposure to 2 and 20 Pa WSS conditions. Although Tukey post-hoc test did not detect significant differences between each of the pairs, statistical significance was determined by two-way ANOVA between 2 and 20 Pa WSS conditions in MMP-2 of ECs (p < 0.05). In MMP-1 expression of SMCs, both the effects of WSS and coculture conditions were also detected (p < 0.05, ANOVA). Regarding TIMPs, there were significant effects of coculture (p < 0.01, ANOVA) and interaction of WSS conditions and coculture on TIMP-1 expression (p < 0.01, ANOVA). The significant effect of WSS conditions was also detected in both TIMP-1 and -2 expressions in ECs (p < 0.01, ANOVA).
Expressions of MMP-1, MMP-2, and MMP-9 after exposure to WSS conditions of 2 and 20 Pa for 24 h. (a) Representative Western blots and results of the densitometry of MMP-1 (b), MMP-2 (c), and MMP-9 (d) of ECs and SMCs in the monoculture and coculture models constructed with C6 (SMC model, n = 6; Coculture model (SMC), n = 7; EC model n = 5–6; Coculture model (EC), n = 6–7). The “Coculture model (SMC) and (EC)” mean the samples of SMCs and ECs extracted from the C6 coculture model, respectively. The expressions are normalized by GAPDH and expressed as relative to statically cultured models. *p < 0.05 (Tukey’s post hoc test).
Expressions of TIMP-1 and TIMP-2 after exposure to WSS conditions of 2 and 20 Pa for 24 h. (a) Representative Western blots and results of the densitometry of TIMP-1 (b) and TIMP-2 (c) of ECs and SMCs in the monoculture and coculture models constructed with C6 (SMC model, n = 6; Coculture model (SMC), n = 7; EC model n = 3-4; Coculture model (EC), n = 7). The “Coculture model (SMC) and (EC)” mean the samples of SMCs and ECs extracted from the C6 coculture model, respectively. The expression levels are normalized by GAPDH and expressed as relative to statically cultured models. **p < 0.01, *p < 0.05 (Tukey’s post hoc test).
To further evaluate the effect of WSS conditions, ratios of MMPs and TIMPs were compared (Fig. 7), because the balance of MMP/TIMP has been shown to play important roles in physiological vascular remodeling and diseases such as aneurysms and atherosclerosis.2,13,15 By referring to previous studies,2,13 we assessed the ratios of MMP-1/TIMP-1, MMP-2/TIMP-2, and MMP-9/TIMP-1. There were no significant changes in MMP/TIMP ratios in EC and SMC monoculture models between the 2 and 20 Pa WSS conditions. MMP-1/TIMP-1 and MMP-9/TIMP-1 ratios were significantly decreased in ECs in the coculture model by applying WSS of 20 Pa compared to 2 Pa. In contrast, the MMP-2/TIMP-2 ratio was increased in ECs exposed to the 20 Pa condition. The MMP-9/TIMP-1 ratio of SMCs in the coculture model was also significantly increased in the 20 Pa WSS condition.
Ratios of MMP-1/TIMP-1 (a, b), MMP-2/TIMP-2 (c, d), and MMP-9/TIMP-1 (e, f) after exposure to the WSS conditions of 2 and 20 Pa for 24 h. (a, c, e) Results for the SMC and EC monocultured models, (b, d, f) results for the cocultured models constructed with C6 (SMC model, n = 5–6; Coculture model (SMC), n = 7; EC model n = 3–4; Coculture model (EC), n = 6–7). The “Coculture model (SMC) and (EC)” mean the samples of SMCs and ECs extracted from the C6 coculture model, respectively. **p < 0.01, *p < 0.05 (Tukey’s post hoc test).
Discussion
EC–SMC coculture models, in which SMCs were 3D cultured in a collagen gel for modeling the structures of native blood vessel walls, have been used for a fundamental understanding of the relationship between vascular pathophysiology and WSS conditions.8,9,10,23 Because the collagen gel does not have sufficient stiffness, it was difficult to use a coculture model constructed with it for the applications of not only higher WSS but also physiological levels of 2 Pa conditions. Previously, we performed flow-exposure experiments with high WSS conditions (~ 10 Pa), using a collagen gel coculture model in which a polymer porous membrane of approximately 10 µm in thickness was incorporated to increase model stiffness.7,8 However, the polymer porous membrane is much stiffer than the native extracellular matrix (ECM) of vessel walls. In addition, the membrane can limit cellular interactions via direct contacts between ECs and SMCs, whereas direct contacts of ECs with SMCs have been shown to play important roles in each cell function.28 To further investigate the roles of higher WSS conditions in vascular pathophysiology, a coculture model that allows direct interaction between ECs and SMCs and enables higher WSS experiments is required. In the present study, using the compressed collagen construct C6, we newly developed a coculture model withstanding WSS conditions of up to 20 Pa without a polymer porous membrane and permitting the direct contact of ECs and SMCs.
