Abstract
Hexavalent chromium [Cr(VI)] has emerged as a prevailing environmental and occupational contaminant over the past few decades. However, the knowledge is sparse regarding Cr(VI)-induced neurological aberrations, and its remediation through natural bioactive compounds has not been fully explored. This study intended to probe the possible invigorative effects of nutraceuticals such as coenzyme Q10 (CoQ10), biochanin A (BCA), and phloretin (PHL) on Cr(VI) intoxicated Swiss albino mice with special emphasis on Nrf2/HO-1/NQO1 gene expressions. Mice received potassium dichromate (75 ppm) through drinking water and were simultaneously co-treated intraperitoneally with CoQ10 (10 mg/kg), BCA, and PHL (50 mg/kg) each for 30-day treatment period. The statistics highlight the elevated levels of lipid peroxidation (LPO) and protein carbonyl content (PCC) with a concomitant reduction in the superoxide dismutase (SOD), glutathione-S-transferase (GST), reduced glutathione (GSH), total thiols (TT), catalase (CAT), and cholinesterase activities in the Cr(VI)-exposed mice. The collateral assessment of DNA fragmentation, DNA breakages, and induced histological alterations was in conformity with the above findings in conjugation with the dysregulation in the Nrf2 and associated downstream HO-1 and NQO1 gene expressions. Co-treatment with the selected natural compounds reversed the above-altered parameters significantly, thereby bringing cellular homeostasis in alleviating the Cr(VI)-induced conciliated impairments. Our study demonstrated that the combination of different bioactive compounds shields the brain better against Cr(VI)-induced neurotoxicity by revoking the oxidative stress-associated manifestations. These compounds may represent a new potential combination therapy due to their ability to modulate the cellular antioxidant responses by upregulating the Nrf2/HO-1/NQO1 signaling pathway against Cr(VI)-exposed population.
Highlights
-
Cr(VI)-associated heavy metal exposure poses a significant threat to the environment, especially to living organisms.
-
Cr(VI) exposure for 30 days resulted in the free radical’s generation that caused neurotoxicity in the Swiss albino mice.
-
Natural compounds such as coenzyme Q10, biochanin A, and phloretin counteracted the neurotoxic effect due to Cr(VI) exposure in scavenging of free radicals by enhancing Nrf2/HO-1/NQO1 gene expressions in maintaining the cellular homeostasis.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the contemporary era, universal well-being is in question because of the exponential upsurge of environmental toxicants. Chromium (Cr) is a naturally existing transition metal found in the hexavalent and trivalent states. It is one of the largest industrial waste, which is discharged into the surroundings through textile industries, metallurgical industries, and leather tanneries, and the release of the untreated effluents in the environment causes serious health problems in both animals and humans [41, 57]. Every year, approximately 33 tons of Cr is laid off in the biosphere [4]. Hexavalent chromium [Cr(VI)], having higher mobility and solubility than the trivalent state of Cr, has resulted in the contamination of water bodies, air, and soil, which further pose threat to the human health and surrounding environment [37]. The trivalent chromium [Cr(III)] is considered an imperative micronutrient in low concentrations and has a vital role in the glucose and other metabolism and is sometimes prescribed as a nutritive supplement [8]. On the contrary, Cr(VI) is a potential toxin and carcinogen causing several health issues ranging from reproductive problems and neural impairments to the increased risks of cancers [9].
The permeability of Cr(VI) is very high, and it easily crosses the plasma membrane via sulfate anion transport system reducing to the intermediate species, i.e., Cr(V), Cr(IV) being metastable, and Cr (III) being most stable [13]. During the reduction of the Cr(VI) to the lower stable forms, several enzymatic and non-enzymatic reactions occur, resulting in the production of hydroxyl radicals. The generation of hydroxyl species and other reactive oxygen species (ROS) such as superoxide anion and nitric oxide leads to the oxidative stress, DNA damage, carcinogenicity, and cytotoxicity [6]. Compared to other organs, the brain absorbs ~ 20% of the body’s oxygen to perform vital functions, yet it has an enormous amount of polyunsaturated fatty acids that makes it vulnerable to ROS attack, thereby causing membrane damage simultaneously [52]. Under such conditions, nuclear factor erythroid 2-related factor 2 (Nrf2) is activated and conjugates with the antioxidants response elements (AREs). It governs the expressions of several antioxidant proteins such as glutathione (GSH), superoxide dismutase (SOD), heme oxygenase1 (HO-1), and NADPH quinone oxidoreductase 1(NQO1) that act as a primary cellular defense mechanism in bringing biochemical equilibrium towards normal functioning [29].
