Abstract
Azithromycin has been reported to increase the risk of death from cardiovascular causes among patients with high baseline risk. Since the information is still limited to bridge the gap between electrophysiological properties of azithromycin in vitro and cardiac death in patients, we initially assessed its electropharmacological effects in doses of 3 and 30 mg/kg, i.v., with the halothane-anesthetized dogs (n = 4). The low dose provided 5.2 times higher than the therapeutic concentration, whereas the high dose attained 17.0 times higher. The high dose delayed the ventricular repolarization in a reverse use-dependent manner, reflecting blockade of the rapid component of delayed rectifier K+ current, and the potency was relatively weak; namely, maximum change in QTc was +20 ms (+5.6 %). The high dose also induced the negative inotropic effect possibly through Ca2+ channel-independent pathway. In order to clarify proarrhythmic risk, 30 mg/kg, i.v., of azithromycin was examined with the chronic atrioventricular block dogs (n = 4). Azithromycin neither induced torsade de pointes nor affected beat-to-beat variability of repolarization. Thus, azithromycin can be considered to lack proarrhythmic potential, but caution has to be paid on its use for patients with left ventricular dysfunction.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Azithromycin has been used for respiratory, urogenital, dermal and other bacterial infections, and expected to exert immunomodulatory effects against chronic inflammatory disorders [1]. In a previous report analyzing a Tennessee Medical cohort [2], azithromycin has been shown to increase the risks of death from cardiovascular causes among patients with high baseline risk, which was attributed to its potential proarrhythmic effect. Indeed, azithromycin has been shown to inhibit the rapid component of delayed rectifier K+ current (I Kr) channel [3], and there have been several clinical case reports showing pronounced QT-interval prolongation and torsade de pointes following the azithromycin administration [4–9]. However, in a recent study [10], such an increased risk of death associated with azithromycin use was not demonstrated in a general population of young and middle-aged adults; thus, information is still limited to bridge the gap between the electrophysiological properties of azithromycin in vitro and clinical adverse events leading to cardiovascular death.
In this study, first we precisely assessed the in vivo cardiohemodynamic and electrophysiological effects of azithromycin together with its pharmacokinetic profile using the halothane-anesthetized dogs [11–15]. Then, the proarrhythmic risk of azithromycin was examined qualitatively and quantitatively using the chronic atrioventricular block dogs [11, 16–19]. Several macrolide antibiotics including erythromycin and clarithromycin have been shown to induce torsade de pointes in the Langendorff-perfused rabbit hearts, which was not observed by azithromycin [20]. Moreover, azithromycin in doses of 2 and 8 mg/kg, i.v., neither affected the electrophysiological parameters nor induced torsade de pointes in the chronic atrioventricular block dogs [3]. The doses of azithromycin in the previous study [3] might not be enough to examine a cardiac toxicity, since the peak plasma concentrations were only at 1.4 and 5.2 times higher than the therapeutic ones, respectively. In this study, we assessed the effects of 30 mg/kg, i.v., of azithromycin in each of the experiments to better analyze the causal link between the azithromycin treatment and its cardiovascular consequences.
Materials and Methods
All animal experiments in this study were approved by The Animal Research Committee for Animal Experimentation of Toho University (Nos. 13-53-151, 13-53-152) and performed in accordance with the Guidelines for the Care and Use of Laboratory Animals of Toho University and the Japanese Pharmacological Society.
Experiment 1
Four beagle dogs of either sex weighing approximately 10 kg were used. The animals were obtained through Kitayama Labes (Nagano, Japan).
Cardiohemodynamic Variables
The dogs were anesthetized initially with thiopental sodium (30 mg/kg, i.v.). After the intubation with a cuffed endotracheal tube, 1 % halothane vaporized with 100 % oxygen was inhaled with a volume-limited ventilator (SN-480-3; Shinano, Tokyo, Japan). The tidal volume and respiratory rate were set at 20 mL/kg and 15 strokes/min, respectively. Heparin calcium (100 IU/kg) was intravenously administered to prevent blood clotting. A clinically available catheter–sheath set (FAST-CATH™, 406108; St. Jude Medical Daig Division, Inc., Minnetonka, MN, USA) was inserted into the right femoral artery to introduce a pig-tail catheter for measuring the left ventricular pressure. The aortic blood pressure was measured at a space between inside of the sheath and outside of the catheter through a flush line. A thermodilution catheter (TC-504NH; Nihon Kohden, Tokyo, Japan) was positioned at the right side of the heart through the right femoral vein. The cardiac output was measured using a standard thermodilution method with a cardiac output computer (MFC-1100; Nihon Kohden). The total peripheral resistance was calculated using the basic equation: total peripheral resistance = mean blood pressure/cardiac output. The maximum upstroke velocity of the left ventricular pressure (LVdP/dt max) and left ventricular end-diastolic pressure were obtained during sinus rhythm to estimate the contractility and preload to the left ventricle, respectively.
