Abstract
The mechanisms underlying arrhythmia induced by the clinical use of azithromycin are poorly understood. We aimed to investigate the proarrhythmic effects of azithromycin using electrocardiogram (ECG) and ion channel models. In vivo and in vitro guinea pig ECG and current and voltage clamp recordings were carried out. Azithromycin at 114.6 mg/kg (three times the clinically relevant dose) reduced heart rate (HR) and prolonged the PR, QRS and rate-corrected QT (QTc) intervals of guinea pig ECG in vivo. In vitro technique revealed that azithromycin at 207.5 and 415 mg/L [five and ten times clinically relevant concentration (CRC)] reduced HR and prolonged the PR, QRS and QTc intervals in the isolated guinea pig heart ECG. Both arrhythmias presented bradyarrhythmic features, mainly with reduced HR and prolonged PR interval. Action potential analysis from the guinea pig cardiomyocytes indicated that azithromycin at 830 mg/L (20 times CRC) significantly prolonged the action potential durations at 50% (APD50) and 90% (APD90) of full repolarization levels with a rectangular pattern. Azithromycin significantly suppressed the L-type Ca2+ and Na+ currents from the left ventricular myocytes of guinea pig at 50% inhibiting concentrations (IC50) of 942.5 ± 68.4 mg/L (22.7 times CRC) and 1123.0 ± 87.7 mg/L (27.1 times CRC), respectively. However, azithromycin at 50 times CRC (2075 mg/L) inhibited IKr current at an inhibition rate of 30.99 ± 5.23% with an undetectable IC50. Azithromycin caused bradyarrhythmia primarily by inhibiting L-type Ca2+ and Na+ currents.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Azithromycin is a macrolide antibiotic, which belongs to the polyketide class of natural products. Azithromycin inhibits bacterial protein synthesis and is commonly used to treat infections caused by Gram-positive bacteria and Haemophilus influenzae, such as respiratory tract and soft tissue infections.
With widespread clinical use, azithromycin has been found to cause an increased risk of abnormal rate-corrected QT (QTc) prolongation and cardiovascular death compared with controls in a retrospective cohort study [1, 2]. Moreover, clinical investigations [3,4,5], case report [6] and animal study [7] have supported azithromycin’s proarrhythmic nature. Given increasing evidence of azithromycin’s proarrhythmic effects, the FDA released a safety announcement on May 12, 2013, stating that azithromycin can cause abnormal changes in the electrical activity of the heart that may lead to a potentially fatal irregular heart rhythm [8].
Other studies have reported that azithromycin induces adverse cardiac events only under certain circumstances. Patient with a high baseline risk [9], who concomitantly used with other drugs [10,11,12,13,14], or both [15] experienced adverse cardiac events. An in vivo anesthetized dog experiment found that azithromycin neither induced torsade de pointes (TdP) nor affected beat-to-beat variability of repolarization, which increases the risk of chronic atrioventricular block, in dogs at a dose of 17.0 times the therapeutic concentration (30 mg/kg) administered intravenously [16].
Many studies, however, have reported that azithromycin is not associated with a higher ventricular arrhythmia risk compared to non-macrolide antibiotics. Population-based retrospective cohort studies [17, 18], systematic review [19] and both in vivo [20] and in vitro study [21, 22] have demonstrated that azithromycin was not associated with a higher risk of ventricular arrhythmia compared with non-macrolide antibiotics, with some investigators arguing that the Food and Drug Administration overstated the proarrhythmic effects of azithromycin [17].
To date, studies evaluating the potential proarrhythmic effects induced by azithromycin are discordant. Potential explanations for these discrepancies include differences in dosages, concomitant drug use, additional proarrhythmic risk factors, genetic predisposition of patients and, in cases, the use of QTc prolongation as the only marker for proarrhythmic effects without a clinical correlate. In order to provide a more thorough understanding of these discrepancies, the current study was undertaken to investigate the basic mechanistic effects of azithromycin on potential arrhythmias using electrocardiogram (ECG) and ion channel models.