Some studies have been performed in the animal model showing the outburst of oxidative stress in mice brain due to the Cr(VI) administration [5, 52]. In a recent study, it was shown that there was association between motor neuron disease death risk and heavy metals (including Cr) in the exposed municipalities in Spain. [43]. Reports related to neurodevelopmental deformities, and a decrease in cholinergic neuronal cells leading to death in the experimental model, have already been documented [47]. In recent years, natural compounds have attracted global consideration regarding their spectrum of beneficial effects on various disease ailments [30, 40, 45]. In past decades, efforts have been made to naturally alleviate the toxic effects of the xenobiotics such as heavy metals, and pesticides, to cite a few [19, 26]. A study focused on the protective role of rosmarinic acid against harmful effects of the Cr(VI) on cultured cerebellar granule neurons in vitro has been documented in this direction [13].
Natural compounds (flavonoids or polyphenols) possess antioxidant properties, thus scavenging free radicals and maintaining regular homeostasis. They work as sequestering agents by binding to the target metal ion and expelling them out from the body. Coenzyme Q10 (CoQ10), also referred as ubiquinone, is naturally present in our body and provides genome stability by scavenging the free radicals through phosphorylation of nuclear factor kappa B, as reported earlier [36]. There are studies reported about beneficial effects of CoQ10 in treating various neurodegenerative disease conditions, viz., Parkinsonism, Alzheimer’s, and Huntington’s disease [59]. Dietary polyphenols such as biochanin A (BCA) and phloretin (PHL) are naturally occurring polyphenols present in the red clover and apples respectively, possess the anti-inflammation properties, and exert a neuroprotective and anti-cancerous functions. A study conducted in the recent past has determined the neuroprotective role of BCA in reducing the oxidative stress outburst through increased gene expression of Nrf2 and HO-1 against cerebral ischemia in rats [17]. While in another study, authors have shown the protective effects of BCA on the dopaminergic neurons and microglia activation, in the lipopolysaccharide-induced rats to mimic Parkinson’s disease model [54]. In another article, authors have reported a decline in the oxidative stress and subsequent neuroprotection by PHL through upregulation of Nrf2 gene expression against cerebral ischemia/reperfusion in the rat model [29].
Despite a large amount of information available from the previous studies, an underlying mechanism regarding Cr(VI)-associated neurotoxicity and the possible intervention strategies has not been fully studied so far. Hence, the present study was conducted to appraise the salutary effects of the CoQ10, BCA, and PHL against the Cr(VI)-induced neurotoxicity in the Swiss albino mice through Nrf2-induced HO-1/NQO1 upregulation.
Material and Methods
Chemicals and Reagents
The chemicals such as potassium dichromate (K2Cr2O7) (19365) from Qualigens Fine Chemicals, co-enzyme Q10 (CoQ10) (≥ 98% purity) (C9538), biochanin A (BCA) (D2016), and phloretin (PHL) (≥ 99% purity) (P7912) were obtained from Sigma-Aldrich Chemicals Company, USA, and other reagents required for the experimental analysis were procured from the local traders such as Himedia, SRL, and Lobachem.
Animals Monitoring
Healthy male Swiss albino mice (Mus musculus) weighing between 25 and 28 g were procured from Zydus Pharmaceutical Research Centre located in Ahmedabad, Gujarat. The ethical consent for conducting the animal experiments complied with the Institutional Animal Ethical Committee (IAEC) of the ICMR-NIOH, Ahmedabad. The animals were confined in the polypropylene cages for the duration of 2 weeks for the acclimatization in the laboratory conditions. The mice were kept under the consistent climatic conditions (22 ± 2 °C with relative humidity of 55–60%) with the 12 h:12 h light/dark schedule. They were provided with the standard dry food pellets and water ad libitum.
Experimental Design
The animals were categorically divided into six groups, with six animals in each group for 30-day treatment duration. The first group was not given any treatment and served as vehicle control. The 2nd group of animals was exposed to Cr(VI) (75 ppm) through drinking water. The animals in groups 3, 4, and 5 were exposed to the Cr(VI) through drinking water and simultaneously were co-treated with intraperitoneal injections of CoQ10 (10 mg/kg), BCA (50 mg/kg), and PHL (50 mg/kg), respectively, as per the body weight of each mouse. Group 6th animals were co-treated with Cr(VI) along with intraperitoneal injections of all three phytochemicals viz., CoQ10, BCA, and PHL for the same treatment schedule and doses. The doses of the Cr(VI) and the antioxidants were selected from the earlier reported studies, with slight alterations as per the route of administration [2, 29, 32, 44, 45, 55].