Electrophysiological Variables
The surface lead II electrocardiogram (ECG) was obtained from the limb electrodes. The corrected QT interval (QTc) was calculated using Van de Water’s formula: QTc = QT − 0.087 × (RR − 1,000) [21]. A quad-polar electrodes catheter was positioned at the non-coronary cusp of the aortic valve through the left femoral artery to obtain the His bundle electrogram. A bidirectional steerable monophasic action potential (MAP) recording/pacing combination catheter (1675P; EP Technologies, Inc., Sunnyvale, CA, USA) was positioned at the endocardium of the right ventricle through the left femoral vein to obtain MAP signals. The signals were amplified with a DC preamplifier (model 300; EP Technologies, Inc.). The duration of the MAP signals was measured as an interval, along a line horizontal to the diastolic baseline, from the MAP upstroke to the desired repolarization level. The interval (ms) at 90 % repolarization level was defined as MAP90.
The heart was electrically driven using a cardiac stimulator (SEC-3102; Nihon Kohden) with the pacing electrodes of the combination catheter placed in the right ventricle. The stimulation pulses were rectangular, 1–2 V of amplitude (about twice the threshold voltage) and of 1 ms duration. The MAP90 was measured during sinus rhythm (MAP90(sinus)) and at a pacing cycle length of 400 ms (MAP90(CL400)) and 300 ms (MAP90(CL300)). The effective refractory period (ERP) of the right ventricle was assessed by the programmed electrical stimulation. The pacing protocol consisted of 5 beats of basal stimuli in a cycle length of 400 ms followed by an extra stimulus of various coupling intervals. Starting in a late diastole, the coupling interval was shortened in 5-ms decrements until refractoriness occurred. The duration of the terminal repolarization period of the ventricle, reflecting phase 3 repolarization of the action potential, was calculated by the difference between the MAP90(CL400) and ERP at the same site, which reflects the extent of electrical vulnerability [11–13, 22].
Experimental Protocol
The aortic blood pressure, left ventricular pressure, ECG, His bundle electrogram and MAP signals were monitored using a polygraph system (RM-6000; Nihon Kohden) and analyzed using a real-time full automatic data analysis system (WinVAS3 version 1.1R24; Physio-Tech, Tokyo, Japan). Each measurement of ECG, MAP as well as atrio-His and His-ventricular intervals was the mean of three recordings of consecutive complexes. The cardiovascular variables were assessed in the following order. The ECG, His bundle electrogram, aortic and left ventricular pressures and MAP signals were recorded under sinus rhythm. Next, the cardiac output was measured three times. Then, MAP signals were recorded during the ventricular pacing at a cycle length of 400 and 300 ms. Finally, the ERP was measured. All variables described above were usually obtained within 1 min at each time point.
After the basal assessment, azithromycin in a low dose of 3 mg/kg, which corresponds to a clinically recommended daily p.o., dose, was intravenously administered over 10 min, and each variable was assessed at 5, 10, 15, 20 and 30 min after the start of the infusion. Then, azithromycin in a high dose of 30 mg/kg was intravenously administered over 10 min, and each variable was assessed at 5, 10, 15, 20, 30, 45 and 60 min after the start of the infusion.