By applying centrifugal compression for the C6 construction, we found an increase in the expression of α-SMA in SMCs. We also obtained the tendencies for decreased expression of MMPs and TIMPs and increased calponin-1 expression of SMCs in C6 compared with the uncompressed construct (Supplementary Figs. S1 and S2). Although the mechanisms causing these changes in SMCs are unclear, the increases of the collagen fiber density and construct stiffness in C6 may affect the expression of α-SMA in SMCs. In addition, since previous studies have shown that mechanical stimuli are important determinants for the expression of SMC phenotype markers,18,21 we think that the mechanical environments accompanied by the centrifugation, including higher gravity and hydrostatic pressure, also contribute to these changes. Because cell growth was still observed in the compressed collagen construct (data not shown), we need a further evaluation of the SMC phenotype in C6 and the investigation of its underlying mechanisms, but centrifugal compression may have beneficial effects on SMCs in the collagen construct for inducing a more typical SMC state as a healthy vessel.
The application of WSS of 2 Pa to the C6 coculture model increased the expression of α-SMA compared to the 20 Pa WSS condition. We could not test the effect of 2 Pa WSS on α-SMA expression of SMCs in the uncompressed construct because the uncompressed collagen gel could not withstand 2 Pa WSS condition. Since changes in α-SMA expression by exposure to WSS were not observed in the SMC monoculture model, responses of ECs to the WSS condition of 2 Pa, but not 20 Pa, induced expression changes in SMCs. this result is consistent with previous studies showing the effect of physiological levels of WSS (approximately 2 Pa) applied to ECs on the cocultured SMC phenotype.27,29 In the present study, we conducted flow-exposure experiments with the model for 24 h and evaluated the changes in the expression of α-SMA as a representative marker of the early stage of SMC differentiation. However, previous studies have performed a 72-h experiment7,8 and used other differentiation markers such as myosin heavy chain, which is a marker for the later stage.1,7 To further evaluate the effects of physiological and pathological levels of WSS conditions on SMC phenotypic changes in the coculture model, we need to perform longer-term experiments and assess the changes in several differentiation markers. In addition, cultured proliferative synthetic SMCs can be thought of as a disease state and have different effects on ECs from quiescent contractile SMCs.16 In our previous study, we successfully controlled SMC phenotypes by arresting cell growth with a serum-free medium.8,22 Constructing with phenotype-controlled SMCs will be also required to understand the role of WSS in the phenotypic changes of SMCs observed in pathological vessel walls.
Higher WSS conditions of more than 10 Pa have been implicated in medial degeneration,11,12 which is a typical pathological change in blood vessel walls accompanying phenotype changes of SMCs and decreases in the extracellular matrix of the vessel wall.30 As described above, we assessed the expression of α-SMA, as an SMC phenotype marker, and found that the expression of α-SMA was significantly lower in the coculture model with the WSS condition of 20 Pa than of the 2 Pa condition, whereas no changes were observed in the SMC monoculture model under both WSS conditions. We also evaluated the ratios of MMP-1, -2, and -9, typical ECM degradation and remodeling enzymes, and their specific inhibitors TIMP-1 and -2, which reflect the net changes in proteolytic activities.17 Although MMP/TIMP ratios in EC and SMC monoculture models showed no differences between the 2 and 20 Pa conditions, the ratios significantly changed in the coculture model by exposure to 2 and 20 Pa WSS. This indicates that cellular interaction between ECs and SMCs under WSS conditions has a great impact on the balance between MMPs and TIMPs. Cellular communications mediated by cytokines and bioactive molecules play important roles in EC and SMC functions. In a previous study using a similar type of a coculture system, we have shown the roles of TGF-β1 secreted by ECs and nitric oxide in MMP productions of SMCs under WSS conditions.8,23 Compared to the physiological conditions of around 2 Pa WSS, under which the MMP/TIMP ratios can be assumed to be balanced, changes in EC production of these bioactive molecules under pathologic high shear stress conditions possibly cause the imbalance of MMP/TIMP ratios, which can be associated with pathological remodeling of arterial walls and the medial degeneration. Compared to the 2 Pa conditions, the MMP-2/TIMP-2 ratio in the ECs and the MMP-9/TIMP-1 ratio in SMCs were significantly increased by exposure to WSS of 20 Pa. MMP-9 primarily contributes to a decrease of extracellular matrix in media, and its activity increases at the site of medial degeneration in the aortic dissection.3,33 Results with the C6 coculture model will be helpful to reveal the role of responses in vascular cells to higher WSS conditions in the pathological degeneration of vessel walls.