Methods
Animals
Male guinea pigs (250–300 g) were used in this study. All experiments were approved by the Animal Care and Use Committee of Nanjing University of Chinese Medicine. Animal breeding was authorized by the local government with certification number SCX(Jiangsu)-2012-0008.
Chemicals
Prescribed azithromycin maleate (lyophilized type; batch number 081406026) was approved by the State Food and Drug Administration (SFDA) with registration number H20020293 and purchased from a local hospital in accordance with relevant local regulations. All other chemicals were purchased from Chinese chemical factories with corresponding purities higher than 99.9% or from Sigma–Aldrich.
Conversions of Doses and Concentrations Between Human and Guinea Pig
Azithromycin maleate, following prescribing specifications, is used in clinical settings at a maximum of 500 mg/day for a human adult, which is equivalent to 8.3 mg/kg/day based on a body weight of 60 kg for a human adult. For in vivo experiments, in accordance with a 4.6 times dose conversion factor between human and guinea pig based on body surface area, a clinically relevant dose (CRD) of azithromycin maleate is matched to 38.2 mg/kg for guinea pigs [23, 24]. Considering human extracellular fluid is 20% of body weight, a human dose of azithromycin maleate at 8.3 mg/kg is converted to a one time clinical concentration of 41.5 mg/L for human subjects (based on intravenous injection, rapidly administered in the absence of any metabolism) [24]. For in vitro experiments, the 41.5 mg/L of azithromycin maleate was utilized as a one time clinically relevant concentration (CRC) for tissue and cell experiments.
In Vivo and In Vitro ECG Recordings
For in vivo ECG recordings, ECG (lead II) was recorded from anesthetized guinea pigs (20% urethane 5 mL/kg, intraperitoneal) by conventional in vivo ECG technique in which the heart rate (HR), PR, QRS and QTc intervals were analyzed [24, 25]. Lyophilized azithromycin maleate was dissolved in saline and administrated intravenously. For in vitro ECG recordings, an isolated guinea pig heart perfusion system was used to record ECG. The hearts from anaesthetized guinea pigs were excised and cannulated through the aorta above the coronary ostia at constant pressure (70 mmHg) with perfusion solution (37 °C) containing (mM): NaCl 117, CaCl2 2.5, KCl 5.7, NaHCO3 4.4, MgCl2 1.7, HEPES 20, NaH2PO4 1.5, glucose 11, gassed with 95% O2 plus 5% CO2, pH 7.4 with NaOH. Drugs were added in the perfusion solution and infused via retrograde perfusion of the coronary artery. The positive electrode was placed at the cardiac apex, the negative electrode at right atrium and the reference electrode at the root of the aorta [24, 25]. Nine guinea pigs were used for in vivo ECG experiments and ten guinea pigs for in vitro ECG experiments. Six effective guinea pig hearts were used to record ECG recording for in vivo or in vitro experiments. Three guinea pigs in vivo and four guinea pigs in vitro were ruled out for experiments due to electrical noise or death secondary to anesthetic accident.
Action Potential Recordings from Single Ventricular Myocyte
Guinea pig ventricular myocytes were obtained by enzymatic dissociation as previously described [24, 25]. A whole-cell current clamp configuration was used to record action potentials with an amplifier (Axopatch 200B, Axon Instruments). The composition of internal solution was (mM): KCl 135, EGTA 10, glucose 5, HEPES 10, Na2-ATP 3, Na-GTP 0.5, pH 7.3 with KOH. The external solution contained (mM): NaCl 117, KCl 5.7, NaHCO3 4.4, MgCl2 1.7, HEPES 20, glucose 20, taurine 20, CaCl2 1.8, pH 7.4 with NaOH. All experiments were performed at room temperature (20–22 °C). Action potentials were elicited by passing appropriate current pulses at 0.1 Hz through the recording electrode. Action potential durations at 50 and 90% full repolarization levels (APD50 and APD90) were measured. Ten guinea pigs were used for single left ventricular myocyte action potential recording experiment.