Plasma Drug Concentration
Blood was sampled from left femoral artery at 5, 10, 15 and 30 min in the low dose, and 5, 10, 15, 30 and 60 min in the high dose after the start of the infusion. The blood samples were centrifuged at 1,500×g for 30 min at 4 °C. The plasma was stored at −80 °C until the drug concentration was measured. The plasma drug concentration was determined as previously reported [23]. Briefly, 200 μL of plasma samples were extracted with 800 μL of ethyl acetate and 0.12 M NaCl. After vortex mixing, the tubes were centrifuged at 900×g for 10 min. Seven hundred microliter of organic phase was collected and evaporated to dryness at 40 °C. The residue was dissolved with 50 μL of a mobile phase consisted of 80 % (v/v) methanol in 0.03 M sodium phosphate buffer (pH 7.5). The supernatant (30 μL) was injected onto an analytical C18 reversed-phase column (250 × 4.6 mm, 5 μm, Mightysil RP-18; Kanto Chemical, Tokyo, Japan) maintained at 40 °C. The elution profiles of azithromycin at a flow rate of 1.5 mL/min were monitored by a UV detector at 210 nm.
Experiment 2
Four beagle dogs of either sex weighing approximately 10 kg were used. The animals were obtained through Kitayama Labes.
Production of Complete Atrioventricular Block
The catheter ablation technique of atrioventricular node was employed as described previously [16, 17]. The dogs were anesthetized with thiopental sodium (30 mg/kg, i.v.). After intubation with a cuffed endotracheal tube, the dogs were artificially ventilated with room air using a volume-limited ventilator (SN-408-3; Shinano). The tidal volume and respiratory rate were set at 20 mL/kg and 15 strokes/min, respectively. To prevent blood clotting, heparin calcium (100 IU/kg) was intravenously administered. The surface lead II ECG was continuously monitored using a polygraph system (RM-6000; Nihon Kohden). A quad-polar electrodes catheter with a large tip of 4 mm (D7-DL-252; Cordis-Webster, Baldwin Park, CA, USA) was inserted through the right femoral vein using the standard percutaneous technique under sterile condition, and positioned around the tricuspid valve, watching the bipolar electrogram from the distal electrodes pair. The optimal site for the atrioventricular node ablation, namely the compact atrioventricular node, was determined by the intracardiac electrogram, of which a very small His deflection was recorded and atrial/ventricular voltage ratio was >2. The site was usually found at 1–2 cm proximal from the position where the largest His bundle electrogram was recorded. The power source for atrioventricular node ablation was obtained from an electrosurgical generator (MS-1500; Mera, Tokyo, Japan) delivering continuous unmodulated radiofrequency energy at a frequency of 500 kHz. After proper positioning, the radiofrequency energy of 20 W was delivered for 10 s from the tip electrode to an indifferent patch electrode positioned on the animal’s back, which was continued then for 30 s, if junctional rhythm was induced. After the induction of complete atrioventricular block, PR interval was measured to determine whether the atrioventricular node conduction had been affected. The endpoint of this procedure was the development of the complete atrioventricular block with an onset of stable idioventricular escaped rhythm. Proper care was taken for the animals, and the proarrhythmic properties of the drugs were assessed >4 weeks after the onset of the complete atrioventricular block based on the previous report [17].
Holter ECG Recording
A Holter recording and analysis system (QR2100 and HS1000; Fukuda ME Kogyo, Tokyo, Japan) was used to obtain information of ECG over 24 h. The effects of drugs on the ventricular rate, QT interval and corrected QT interval (QTc) as well as the proarrhythmic effects were assessed without anesthesia. These values were expressed as the mean of ten consecutive complexes. QTc was calculated using Fridericia’s formula [24]. Torsade de pointes was defined as a polymorphic ventricular tachycardia, of which QRS complex twisted around the baseline, lasting ≥5 consecutive beats [25].
Experimental Protocol
Experiments were conducted ≥4 weeks after the induction of complete atrioventricular block [17, 18]. A single dose of each drug was assessed using the atrioventricular block dogs without anesthesia. Namely, 2 h after the start of ECG monitoring, azithromycin (30 mg/kg, i.v., over 10 min) was administered. Ventricular rate and QT interval were assessed at 1, 2, 3, 4, 6, 8, 12 and 21 h after the start of the administration.
Beat-to-Beat Analysis
ECG of 51 consecutive beats under stable idioventricular automaticity was recorded before and for 0.8–2.5 h after the administration of azithromycin. Poincaré plots with QT n versus QT n+1 were prepared for each of two analysis time points. The mean orthogonal distance from the diagonal to the points of the Poincaré plot was determined as short-term variability (=\(\sum |{\text{QT}}_{n + 1} {-}{\text{QT}}_{n} |/[50 \times \sqrt 2 ]\)). On the other hand, the mean distance to the mean of the parameter parallel to the diagonal of the Poincaré plot was determined as long-term variability (=\(\sum |{\text{QT}}_{n + 1} {\text{ + QT}}_{n} - 2{\text{QT}}_{\text{mean}} |/[50 \times \sqrt 2 ]\)). These nomenclatures are adopted from the investigations of heart rate variability with Holter ECG monitoring in humans [26], which have been applied to the QT interval of normal dogs and chronic atrioventricular block dogs [16, 18, 19].
