Abstract
Drug combinations may elevate the risk of proarrhythmia. The aim of the present study was to investigate whether combinations of non-cardiovascular agents induce an additive increase in the proarrhythmic risk. In 12 female rabbit hearts, a drug combination of cotrimoxazole (300 µM), ondansetron (5 µM) and domperidone (1 µM) was infused after obtaining baseline data. In another 13 hearts, a combination of cotrimoxazole (300 µM), ondansetron (5 µM) and erythromycin (300 µM) was infused. Monophasic action potentials and ECG displayed a significant QT prolongation in all groups. This was accompanied by a significant increase in action potential duration. Of note, addition of each drug resulted in a further increase in the QT interval. Furthermore, a significant elevation of spatial dispersion of repolarization was observed. Lowering of potassium concentration in bradycardic AV-blocked hearts provoked early afterdepolarizations and torsade de pointes (TDP) in both study groups. Under baseline conditions, no episodes of TDP recorded. After administration of the first agent, TDP occurred in 5 of 12 hearts (37 episodes) and 5 of 13 hearts (26 episodes), respectively. After additional infusion of the second drug, TDP were recorded in 7 of 12 hearts (55 episodes) and 8 of 13 hearts (111 episodes). After additional infusion of the third drug, TDP occurred in 11 of 12 hearts (118 episodes) and 9 of 13 hearts (88 episodes). Combined treatment with several non-cardiovascular QT-prolonging agents resulted in a remarkable occurrence of proarrhythmia. An additive and significant prolongation of cardiac repolarization combined with an increased spatial dispersion of repolarization represents the underlying electrophysiological mechanism.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Various non-cardiovascular drugs have the potential to prolong cardiac repolarization. This effect is clinically mirrored as a prolongation of the QT interval. However, a sole consideration of the QT interval is not sufficient to precisely assess the true proarrhythmic risk [1]. In the past, spatial dispersion was identified as an important predictor of proarrhythmia [1, 2].
Of note, potentially QT-prolonging non-cardiovascular agents are routinely administered in patients undergoing polypharmacotherapy. Patients with haematologic diseases receiving chemotherapy and, therefore, requiring supportive medication such as antimicrobial agents, antiemetic agents or other drugs are a common example. If other risk factors such as heart failure coincide, the proarrhythmic risk is even higher based on a reduced repolarization reserve [3].
Proarrhythmic effects have been described for different antibiotic agents such as macrolides [4] or quinolones [5], antimycotic agents [6] and antiemetic agents [7]. However, no thorough data are available on the combination of different QT-prolonging agents. Depending on the underlying proarrhythmic mechanisms, a combination of QT-prolonging drugs may further increase the risk of proarrhythmia as compared with monotherapy.
Therefore, the aim of this proof-of-concept study was to assess the effects of a combination therapy with different QT-prolonging agents on cardiac repolarization in a sensitive whole-heart model of proarrhythmia [6, 8].
Methods
All experimental protocols were approved by the local animal care committee and conformed to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 852-3, revised 1996).
The method of heart isolation employing a Langendorff apparatus has previously been described [8]. Hearts were perfused with tempered Krebs–Henseleit solution (composition in mM: CaCl2 1.80, KCl 4.70, KH2PO4 1.18, MgSO4 0.83, NaCl 118, NaHCO3 24.88, Na-pyruvate 2.0 and d-glucose 5.55). The atrioventricular node was mechanically ablated using surgical tweezers resulting in a complete atrioventricular dissociation.
Seven epicardial and one endocardial monophasic action potentials (MAP) as well as an ECG were recorded. Pacing at twice diastolic threshold was performed for 1 min at each cycle length (CL) from 900 to 300 ms for determination of action potential duration at 90% of repolarization (APD90) and QT interval. Dispersion of repolarization was measured between different epicardial electrodes as well as between endocardial and epicardial electrodes. After recording of electrophysiological parameters, the extracellular K+ concentration was lowered to 1.5 mM for 5 min per protocol part in order to enhance the occurrence of early afterdepolarizations and torsade de pointes in spontaneously beating hearts. Then, the antibiotic agent cotrimoxazole (300 µM) was administered over a period of 15 min in both study groups, and measurements were repeated. Subsequently, the antiemetic agent ondansetron (5 µM) was infused in both study groups, and measurements were repeated. Thereafter, either the antiemetic drug domperidone (1 µM, group 1, n = 12) or the macrolide antibiotic erythromycin (300 µM, group 2, n = 13) were administered, and measurements were repeated.
Statistical analysis was performed using the SPSS software for Windows, release 23.0.0. (SPSS Inc., Chicago, USA). Each continuous variable was analysed for normal distribution using the Kolmogorov–Smirnov test. Drug effects on electrophysiological parameters were assessed using general linear model (GLM) for repeated measures. The Chi-squared test and the Fisher test were used to compare the incidences of polymorphic VT. Continuous data are presented as mean ± standard deviation. Differences are considered significant at p < 0.05.