Mechanical compression is an effective method to increase the strength of the collagen gels.5,6 By compression using only a normal plate centrifuge, we also made a construct with high collagen concentration and a stiffer construct. Our protocol would be beneficial to not only bioengineers but also researchers who do not have special mechanical devices and are not familiar with physics and mechanics. However, there are several limitations. Compression of the collagen gel increases not only the construct stiffness but also the density of collagen fibers surrounding SMCs in the construct, which can induce an elevated receptor-ECM interaction and modulate cell responses to environmental stimuli. Although this is important for regulating cellular conditions in the construct, our method cannot control independently both the collagen concentration and the gel stiffness. In addition, as a model of aortic walls, there are still differences between our C6 coculture model and native vessels. Mechanical stiffness of the C6 construct of about 7 kPa is much lower than that of physiological aortic walls (> 30 kPa),20 and the cell density in the C6 is thought to be not enough to mimic native vessel wall conditions. Since the SMCs secrete intercellular signaling molecules, the ratio of EC and SMC densities may be important for investigating cellular interactions. The stiffness of the extracellular matrix is also known to affect both EC and SMC functions.14,19 We need to clarify the effects of these factors on functions of ECs and SMCs under coculture conditions. Furthermore, aortic walls consist of not only ECs, SMCs, and collagen type I but also fibroblasts in mainly adventitia and the other types of ECM components. Although the effects of these components are thought to be not well studied yet, we need to incorporate other intervening cells and ECMs for the improvement of the coculture model.
In summary, we constructed a novel EC–SMC coculture model with a compressed collagen construct C6 that enables the performance of higher WSS experiments. The C6 showed a potential to induce SMC phenotype modulation from synthetic to contractile states. We found that the high WSS condition of 20 Pa led to a significantly different expression of MMP-1/TIMP-1, MMP-2/TIMP-2, and MMP-9/TIMP-1 in ECs and SMCs in the C6 coculture model compared with a physiological level of 2 Pa. The coculture model constructed in the present study will be useful to investigate the relationship between media degeneration and a wide range of WSS magnitudes.
References
Abberton, K. M., D. L. Healy, and P. A. Rogers. Smooth muscle alpha actin and myosin heavy chain expression in the vascular smooth muscle cells surrounding human endometrial arterioles. Hum. Reprod. 14(12):3095–3100, 1999.
Aoki, T., H. Kataoka, T. Moriwaki, K. Nozaki, and N. Hashimoto. Role of TIMP-1 and TIMP-2 in the progression of cerebral aneurysms. Stroke. 38(8):2337–2345, 2007.
Chen, L., X. Wang, S. A. Carter, Y. H. Shen, H. R. Bartsch, R. W. Thompson, J. S. Coselli, D. L. Wilcken, X. L. Wang, and S. A. LeMaire. A single nucleotide polymorphism in the matrix metalloproteinase 9 gene (-8202A/G) is associated with thoracic aortic aneurysms and thoracic aortic dissection. J. Thorac. Cardiovasc. Surg. 131(5):1045–1052, 2006.
Chiu, J. J., L. J. Chen, P. L. Lee, C. I. Lee, L. W. Lo, S. Usami, and S. Chien. Shear stress inhibits adhesion molecule expression in vascular endothelial cells induced by coculture with smooth muscle cells. Blood. 101(7):2667–2674, 2003.
Ghezzi, C. E., B. Marelli, N. Muja, and S. N. Nazhat. Immediate production of a tubular dense collagen construct with bioinspired mechanical properties. Acta Biomater. 8(5):1813–1825, 2012.
Ghezzi, C. E., P. A. Risse, B. Marelli, N. Muja, J. E. Barralet, J. G. Martin, and S. N. Nazhat. An airway smooth muscle cell niche under physiological pulsatile flow culture using a tubular dense collagen construct. Biomaterials. 34(8):1954–1966, 2013.
Han, X., N. Sakamoto, N. Tomita, H. Meng, M. Sato, and M. Ohta. Influence of shear stress on phenotype and MMP production of smooth muscle cells in a co-culture model. J. Biorheol. 31(2):50–56, 2017.