Drugs
Commercially available azithromycin was used (Zythromac® Intravenous use, Pfizer Japan Inc., Tokyo, Japan), which was dissolved with saline in concentrations of 3 and 30 mg/mL. Other drugs used were thiopental sodium (Ravonal® 0.5 g for Injection, Mitsubishi Tanabe Pharma Corporation, Osaka, Japan), halothane (Fluothane®, Takeda Pharmaceutical Co., Ltd., Osaka, Japan) and heparin calcium (Caprocin®, Sawai Pharmaceutical Co., Ltd., Osaka, Japan).
Statistical Analysis
Data are presented as the mean ± SE. The statistical significances within a parameter were evaluated by one-way repeated-measures analysis of variance (ANOVA) followed by Contrasts for mean values comparison, whereas those of paired data within a parameter were evaluated by paired t-test and those of unpaired data between the groups were evaluated by unpaired t-test. A p value <0.05 was considered significant.
Results
Experiment 1
No animals exerted any lethal ventricular arrhythmias or cardiohemodynamic collapse, leading to the animal’s death during the experimental period.
Plasma Drug Concentration
The time course of the plasma drug concentration is summarized in Fig. 1. The peak plasma concentrations of azithromycin after 3 and 30 mg/kg infusion were 20.8 ± 5.9 µg/mL (27.8 µM) and 68.5 ± 4.8 µg/mL (91.5 µM), respectively.
Time courses of plasma concentration (Conc.), heart rate (HR), mean blood pressure (MBP), cardiac output (CO), total peripheral resistance (TPR), maximum upstroke velocity of the left ventricular pressure (LVdP/dt max) and left ventricular end-diastolic pressure (LVEDP). Data are presented as the mean ± SE. Closed symbols represent significant differences from the corresponding pre-drug control value (C) by p < 0.05
Effects on Hemodynamic Variables
The time courses of changes in the hemodynamic variables are summarized in Fig. 1. The pre-drug control values (C) of the heart rate, mean blood pressure, cardiac output, total peripheral resistance, LVdP/dt max and left ventricular end-diastolic pressure were 102 ± 8 beats/min, 107 ± 2 mmHg, 1.63 ± 0.09 L/min, 66.6 ± 4.9 mmHg min/L, 2,151 ± 276 mmHg/s and 8.6 ± 1.8 mmHg, respectively. After the low dose of 3 mg/kg administration, no significant change was detected in them. After the high dose of 30 mg/kg administration, the mean blood pressure, cardiac output and LVdP/dt max decreased for 10–15 min, for 10–20 min and for 10–60 min, respectively, whereas total peripheral resistance increased for 45–60 min.
Effects on the ECG Variables During the Sinus Rhythm
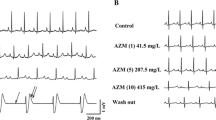
Typical tracings of the ECG are depicted in Fig. 2, and the time courses of changes in the ECG variables are summarized in Fig. 3. The pre-drug control values (C) of the PR interval, QRS width, QT interval and QTc were 118 ± 10, 69 ± 2, 324 ± 24 ms and 359 ± 20, respectively. After the low dose, no significant change was detected in them. After the high dose, the QT interval and QTc were prolonged for 10–20 min, whereas no significant change was detected in the PR interval or QRS width.