Results
Drug Effects on Action Potential Duration and QT Interval
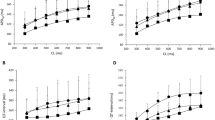
Infusion of the employed agents led to an additive prolongation of the QT interval. This is illustrated in Fig. 1. Infusion of cotrimoxazole (300 µM) similarly increased the QT interval in both groups compared to baseline values (group 1: 230 ± 11 ms vs. 249 ± 8 ms, p < 0.01; group 2: 235 ± 14 ms vs. 248 ± 15 ms, p < 0.05). Additional infusion of ondansetron (5 µM) in the presence of continuous infusion of cotrimoxazole further increased the QT interval (group 1: 280 ± 11 ms, p < 0.01; group 2: 278 ± 17 ms; p < 0.01). Further infusion of domperidone (1 µM) during continuous infusion of cotrimoxazole and ondansetron in group 1 resulted in a further significant increase in QT interval (295 ± 10 ms, p < 0.05). In group 2, additional treatment with erythromycin also resulted in a further significant increase in QT interval (313 ± 14 ms, p < 0.01). Similar results were obtained for APD90 in both study groups (Fig. 1).
Cycle length-dependent effects on action potential duration (APD90) and QT interval: left group 1 (n = 12): filled circle baseline, filled triangle cotrimoxazole, filled square cotrimoxazole + ondansetron and filled plus cotrimoxazole + ondansetron + domperidone; right group 2 (n = 13): filled circle baseline, filled triangle cotrimoxazole, filled square cotrimoxazole + ondansetron and filled plus cotrimoxazole + ondansetron + erythromycin
Drug Effects on Dispersion of Repolarization
Drug treatment led to an increase in spatial dispersion of repolarization in both study groups. Of note, sole infusion of cotrimoxazole did not significantly increase spatial dispersion of repolarization (group 1: baseline: 45 ± 13 ms, cotrimoxazole: 47 ± 14 ms; group 2: baseline: 41 ± 17 ms, cotrimoxazole 42 ± 13 ms). Additional infusion of ondansetron and domperidone led to a significant increase in spatial dispersion (group1: 51 ± 12 ms and 60 ± 15 ms, p < 0.05 compared with baseline). Similar results were observed after additional infusion of ondansetron and erythromycin (49 ± 12 ms and 74 ± 22 ms, p < 0.01, Fig. 2).
Occurrence of Proarrhythmia
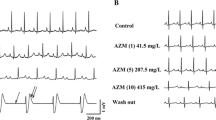
After lowering [K+] concentration to 1.5 mM, no early afterdepolarizations (EAD) and/or torsade de pointes (TDP) were observed under baseline conditions in both groups. After infusion of cotrimoxazole, 5 of 12 hearts (group 1, p < 0.05 vs. baseline, 37 episodes) and 5 of 13 hearts (group 2, p < 0.05 vs. baseline, 26 episodes, Fig. 3) presented EAD and TDP. Additional treatment with ondansetron resulted in EAD and TDP in 7 of 12 hearts (group 1, p < 0.01 vs. baseline, 55 episodes) and 8 of 13 hearts (group 2, p < 0.01 vs. baseline, 111 episodes). Under the additional influence of domperidone, EAD and TDP were observed in 11 of 12 hearts (group 1, p < 0.001 vs. baseline, 118 episodes) while additional treatment with erythromycin resulted in the occurrence of EAD and TDP in 9 of 13 hearts (group 2, p < 0.001 vs. baseline, 88 episodes, Figs. 4, 5).
Discussion
The results obtained in this proof-of-concept study underline that a combined administration of several QT-prolonging agents may significantly increase the proarrhythmic risk. The present data demonstrate that addition of further QT-prolonging drugs can result in a further increase in QT prolongation and a more marked increase in spatial dispersion of repolarization. Consequently, an increased incidence of torsade de pointes highlights the increased proarrhythmic risk.
A reduced repolarization reserve represents a possible explanation for the increased proarrhythmic risk under the influence of several QT-prolonging agents. This concept is defined as an individual response to a prolonged repolarization period mediated by cardiovascular and non-cardiovascular drugs [3]. A reduced repolarization reserve regularly occurs as a result of treatment with different potentially QT-prolonging agents or severe heart failure. Patients with genetic aberrations may also be exposed to a particular risk [1].
The assumption that a combination of several potentially QT-prolonging agents may result in an increased proarrhythmic risk is not new [1]. However, a thorough experimental analysis employing several drugs in order to prove potential additive proarrhythmic effects was not conducted before.
The employed agents are all known to induce a relevant prolongation of the QT interval and to bear a significant proarrhythmic potential when administered alone in identical concentrations as employed in the present study. In the same experimental model, ondansetron and domperidone presented a severe proarrhythmic potential [7]. In accordance, proarrhythmic effects have been reported for erythromycin [4] and cotrimoxazole [9].