Recordings of L-type Ca2+, Na+ and IKr Currents from Guinea Pig Left Ventricular Myocytes
Whole-cell voltage clamp technique was used to record L-type Ca2+, Na+ and IKr currents from guinea pig left ventricular myocytes. For L-type Ca2+ current, the recording external solution contained (mM): NaCl 132, CsCl 5.4, CaCl2 1.8, MgCl2 1.8, NaH2PO4 0.6, 4-AP 5, HEPES 10, glucose 10, Na-pyruvate 5, pH 7.4 with NaOH. The internal solution contained (mM): CsCl 130, MgCl2 2, EGTA 11, HEPES 20, glucose 10, Na2-ATP 2, Na-GTP 0.1, pH 7.3 with CsOH. To record the L-type Ca2+ current, the depolarizing pulses applied from a holding potential of −80 mV were stepped to −40 mV for 30 ms (to inactivate Na+ current) and then to 0 mV for 300 ms at 0.1 Hz.
For Na+ current recordings, the external solution contained (mM): NaCl 70, choline-Cl 70, KCl 5.4, MgCl2 1, CaCl2 0.1, HEPES 10, glucose 10, NaH2PO4 0.33, pH 7.4 with NaOH. The internal solution contained (mM): CsCl 120, EGTA 11, HEPES 10, Na2-ATP 5, MgCl2 5, CaCl2 1, glucose 11, pH 7.3 with CsOH. The stimulation pulse for recording Na+ current was stepped −40 mV for 50 ms from a holding potential of −80 mV at 0.1 Hz.
For IKr current recordings, the external solution contained (mM): NaCl 140, KCl 5.4, MgCl2 1, CaCl2 1.5, glucose 10, HEPES 10, pH 7.4 with NaOH. The internal solution contained (mM): KCl 140, MgCl2 1, Na2-ATP 4, EGTA 5, HEPES 10, pH 7.30 with KOH. To record IKr, 11 depolarizing pulses applied from a holding potential of −80 mV were stepped from −40 to +60 mV with 10 mV increment for each for 2000 ms and then to −40 mV for 2000 ms at 0.03 Hz. Moreover, in IKr current recording, 4-AP (200 μM), chromanol 293B (20 μM), BaCl2 (200 μM) and nifedipine (10 μM) were added to the external solution to block Ito, IKs, IK1 and L-type Ca2+ currents, respectively. Nineteen guinea pigs were used for L-type Ca2+, Na+ and IKr current recording experiments.
Statistical Analyses
All values are expressed as mean ± SE. One-way ANOVA was performed to analyze data using SigmaPlot 12.5. Differences with P < 0.05 were deemed statistically significant.
Results
Effects of Azithromycin on the HR, PR, QRS and QTc Intervals in In Vivo and In Vitro Guinea Pig ECG
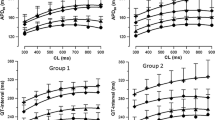
The effects of jugular intravenous injection of azithromycin at one (38.2 mg/kg), three (114.6 mg/kg) and five times CRD (191 mg/kg), cumulatively, were analyzed. Azithromycin at three times CRD (114.6 mg/kg) significantly reduced the HR and caused the prolongations on PR, QRS and QTc intervals (Table 1, upper panel). Moreover, azithromycin at five times CRD (191 mg/kg) further reduced the HR. P and T waves were undetectable and a deformed QRS occurred at this dose due to the complete atrioventricular blockage and the merging of the QRS complex and T wave (Fig. 1a). Administration of equivalent volumes of saline had no effect on the above parameters. To test whether arrhythmias induced by azithromycin, as measured by in vivo ECG, occur independently of the neurohumoral system, metabolites and/or allergic reactions, an isolated guinea pig heart was used to analyze the effects of azithromycin on ECG. Azithromycin, starting from one time CRC (41.5 mg/L), significantly reduced the HR in a concentration-dependent manner. Azithromycin, at five (207.5 mg/L) and ten (415 mg/L) times CRCs, also significantly prolonged the PR, QRS and QTc intervals. These prolonged PR and QRS intervals, but not QTc, presented in a concentration-dependent manner. (Table 1, lower panel). Washout partially abolished the effects of azithromycin on reduced HR and prolonged PR, QRS and QTc intervals (Fig. 1b).