Typical tracings of the His bundle electrogram (His), lead II surface electrocardiogram (ECG), aortic blood pressure (AoP), left ventricular pressure (LVP) and monophasic action potential (MAP) recorded from the right ventricle during sinus rhythm at pre-drug control (Control) and 15 min after the start of 30 mg/kg of azithromycin infusion (15 min after 30 mg/kg)
Time courses of PR interval (PR), QRS width (QRS), QT interval (QT), corrected QT interval by Van de Water’s formula (QTc), atrio-His (AH) and His-ventricular (HV) intervals and duration of monophasic action potential at 90 % repolarization level during sinus rhythm (MAP90(sinus)). Data are presented as the mean ± SE. Closed symbols represent significant differences from the corresponding pre-drug control value (C) by p < 0.05
Effects on the Atrio-His and His-Ventricular Intervals, and MAP Duration During the Sinus Rhythm
The time courses of changes in the atrio-His and His-ventricular intervals, and MAP90(sinus) are summarized in Fig. 3, of which pre-drug control values (C) were 80 ± 9, 34 ± 6 and 258 ± 27 ms, respectively. After the low dose, the MAP90(sinus) was shortened for 5–10 and at 20 min, whereas no significant change was detected in the atrio-His or His-ventricular interval. After the high dose, MAP90(sinus) was prolonged for 10–30 min, whereas no significant change was detected in the atrio-His or His-ventricular interval.
Effects on the MAP90 During the Ventricular Pacing
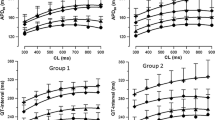
The time courses of changes in MAP90(CL300) and MAP90(CL400) are summarized in Fig. 4, of which pre-drug control values (C) were 228 ± 9 and 250 ± 20 ms, respectively. After the low dose, no significant change was observed in either of them. After the high dose, the MAP90(CL300) and MAP90(CL400) were prolonged in 10–20 and 10–30 min, respectively.
Time courses of duration of the monophasic action potential at 90 % repolarization level during the ventricular pacing at a cycle length of 300 ms (MAP90(CL300)) and 400 ms (MAP90(CL400)); effective refractory period of the right ventricle (ERP); and terminal repolarization period (TRP). Data are presented as the mean ± SE. Closed symbols represent significant differences from the corresponding pre-drug control value (C) by p < 0.05
Effects on the ERP and Terminal Repolarization Period
The time courses of changes in the ERP and terminal repolarization period are summarized in Fig. 4, of which pre-drug control values (C) were 221 ± 13 and 29 ± 8 ms, respectively. After the low dose, no significant change was detected in either of them. After the high dose, the ERP was prolonged for 5–30 min and at 60 min, whereas the terminal repolarization period was shortened at 60 min.
Experiment 2
Torsadogenic Actions
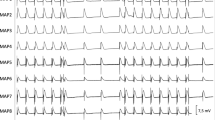
Typical tracings of the ECG before and after the administration of azithromycin are depicted in Fig. 5a, whereas the time courses of the changes in the ECG parameters are summarized in Fig. 5b. The pre-drug control values (C) of the ventricular rate, QT interval and QTc were 33 ± 3 bpm, 331 ± 17 ms and 269 ± 11, respectively. Significant change was not observed in any of the parameters after the administration of azithromycin. Torsade de pointes was not induced during the experimental period, either.
Effects of azithromycin on the chronic atrioventricular block dogs. a Typical tracings of ECG demonstrating lack of torsadogenic action. Neither torsade de pointes nor QT prolongation was detected after the intravenous administration of 30 mg/kg of azithromycin. b Time courses of the effects of azithromycin on the ventricular rate, QT interval (QT) and corrected QT interval by Fridericia’s formula (QTc). Data are presented as mean ± SE
Beat-to-Beat Analysis
Poincaré plots of QT interval are depicted in Fig. 6. Pre-drug control values of short- and long-term variability of repolarization were 4.7 ± 1.0 and 11.4 ± 3.9 ms, respectively. After the administration of azithromycin, no significant change was detected in the short- or long-term variability of repolarization, which was 5.2 ± 1.5 and 11.6 ± 6.2 ms, respectively.
Effect of azithromycin on the Poincaré plots of the QT interval in the chronic atrioventricular block canine model. A total of 51 beats were plotted for each of the two analysis time points: at pre-drug control (gray) and after (black) 30 mg/kg i.v., of azithromycin. Torsade de pointes was not observed by azithromycin. STV short-term variability, LTV long-term variability
Discussion
In this study, electropharmacological effects and pharmacokinetic profile of azithromycin were simultaneously assessed with the halothane-anesthetized dogs, whereas its proarrhythmic risk was examined with the chronic atrioventricular block dogs. Supratherapeutic doses of azithromycin prolonged the QT interval and suppressed the ventricular contraction, but it did not induce torsade de pointes.