Dispersion of Repolarization, Early After Depolarizations and Torsade de Pointes
All employed drugs are known to significantly increase spatial dispersion of repolarization. This effect could be reproduced in the present study. The importance of spatial dispersion of repolarization for the origination and perpetuation of torsade de pointes has been described extensively in the existing literature [2, 10]. In accordance, previous studies in the same experimental model have underlined the close association between an elevated spatial dispersion and the proarrhythmic risk [6, 11, 12]. However, in the present study an additive effect on dispersion of repolarization under the influence of different QT-prolonging agents was observed for the first time. This additive increase in spatial dispersion resulted in a further increased incidence of torsade de pointes.
In the present study, torsade de pointes always originated from early afterdepolarizations while no arrhythmias occurred in the absence of early afterdepolarizations. This finding is consistent with previous experimental studies [6, 12].
Conclusion
The present study underlines that concomitant treatment with several potentially QT-prolonging agents may exert an additive effect on cardiac repolarization and therefore increase the proarrhythmic risk. Additional infusion of each drug resulted in an increase in spatial dispersion that was accompanied by an increased incidence of torsade de pointes.
References
Frommeyer, G., & Eckardt, L. (2016). Drug-induced proarrhythmia: Risk factors and electrophysiological mechanisms. Nature reviews. Cardiology, 13, 36–47.
Antzelevitch, C. (2008). Drug-induced spatial dispersion of repolarization. Cardiology Journal, 15, 100–121.
Roden, D. M. (1998). Taking the “idio” out of “idiosyncratic”: Predicting torsades de pointes. Pacing and Clinical Electrophysiology: PACE, 21, 1029–1034.
Milberg, P., Eckardt, L., Bruns, H. J., Biertz, J., Ramtin, S., Reinsch, N., et al. (2002). Divergent proarrhythmic potential of macrolide antibiotics despite similar QT prolongation: Fast phase 3 repolarization prevents early afterdepolarizations and torsade de pointes. Journal of Pharmacology and Experimental Therapeutics, 303, 218–225.
Milberg, P., Hilker, E., Ramtin, S., Cakir, Y., Stypmann, J., Engelen, M. A., et al. (2007). Proarrhythmia as a class effect of quinolones: Increased dispersion of repolarization and triangulation of action potential predict torsades de pointes. Journal of Cardiovascular Electrophysiology, 18, 647–654.
Frommeyer, G., Fischer, C., Lange, P. S., Leitz, P., Fehr, M., Bogossian, H., et al. (2016). Divergent electrophysiologic profile of fluconazole and voriconazole in an experimental whole-heart model of proarrhythmia. European Journal of Pharmacology, 776, 185–190.
Frommeyer, G., Fischer, C., Ellermann, C., Lange, P. S., Dechering, D. G., Kochhauser, S., et al. (2017). Severe proarrhythmic potential of the antiemetic agents ondansetron and domperidone. Cardiovascular Toxicology. doi:10.1007/s12012-017-9403-5.
Frommeyer, G., Milberg, P., Witte, P., Stypmann, J., Koopmann, M., Lucke, M., et al. (2011). A new mechanism preventing proarrhythmia in chronic heart failure: Rapid phase-III repolarization explains the low proarrhythmic potential of amiodarone in contrast to sotalol in a model of pacing-induced heart failure. European Journal of Heart Failure, 13, 1060–1069.
Lopez, J. A., Harold, J. G., Rosenthal, M. C., Oseran, D. S., Schapira, J. N., & Peter, T. (1987). QT prolongation and torsades de pointes after administration of trimethoprim-sulfamethoxazole. The American Journal of Cardiology, 59, 376–377.
Verduyn, S. C., Vos, M. A., van der Zande, J., Kulcsar, A., & Wellens, H. J. (1997). Further observations to elucidate the role of interventricular dispersion of repolarization and early afterdepolarizations in the genesis of acquired torsade de pointes arrhythmias: A comparison between almokalant and d-sotalol using the dog as its own control. Journal of the American College of Cardiology, 30, 1575–1584.
Frommeyer, G., Kaiser, D., Uphaus, T., Kaese, S., Osada, N., Rajamani, S., et al. (2012). Effect of ranolazine on ventricular repolarization in class III antiarrhythmic drug-treated rabbits. Heart Rhythm, 9, 2051–2058.
Frommeyer, G., Brucher, B., von der Ahe, H., Kaese, S., Dechering, D. G., Kochhauser, S., et al. (2016). Low proarrhythmic potential of citalopram and escitalopram in contrast to haloperidol in an experimental whole-heart model. European Journal of Pharmacology, 788, 192–199.
Acknowledgements
This study was supported by the German Cardiac Society (to G.F.) and the Dr. Peter Osypka Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Frommeyer, G., Fischer, C., Ellermann, C. et al. Additive Proarrhythmic Effect of Combined Treatment with QT-Prolonging Agents. Cardiovasc Toxicol 18, 84–90 (2018). https://doi.org/10.1007/s12012-017-9416-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12012-017-9416-0