Han, X., N. Sakamoto, N. Tomita, H. Meng, M. Sato, and M. Ohta. Influence of TGF-beta1 expression in endothelial cells on smooth muscle cell phenotypes and MMP production under shear stress in a co-culture model. Cytotechnology. 71(2):489–496, 2019.
Hong, M., H. Jo, R. F. Ankeny, C. J. Holliday-Ankeny, H. Kim, G. Khang, and R. M. Nerem. Influence of mesenchymal stem cells on the response of endothelial cells to laminar flow and shear stress. Cells Tissues Organs. 198(4):289–299, 2013.
Imberti, B., D. Seliktar, R. M. Nerem, and A. Remuzzi. The response of endothelial cells to fluid shear stress using a co-culture model of the arterial wall. Endothelium. 9(1):11–23, 2002.
Karmonik, C., S. Partovi, M. Muller-Eschner, J. Bismuth, M. G. Davies, D. J. Shah, M. Loebe, D. Bockler, A. B. Lumsden, and H. von Tengg-Kobligk. Longitudinal computational fluid dynamics study of aneurysmal dilatation in a chronic DeBakey type III aortic dissection. J. Vasc. Surg. 56(1):260–263, 2012.
Kimura, N., M. Nakamura, K. Komiya, S. Nishi, A. Yamaguchi, O. Tanaka, Y. Misawa, H. Adachi, and K. Kawahito. Patient-specific assessment of hemodynamics by computational fluid dynamics in patients with bicuspid aortopathy. J. Thorac. Cardiovasc. Surg. 153(4):S52–S62, 2017.
Knox, J. B., G. K. Sukhova, A. D. Whittemore, and P. Libby. Evidence for altered balance between matrix metalloproteinases and their inhibitors in human aortic diseases. Circulation. 95(1):205–212, 1997.
Kohn, J. C., D. W. Zhou, F. Bordeleau, A. L. Zhou, B. N. Mason, M. J. Mitchell, M. R. King, and C. A. Reinhart-King. Cooperative effects of matrix stiffness and fluid shear stress on endothelial cell behavior. Biophys. J. 108(3):471–478, 2015.
Koullias, G. J., P. Ravichandran, D. P. Korkolis, D. L. Rimm, and J. A. Elefteriades. Increased tissue microarray matrix metalloproteinase expression favors proteolysis in thoracic aortic aneurysms and dissections. Ann. Thorac. Surg. 78(6):2106–2110, 2004.
Lavender, M. D., Z. Pang, C. S. Wallace, L. E. Niklason, and G. A. Truskey. A system for the direct co-culture of endothelium on smooth muscle cells. Biomaterials. 26(22):4642–4653, 2005.
Moore, C. S., and S. J. Crocker. An alternate perspective on the roles of TIMPs and MMPs in pathology. Am. J. Pathol. 180(1):12–16, 2012.
Nagayama, K., N. Morishima, and T. Matsumoto. Effects of three-dimensional culture and cyclic stretch stimulation on expression of contractile proteins in freshly isolated rat aortic smooth muscle cells. J. Biomech. Sci. Eng. 4(2):286–297, 2009.
Nagayama, K., and K. Nishimiya. Moderate substrate stiffness induces vascular smooth muscle cell differentiation through cellular morphological and tensional changes. Biomed. Mater. Eng. 31(3):157–167, 2020.
Rezvani-Sharif, A., M. Tafazzoli-Shadpour, and A. Avolio. Mechanical characterization of the lamellar structure of human abdominal aorta in the development of atherosclerosis: an atomic force microscopy study. Cardiovasc. Eng. Technol. 10(1):181–192, 2019.
Roby, T., S. Olsen, and J. Nagatomi. Effect of sustained tension on bladder smooth muscle cells in three-dimensional culture. Ann. Biomed. Eng. 36(10):1744–1751, 2008.
Sakamoto, N., T. Kiuchi, and M. Sato. Development of an endothelial-smooth muscle cell coculture model using phenotype-controlled smooth muscle cells. Ann. Biomed. Eng. 39(11):2750–2758, 2011.
Sakamoto, N., T. Ohashi, and M. Sato. Effect of fluid shear stress on migration of vascular smooth muscle cells in cocultured model. Ann. Biomed. Eng. 34(3):408–415, 2006.