Plasma Drug Concentration
In experiment 1, the peak plasma concentrations of azithromycin after 3 and 30 mg/kg infusion were 20.8 ± 5.9 and 68.5 ± 4.8 µg/mL, respectively. The decrease in the plasma concentration of the drug followed a pattern that could be predicted by the two-compartment theory of pharmacokinetics. In the interview form from the manufacturer, the peak plasma concentration of azithromycin was 4.03 ± 0.61 µg/mL after the intravenous administration dose of 500 mg over 1 h to healthy subjects. Therefore, the low and high doses of azithromycin used in experiment 1 can be considered to provide approximately 5.2 and 17.0 times higher than the therapeutic plasma concentration, respectively. Furthermore, in a previous report [3], the peak plasma concentrations of azithromycin after 2 and 8 mg/kg infusion over 5 min in anesthetized dogs with atrioventricular block were 5.4 ± 1.3 and 20.8 ± 4.9 µg/mL, respectively, indicating that the plasma concentration of azithromycin after the high dose in experiment 1 was >3.3 times greater than those.
Cardiohemodynamic Effects
The low dose of azithromycin hardly affected any of the cardiohemodynamic variables, whereas the high dose decreased the ventricular contraction together with the mean blood pressure and cardiac output. Although the negative inotropic effect of azithromycin has not been reported before, an immunosuppressant tacrolimus and antibiotics josamycin and erythromycin, which have macrolide structure, have been shown to decrease the cardiac contractility in our previous study and other’s [14, 27]. These macrolides were demonstrated to inhibit Ca2+ channels [14, 27], which can explain their negative inotropic effect. However, in this study, azithromycin might not affect cardiac Ca2+ channel as discussed in the next paragraph of electrophysiological effects. Thus, azithromycin may induce the negative inotropic effect through Ca2+ channel-independent pathway, including neurogenic and/or autacoidal modifications [1], and further studies will be needed to clarify its mechanisms. It should be noted that total peripheral resistance increased in experiment 1 after the high-dose infusion of azithromycin, which may be induced by the decrease of mean blood pressure via reflex-mediated increase of sympathetic tone.
Electrophysiological Effects
The low and high doses of azithromycin hardly affected the atrioventricular nodal or intraventricular conductions, indicating that azithromycin would modify neither the cardiac Ca2+ nor Na+ channel function, since selective Ca2+ and Na+ channel blockers have been reported to delay the atrioventricular nodal (PR interval and AH interval) and intraventricular conductions (QRS width and HV interval), respectively, in the halothane-anesthetized dogs [13, 28]. Although the low dose of azithromycin slightly shortened the MAP90(sinus) without affecting the QT/QTc interval, MAP90(CL300) or MAP90(CL400), the high dose prolonged the QT/QTc interval, MAP90(sinus), MAP90(CL300) and MAP90(CL400). Increment of MAP90(CL400) was greater than that of MAP90(CL300) at 15 min after the high dose, showing the reverse use-dependent prolongation of repolarization, which may indicate the blockade of I Kr [11]. This observation is in good accordance with a previous study [12] with the Langendorff-perfused rabbit hearts, in which reverse-use dependence of repolarization delay was induced by azithromycin; and one [3] with human embryonic kidney (HEK) 293 cells transfected with human ether-a-go–go-related gene (hERG), in which 300 µM of azithromycin inhibited I Kr by 22.5 ± 6.5 %. The degree of inhibition by azithromycin was 11 times less potent than that by moxifloxacin [3]. Furthermore, the degree of prolongation of QT/QTc interval after the high dose in this study was +20 ms (+5.6 %), which was less great than that of 3 mg/kg, i.v., of moxifloxacin assessed in the same model by us [15]. These results suggest that the potency of azithromycin for the repolarization delay may be weaker than that of moxifloxacin.
Proarrhythmic Potential
Although administration of 30 mg/kg of azithromycin prolonged QT/QTc interval, the terminal repolarization period was shortened in experiment 1, suggesting the antiarrhythmic potential [11]. Moreover, the same dose of the drug did not induce torsade de pointes or hardly affected the short- or long-term variability of repolarization in the atrioventricular block dogs in experiment 2. Since the short-term variability of repolarization has been known to be a reliable marker for quantifying the extent of torsadogenic potential [18, 19], these results indicate that azithromycin may lack potential of proarrhythmia. These results are in good accordance with previous studies with the Langendorff-perfused rabbit hearts [20] and chronic atrioventricular block dogs [3].