Representative tracings of guinea pig in vivo and in vitro ECG showing that azithromycin reduced the HR and prolonged PR, QRS and QTc intervals. The numbers in parentheses indicate the corresponding multiple(s) of clinically relevant doses or concentrations. AZM azithromycin. The single arrow and double arrows indicate the absence of a P wave and the merging of the QRS complex and T wave, respectively
Azithromycin-Induced Action Potential Prolongation in Guinea Pig Left Ventricular Myocytes
To investigate the cellular mechanisms underlying the effects of azithromycin on ECG in both in vivo and in vitro models, single left ventricular myocytes were used to record action potentials. Azithromycin, at one (41.5 mg/L) and ten times CRC (415 mg/L), did not significantly prolong the APD50 (from control 420.40 ± 19.84 to 438.20 ± 22.82 ms for one time CRC and 498.06 ± 34.98 ms for ten times CRC, respectively) and APD90 (from control 435.04 ± 19.81 to 454.24 ± 22.25 ms for one time CRC and 514.92 ± 35.05 ms for ten times CRC, respectively). However, azithromycin at 20 times CRC (830 mg/L) prolonged APD50 (from control 420.40 ± 19.84 to 562.58 ± 34.82 ms, P < 0.05, vs. control or one time CRC group, n = 5) and APD90 (from control 435.04 ± 19.81 to 579.96 ± 34.87 ms, P < 0.05, vs. control or one time CRC group, n = 5). Moreover, prolonged action potentials presented a rectangular pattern (Fig. 2), indicating a weak blocking effect of azithromycin on IKr current.
Representative tracings of action potentials indicating that azithromycin prolonged the action potential durations at 50% (APD50) and 90% (APD90) of full repolarization levels in isolated guinea pig left ventricular myocytes. The numbers in parentheses indicate the corresponding multiple(s) of clinically relevant concentrations. AZM azithromycin
Effects of Azithromycin on L-Type Ca2+, Na+ and IKr Currents from Guinea Pig Left Ventricular Myocytes
L-type Ca2+ currents from guinea pig left ventricular myocytes were tested to assess the potential underlying mechanism of azithromycin effects on HR reduction and PR interval prolongation. Azithromycin at one (41.5 mg/L), ten (415 mg/L), 30 (1245 mg/L) and 50 times CRC (2075 mg/L) blocked L-type Ca2+ current by 16.94 ± 1.15, 31.82 ± 1.08, 53.72 ± 2.38 and 67.14 ± 1.85%, respectively, in a concentration-dependent manner with an IC50 of 942.5 ± 68.4 mg/L (22.7 times CRC) (Fig. 3a, d for representative traces and the relationship between % inhibition of L-type Ca2+ current and concentrations, respectively).
Representative tracings showing that azithromycin inhibited L-type Ca2+, Na+ and IKr currents. a–c Suppression tracings of L-type Ca2+, Na+ and IKr currents from guinea pig left ventricular myocytes by azithromycin, respectively. d–f Relationships between % inhibition of L-type Ca2+, Na+ and IKr currents and concentrations, respectively. The numbers in parentheses indicate the corresponding multiple(s) of clinically relevant concentrations. AZM azithromycin
Given that azithromycin induced PR prolongation and merging of the QRS and T waves in guinea pig hearts, Na+ current was then recorded to test the possible underlying mechanism. Azithromycin at one (41.5 mg/L), ten (415 mg/L), 30 (1245 mg/L) and 50 times CRC (2075 mg/L) blocked Na+ current by 10.65 ± 0.78, 28.70 ± 0.79, 51.71 ± 2.41 and 66.36 ± 2.60%, respectively, in a concentration-dependent manner with an IC50 of 1123.0 ± 87.7 mg/L (27.1 times CRC) (Fig. 3b, e).
IKr current, which mediates the action potential repolarization, was recorded to investigate the potential mechanism of azithromycin’s effect on QTc prolongations for both in vivo and in vitro ECG. Taking the depolarization pulse at +60 mV for analysis, azithromycin at one (41.5 mg/L), ten (415 mg/L), 30 (1245 mg/L) and 50 times CRC (2075 mg/L) blocked IKr current by 8.80 ± 2.43, 15.94 ± 2.51, 24.86 ± 4.20 and 30.99 ± 5.23%, respectively, in a concentration-dependent manner with an undetectable IC50 up to 50 times CRC (2075 mg/L) (Fig. 3c, f). For L-type Ca2+, Na+, and IKr current recordings, a total of four cells were evaluated with each cell isolated from a different guinea pig heart.