Sakamoto, N., Y. Ueki, M. Oi, T. Kiuchi, and M. Sato. Fluid shear stress suppresses ICAM-1-mediated transendothelial migration of leukocytes in coculture model. Biochem. Biophys. Res. Commun. 502(3):403–408, 2018.
Shav, D., R. Gotlieb, U. Zaretsky, D. Elad, and S. Einav. Wall shear stress effects on endothelial–endothelial and endothelial–smooth muscle cell interactions in tissue engineered models of the vascular wall. PLoS ONE.9(2):e88304, 2014.
Sneddon, I. N. The relation between load and penetration in the axisymmetric boussinesq problem for a punch of arbitrary profile. Int. J. Eng. Sci. 3(1):47–57, 1965.
van Engeland, N. C. A., A. Pollet, J. M. J. den Toonder, C. V. C. Bouten, O. Stassen, and C. M. Sahlgren. A biomimetic microfluidic model to study signalling between endothelial and vascular smooth muscle cells under hemodynamic conditions. Lab Chip. 18(11):1607–1620, 2018.
Wallace, C. S., and G. A. Truskey. Direct-contact co-culture between smooth muscle and endothelial cells inhibits TNF-alpha-mediated endothelial cell activation. Am. J. Physiol. Heart Circ. Physiol. 299(2):H338-346, 2010.
Wang, L., Y. Han, Y. Shen, Z. Q. Yan, P. Zhang, Q. P. Yao, B. R. Shen, L. Z. Gao, Y. X. Qi, and Z. L. Jiang. Endothelial insulin-like growth factor-1 modulates proliferation and phenotype of smooth muscle cells induced by low shear stress. Ann. Biomed. Eng. 42(4):776–786, 2014.
Wu, D., Y. H. Shen, L. Russell, J. S. Coselli, and S. A. LeMaire. Molecular mechanisms of thoracic aortic dissection. J. Surg. Res. 184(2):907–924, 2013.
Yamasaki, M., K. Oya, M. Numao, and H. Fujie. Development of a mesenchymal stem cell-based aggregate reinforced by fiberized collagen fibrils for tissue repair. In European Chapter Meeting of the Tissue Engineering and Regenerative Medicine International Society. 2017. Switzerland: European Cells and Materials.
Yamasaki, M., K. Oya, M. Numao, and H. Fujie. Development of a novel tissue-engineered material composed of mesenchymal stem cells and collagen fibril. In 26th International Society of Biomechanics. 2017.
Zhang, X., D. Wu, J. C. Choi, C. G. Minard, X. Hou, J. S. Coselli, Y. H. Shen, and S. A. LeMaire. Matrix metalloproteinase levels in chronic thoracic aortic dissection. J. Surg. Res. 189(2):348–358, 2014.
Acknowledgments
The present study was supported in part by Grants-in-Aid for Scientific Research from the MEXT of Japan (Grant Nos. 17K10763, 18K08770, 18K19934, 20K09154, and 21K08828) and Tokyo Metropolitan Government Advanced Research Grant (Grant No. R2-2).
Funding
Funding was provided by Ministry of Education, Culture, Sports, Science and Technology and Tokyo Metropolitan Government (Grant No. R2-2).
Conflict of interest
The authors declare that they have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Jane Grande-Allen oversaw the review of this article.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file1 (TIFF 10263 KB)
Supplementary Figure S1 Relative expressions of MMP-2, MMP-9, TIMP-1, and TIMP-2 of SMCs in compressed collagen constructs. Results are normalized by GAPDH and expressed as relative to the expression of SMCs in the uncompressed collagen constructs. *p < 0.05 (Mann-Whitney U test).
Supplementary file2 (TIFF 10263 KB)
Supplementary Figure S2 Representative fluorescence images of calponin-1 of SMCs in the uncompressed (left) and the compressed (C6)(right) collagen constructs. Bars = 100 µm. With a primary antibody for calponin-1 (1:200, Cell Signaling Technology), fluorescent staining was performed according to the method stated in the manuscript. By counting the number of cells in the images, we found the ratio of calponin-1 expressing cells in the compressed (C6) construct was 0.29 ± 0.02, while that in the uncompressed gel was 0.23 ± 0.02 (mean ± SEM, n = 3).
Rights and permissions
About this article
Cite this article
Hiroshima, Y., Oyama, Y., Sawasaki, K. et al. A Compressed Collagen Construct for Studying Endothelial–Smooth Muscle Cell Interaction Under High Shear Stress. Ann Biomed Eng 50, 951–963 (2022). https://doi.org/10.1007/s10439-022-02972-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-022-02972-7