Conclusion
Supratherapeutic dose of azithromycin showed a reverse use-dependent repolarization delay reflecting I Kr blockade, of which potency was relatively weak, whereas it induced the negative inotropic effect possibly through Ca2+ channel-independent pathway in the halothane-anesthetized dogs. Azithromycin neither induced torsade de pointes nor affected beat-to-beat variability of repolarization in the chronic atrioventricular block dogs. Thus, azithromycin can be considered to lack proarrhythmic potential, but caution has to be paid on its use for patients with left ventricular dysfunction.
References
Parnham, M. J., Haber, V. E., Giamarellos-Bourboulis, E. J., Perletti, G., Verleden, G. M., & Vos, R. (2014). Azithromycin: Mechanisms of action and their relevance for clinical applications. Pharmacology & Therapeutics, 143, 225–245.
Ray, W. A., Murray, K. T., Hall, K., Arbogast, P. G., & Stein, C. M. (2012). Azithromycin and the risk of cardiovascular death. New England Journal of Medicine, 366, 1881–1890.
Thomsen, M. B., Beekman, J. D., Attevelt, N. J., Takahara, A., Sugiyama, A., Chiba, K., et al. (2006). No proarrhythmic properties of the antibiotics moxifloxacin or azithromycin in anaesthetized dogs with chronic-AV block. British Journal of Pharmacology, 149, 1039–1048.
Santos, N., Oliveira, M., Galrinho, A., Oliveira, J. A., Ferreira, L., & Ferreira, R. (2010). QT interval prolongation and extreme bradycardia after a single dose of azithromycin. Revista Portuguesa de Cardiologia, 29, 139–142.
Huang, B. H., Wu, C. H., Hsia, C. P., & Yin Chen, C. (2007). Azithromycin-induced torsade de pointes. Pacing and Clinical Electrophysiology, 30, 1579–1582.
Kezerashvili, A., Khattak, H., Barsky, A., Nazari, R., & Fisher, J. D. (2007). Azithromycin as a cause of QT-interval prolongation and torsade de pointes in the absence of other known precipitating factors. Journal of Interventional Cardiac Electrophysiology, 18, 243–246.
Tilelli, J. A., Smith, K. M., & Pettignano, R. (2006). Life-threatening bradyarrhythmia after massive azithromycin overdose. Pharmacotherapy, 26, 147–150.
Kim, M. H., Berkowitz, C., & Trohman, R. G. (2005). Polymorphic ventricular tachycardia with a normal QT interval following azithromycin. Pacing and Clinical Electrophysiology, 28, 1221–1222.
Samarendra, P., Kumari, S., Evans, S. J., Sacchi, T. J., & Navarro, V. (2001). QT prolongation associated with azithromycin/amiodarone combination. Pacing and Clinical Electrophysiology, 24, 1572–1574.
Svanstrom, H., Pasternak, B., & Hviid, A. (2013). Use of azithromycin and death from cardiovascular causes. New England Journal of Medicine, 368, 1704–1712.
Sugiyama, A. (2008). Sensitive and reliable proarrhythmia in vivo animal models for predicting drug-induced torsades de pointes in patients with remodelled hearts. British Journal of Pharmacology, 154, 1528–1537.
Ishizaka, T., Takahara, A., Iwasaki, H., Mitsumori, Y., Kise, H., Nakamura, Y., et al. (2008). Comparison of electropharmacological effects of bepridil and sotalol in halothane-anesthetized dogs. Circulation Journal, 72, 1003–1011.
Yoshida, H., Sugiyama, A., Satoh, Y., Ishida, Y., Kugiyama, K., & Hashimoto, K. (2002). Effects of disopyramide and mexiletine on the terminal repolarization process of the in situ heart assessed using the halothane-anesthetized in vivo canine model. Circulation Journal, 66, 857–862.
Kise, H., Nakamura, Y., Hoshiai, M., Sugiyama, H., Sugita, K., & Sugiyama, A. (2010). Cardiac and haemodynamic effects of tacrolimus in the halothane-anaesthetized dog. Basic & Clinical Pharmacology & Toxicology, 106, 288–295.
Chiba, K., Sugiyama, A., Hagiwara, T., Takahashi, S., Takasuna, K., & Hashimoto, K. (2004). In vivo experimental approach for the risk assessment of fluoroquinolone antibacterial agents-induced long QT syndrome. European Journal of Pharmacology, 486, 189–200.