Discussion
Azithromycin at three times CRD (114.6 mg/kg) induced a significant reduction in HR, and a prolongation of PR, QRS and QTc intervals. Extending the dosage to five times CRD (191 mg/kg) caused the disappearance of sinus rhythm and induced ventricular arrhythmia with an ectopic pacemaker and deformed QRS wave, which may cause cardiac arrest of in vivo guinea pig heart.
In the isolated guinea pig heart ECG study, azithromycin reduced HR and caused PR and QRS interval prolongation in a concentration-dependent manner. Azithromycin at five times CRC (207.5 mg/L) also prolonged the QTc interval. Changes in HR, PR, QRS and QTc could be washed out, indicating that the effect of azithromycin on the acting target(s) is reversible. The proarrhythmic effect of azithromycin on the in vitro ECG, which was independent of the neurohumoral system, metabolites and/or an allergic reaction, was mediated by an inhibition of ion channels in the cardiac myocyte. In addition, in the isolated guinea pig heart ECG study, azithromycin at five times CRC (207.5 mg/L) induced a similar effect as three times CRD (114.6 mg/kg) on in vivo ECG, indicating that dose conversions from in vivo to in vitro concentrations are suitable in this study.
The action potential of cardiomyocyte is the basis of ECG waveforms and results from the integration of various ion channel activities. The action potential experiments in this study not only further confirmed the effects of azithromycin on ECG but also explored the underlying mechanism. Azithromycin had little prolonging effects on APD50 and APD90 and significantly prolonged APD50 and APD90 only at up to 20 times CRC (830 mg/L) with a rectangular, not triangular pattern [22], indicating that azithromycin hardly induced early afterdepolarization (EAD), which is the basis of TdP in ventricular myocytes. To investigate the mechanism behind azithromycin’s effects on ECG and action potentials, the roles of L-type Ca2+, Na+ and IKr channels were examined. These three ion channels shape the action potential pattern not only from the ventricular myocytes but also from P cells of the sinoatrial node and cells of the atrioventricular node. In concordance with the ECG and action potential studies, the primary ion channel currents, not cloned human cDNA ion channel currents such as hERG and hNav1.5, were recorded from guinea pig ventricular myocytes. Results demonstrated that azithromycin blocked L-type Ca2+, Na+ and IKr currents in a concentration-dependent manner and blocked L-type Ca2+ and Na+ currents first at relatively low concentrations based on the IC50 values of the three ion channel currents. Although azithromycin blocked IKr current, which prolongs the APD and may mediate EAD, azithromycin might not induce the EAD due to the coexisting of blocking L-type Ca2+ and Na+ currents. The inhibitions of L-type Ca2+ and Na+ currents were stronger than that of IKr current. It is apparent from the current results that azithromycin-induced inhibition of these three ion channels was mediated by its direct action on these channels, based on the washout-induced abolition of the effects on in vitro ECG. An additional possibility is that effects may be partially mediated by the azithromycin-induced destruction of cardiomyocyte mitochondria [26].
The current results for azithromycin tested at one time clinical dose multiples may not generalize to clinical outcomes, and a potential arrhythmic risk remains for patients even without heart disease risk factors. Moreover, according to standard criteria for drug concentration selection for preclinical cardiac safety evaluations [27], up to 30 times the clinical dose for treating moderate disease should be evaluated to rule out the potential proarrhythmic risk for patients with heart disease risk factors in clinics. Interactions with additional drugs and clinic-to-clinic treatment variations may pose additional potential for azithromycin to cause severe arrhythmia.
A limitation of this study is that the concentrations of azithromycin in some tissues, such as white blood cells [28], are orders of magnitude higher than that in plasma. To date, we do not know azithromycin’s concentration in myocardial tissue after intravenous administration in humans. Therefore, the one time CRCs in this study may not be equal to concentrations of azithromycin in clinic.