Takahara, A., Sugiyama, A., Ishida, Y., Satoh, Y., Wang, K., Nakamura, Y., et al. (2006). Long-term bradycardia caused by atrioventricular block can remodel the canine heart to detect the histamine H1 blocker terfenadine-induced torsades de pointes arrhythmias. British Journal of Pharmacology, 147, 634–641.
Sugiyama, A., Ishida, Y., Satoh, Y., Aoki, S., Hori, M., Akie, Y., et al. (2002). Electrophysiological, anatomical and histological remodeling of the heart to AV block enhances susceptibility to arrhythmogenic effects of QT-prolonging drugs. Japanese Journal of Pharmacology, 88, 341–350.
Thomsen, M. B., Verduyn, S. C., Stengl, M., Beekman, J. D., de Pater, G., van Opstal, J., et al. (2004). Increased short-term variability of repolarization predicts d-sotalol-induced torsades de pointes in dogs. Circulation, 110, 2453–2459.
Thomsen, M. B., Volders, P. G., Beekman, J. D., Matz, J., & Vos, M. A. (2006). Beat-to-beat variability of repolarization determines proarrhythmic outcome in dogs susceptible to drug-induced torsades de pointes. Journal of the American College of Cardiology, 48, 1268–1276.
Milberg, P., Eckardt, L., Bruns, H. J., Biertz, J., Ramtin, S., Reinsch, N., et al. (2002). Divergent proarrhythmic potential of macrolide antibiotics despite similar QT prolongation: Fast phase 3 repolarization prevents early afterdepolarizations and torsade de pointes. Journal of Pharmacology and Experimental Therapeutics, 303, 218–225.
Van de Water, A., Verheyen, J., Xhonneux, R., & Reneman, R. S. (1989). An improved method to correct the QT interval of the electrocardiogram for changes in heart rate. Journal of Pharmacological Methods, 22, 207–217.
Sugiyama, A., & Hashimoto, K. (2002). Effects of a typical IKr channel blocker sematilide on the relationship between ventricular repolarization, refractoriness and onset of torsades de pointes. Japanese Journal of Pharmacology, 88, 414–421.
Sharma, K., & Mullangi, R. (2013). A concise review of HPLC, LC-MS and LC-MS/MS methods for determination of azithromycin in various biological matrices. Biomedical Chromatography, 27, 1243–1258.
Fridericia, L. S. (2003). The duration of systole in an electrocardiogram in normal humans and in patients with heart disease. 1920. Annals of Noninvasive Electrocardiology, 8, 343–351.
Satoh, T., & Zipes, D. P. (1996). Rapid rates during bradycardia prolong ventricular refractoriness and facilitate ventricular tachycardia induction with cesium in dogs. Circulation, 94, 217–227.
Brennan, M., Palaniswami, M., & Kamen, P. (2001). Do existing measures of Poincare plot geometry reflect nonlinear features of heart rate variability? IEEE Transactions on Biomedical Engineering, 48, 1342–1347.
Tamargo, J., De Miguel, B., & Tejerina, M. T. (1982). A comparison of josamycin with macrolides and related antibiotics on isolated rat atria. European Journal of Pharmacology, 80, 285–293.
Shiina, H., Sugiyama, A., Takahara, A., Satoh, Y., & Hashimoto, K. (2000). Comparison of the electropharmacological effects of verapamil and propranolol in the halothane-anesthetized in vivo canine model under monophasic action potential monitoring. Japanese Circulation Journal, 64, 777–782.
Acknowledgments
This study was partially supported by the Japan Science and Technology Agency (#AS2116907E), the Project Research Grant of Toho University School of Medicine (No. 25-25) and Toho University Joint Research Fund (H25-3, H26-2). The authors thank Ms. Misako Nakatani, Mr. Yuki Suzuki and Dr. Norie Murayama for their technical supports.
Conflict of interest
The authors declare no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ohara, H., Nakamura, Y., Watanabe, Y. et al. Azithromycin Can Prolong QT Interval and Suppress Ventricular Contraction, but Will Not Induce Torsade de Pointes. Cardiovasc Toxicol 15, 232–240 (2015). https://doi.org/10.1007/s12012-014-9289-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12012-014-9289-4