In conclusion, azithromycin may cause bradyarrhythmia by inhibiting L-type Ca2+ and Na+ currents. The use of QTc prolongation as the sole marker for clinical proarrhythmic risk evaluation may result in an underestimation of the proarrhythmic liability of azithromycin in patients.
References
Lee, R. A., Guyton, A., Kunz, D., Cutter, G. R., & Hoesley, C. J. (2016). Evaluation of baseline corrected QT interval and azithromycin prescriptions in an academic medical center. Journal of Hospital Medicine, 11, 15–20.
Huang, B. H., Wu, C. H., Hsia, C. P., & Yin Chen, C. (2007). Azithromycin-induced torsade de pointes. Pacing and Clinical Electrophysiology, 30, 1579–1582.
Ray, W. A., Murray, K. T., Hall, K., Arbogast, P. G., & Stein, C. M. (2012). Azithromycin and the risk of cardiovascular death. The New England Journal of Medicine, 366, 1881–1890.
Rao, G. A., Mann, J. R., Shoaibi, A., Bennett, C. L., Nahhas, G., Sutton, S. S., et al. (2014). Azithromycin and levofloxacin use and increased risk of cardiac arrhythmia and death. Annals of Family Medicine, 12, 121–127.
Chou, H. W., Wang, J. L., Chang, C. H., Lai, C. L., Lai, M. S., & Chan, K. A. (2015). Risks of cardiac arrhythmia and mortality among patients using new-generation macrolides, fluoroquinolones, and β-lactam/β-lactamase inhibitors: A Taiwanese nationwide study. Clinical Infectious Diseases, 2015(60), 566–577.
Tilelli, J. A., Smith, K. M., & Pettignano, R. (2006). Life-threatening bradyarrhythmia after massive azithromycin overdose. Pharmacotherapy, 26, 147–150.
Nilsson, M. F., & Webster, W. S. (2014). Effects of macrolide antibiotics on rat embryonic heart function in vitro. Birth Defects Research Part B, Developmental and Reproductive Toxicology, 101, 189–198.
FDA Drug Safety Communication. Azithromycin (Zithromax or Zmax) and the risk of potentially fatal heart rhythms. http://www.fda.gov/drugs/drugsafety/ucm341822.htm. Accessed March 12, 2013.
Maisch, N. M., Kochupurackal, J. G., & Sin, J. (2014). Azithromycin and the risk of cardiovascular complications. Journal of Pharmacy Practice, 27, 496–500.
Granowitz, E. V., Tabor, K. J., & Kirchhoffer, J. B. (2000). Potentially fatal interaction between azithromycin and disopyramide. Pacing and Clinical Electrophysiology, 23, 1433–1435.
Samarendra, P., Kumari, S., Evans, S. J., Sacchi, T. J., & Navarro, V. (2001). QT prolongation associated with azithromycin/amiodarone combination. Pacing and Clinical Electrophysiology, 24, 1572–1574.
Cocco, G., & Jerie, P. (2015). Torsades de pointes induced by the concomitant use of ivabradine and azithromycin: An unexpected dangerous interaction. Cardiovascular Toxicology, 15, 104–106.
Winton, J. C., & Twilla, J. D. (2013). Sudden cardiac arrest in a patient on chronic methadone after the addition of azithromycin. The American Journal of the Medical Sciences, 345, 160–162.
Niedrig, D., Maechler, S., Hoppe, L., Corti, N., Kovari, H., & Russmann, S. (2016). Drug safety of macrolide and quinolone antibiotics in a tertiary care hospital: Administration of interacting co-medication and QT prolongation. European Journal of Clinical Pharmacology, 72, 859–867.
Howard, P. A. (2013). Azithromycin-induced proarrhythmia and cardiovascular death. The Annals of Pharmacotherapy, 47, 1547–1551.
Ohara, H., Nakamura, Y., Watanabe, Y., Cao, X., Yamazaki, Y., Izumi-Nakaseko, H., et al. (2015). Azithromycin can prolong QT interval and suppress ventricular contraction, but will not induce torsade de pointes. Cardiovascular Toxicology, 15, 232–240.
Trac, M. H., McArthur, E., Jandoc, R., Dixon, S. N., Nash, D. M., Hackam, D. G., et al. (2016). Macrolide antibiotics and the risk of ventricular arrhythmia in older adults. Canadian Medical Association Journal, 188, E120–E129.
Svanström, H., Pasternak, B., & Hviid, A. (2013). Use of azithromycin and death from cardiovascular causes. The New England Journal of Medicine, 368, 1704–1712.
Albert, R. K., & Schuller, J. L. (2014). COPD Clinical Research Network. Macrolide antibiotics and the risk of cardiac arrhythmias. American Journal of Respiratory and Critical Care Medicine, 189, 1173–1180.
Fossa, A. A., Wisialowski, T., Duncan, J. N., Deng, S., & Dunne, M. (2007). Azithromycin/chloroquine combination does not increase cardiac instability despite an increase in monophasic action potential duration in the anesthetized guinea pig. The American Journal of Tropical Medicine and Hygiene, 77, 929–938.
Thomsen, M. B., Beekman, J. D., Attevelt, N. J., Takahara, A., Sugiyama, A., Chiba, K., et al. (2006). No proarrhythmic properties of the antibiotics Moxifloxacin or Azithromycin in anaesthetized dogs with chronic-AV block. British Journal of Pharmacology, 149, 1039–1048.
Milberg, P., Eckardt, L., Bruns, H. J., Biertz, J., Ramtin, S., Reinsch, N., et al. (2002). Divergent proarrhythmic potential of macrolide antibiotics despite similar QT prolongation: Fast phase 3 repolarization prevents early afterdepolarizations and torsade de pointes. The Journal of Pharmacology and Experimental Therapeutics, 303, 218–225.
Reagan-Shaw, S., Nihal, M., & Ahmad, N. (2008). Dose translation from animal to human studies revisited. FASEB Journal, 22, 659–661.
Chen, L., Titch, T., Luo, Z., Xu, Y., Li, X., Huang, F., et al. (2012). Confirmation of a proarrhythmic risk underlying the clinical use of common Chinese herbal intravenous injections. Journal of Ethnopharmacology, 142, 829–835.
Chen, L., Wang, L., Xu, B., Ni, G., Yu, L., Han, B., et al. (2009). Mechanisms of alpha1-adrenoceptor mediated QT prolongation in the diabetic rat heart. Life Sciences, 84, 250–256.
Salimi, A., Eybagi, S., Seydi, E., Naserzadeh, P., Kazerouni, N. P., & Pourahmad, J. (2016). Toxicity of macrolide antibiotics on isolated heart mitochondria: A justification for their cardiotoxic adverse effect. Xenobiotica, 46, 82–93.
Redfern, W. S., Carlsson, L., Davis, A. S., Lynch, W. G., MacKenzie, I., Palethorpe, S., et al. (2003). Relationships between preclinical cardiac electrophysiology, clinical QT interval prolongation and torsade de pointes for a broad range of drugs: Evidence for a provisional safety margin in drug development. Cardiovascular Research, 58, 32–45.
Matzneller, P., Krasniqi, S., Kinzig, M., Sörgel, F., Hüttner, S., Lackner, E., et al. (2013). Blood, tissue, and intracellular concentrations of azithromycin during and after end of therapy. Antimicrobial Agents and Chemotherapy, 57, 1736–1742.
Acknowledgements
This study was supported by “The major project of natural science for Jiangsu province high education institution (14KJA360002)” and “The project supported by natural science foundation of Jiangsu province (BK20131262, BK20151355).” The authors thank Mr. Tom Fitch of Eli Lilly and Company for assistance with English editing.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Rights and permissions
About this article
Cite this article
Zhang, M., Xie, M., Li, S. et al. Electrophysiologic Studies on the Risks and Potential Mechanism Underlying the Proarrhythmic Nature of Azithromycin. Cardiovasc Toxicol 17, 434–440 (2017). https://doi.org/10.1007/s12012-017-9401-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12012-017-9401-7