After the completion of the treatment period, the mice were sacrificed under anesthesia by the cervical dislocation. The blood was drawn from the retro-orbital sinus for further analysis, and brain tissues were dissected. They were weighed, and homogenates were made in the solution of 100 mM phosphate buffer and 0.1% TX-100 for all the biochemical assays.
Biochemical Analysis
All the biochemical indices were evaluated in homogenates of brain tissues using Synergy H1MD, BioTek, USA, multimode analyzer. Lipid peroxidation (LPO) was determined at 532 nm by calculating the malondialdehyde (MDA) content and expressed as nM MDA/mg tissue weight [11]. Protein carbonyl content (PCC) was measured at 450 nm, and the values were expressed in micromoles of PCC formed/mg protein [25]. The enzymatic activity of SOD was evaluated at 420 nm as described earlier [33], and the values were expressed as U/min/mg protein. Enzymatic activity of glutathione-S-transferase (GST) was calculated based on the protocol [18] and was detected at 340 nm. The values were expressed as nmol/min/mg protein. Reduced glutathione (GSH) analysis was per the previously described method [14] at 412 nm and expressed as nmol/mg tissue weight. Total thiol (TT) activity was evaluated as per the method at 412 nm [21]. Catalase (CAT) activity was analyzed at 240 nm and the values were expressed as µmol/min/mg protein as per the method [1].
Cholinesterase Enzyme Detection
The activity of acetylcholinesterase (AChE) and butyrylcholinesterase (BChE) were calculated by the methods of [15] and [48], respectively, at 412 nm optical density using Synergy H1MD, BioTek, USA, multimode analyzer.
DNA Isolation and Gel Electrophoresis
The genomic DNA from the brain tissues of different experimental groups was extracted using the phenol/chloroform method and precipitated with ethanol/2.5 M sodium acetate. The pellets were dissolved in TE buffer (50 µL). The DNA from the different experimental groups was quantified at 260 nm to check the yield, loaded onto a (1%) agarose gel, and finally electrophoresed at 80 V for 45 min to visualize the bands.
Comet Assay
The DNA damage of the eukaryotic cells was performed as per the following method [46]. The slides were prepared with layers of agarose and blood collected from the mice. After the electrophoresis, the slides were air-dried, and about 100–120 cells were analyzed under Leica, Germany, fluorescence microscope in the presence of ethidium bromide fluorescent dye. The cells were analyzed in comet assay software project, and the data was calculated in terms of % as head DNA, tail DNA, and olive tail moment.
Histological Evaluation
Regarding the histopathological findings, the brain samples prior stored in the 10% buffered formalin were sliced around 5-µm-thick sections and were stained later with hematoxylin and eosin (H&E) for the detection of any morphological abnormality(s). The evaluation was performed under light microscopy as per the method described earlier [3].
Gene Expression Analysis
Total cellular RNA of brain tissues was extracted as per the manufacturer’s instructions (Nucleospin RNA Plus kit). RNA quantification was done by evaluating the absorbance at 260/280 ratio in Synergy H1 MD Biotek, USA, multi-mode analyzer. Total RNA (< 5 µg) was taken to reverse transcribe into cDNA using cDNA synthesis kit as per the instruction manual (Takara PrimeScript 1st strand). The amplification and analyses of cDNA were done by RT-qPCR (Applied Biosystems) using SYBR green as the detection dye with specific gene primers. The values of the specific gene expression were evaluated by the 2−ΔΔCT method taking the difference in CT values of the target genes and housekeeping gene. Table 1 describes the primer sequences used for the analysis of specific gene expressions.
Statistical Analysis
The statistical evaluations were performed among different groups by one-way analysis of variance (ANOVA) with Dunnett’s as the post hoc analysis through GraphPad Prism software, USA. The results are represented as mean ± SEM. The values with p < 0.05 were considered statistically significant.
Results
Effect of Cr(VI) and Natural Compounds on Mice Weight
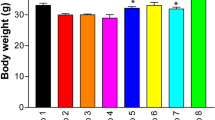
To determine the changes due to the Cr(VI) and the remedial effects of natural compounds, the weight of mice was measured regularly for the entire experimental period. The weight in the Cr(VI)-treated mice was reduced in comparison with the sham control and other co-treatment groups. However, the combined treatment with CoQ10, BCA, and PHL restored the bodyweight of the experimental mice to the normal levels as compared to the Cr(VI) treatment group (p < 0.05) (Table 2).
Effect of Cr(VI) and Natural Compounds on Oxidative Stress Parameters
Cr(VI) curtails redox balance and cellular bioenergetics leads to metabolic integration and subsequent cell cessation. Our results indicated that Cr(VI) generated ROS, while treatment with the CoQ10, BCA, and PHL counteract the oxidative stress, thereby reducing the free radical’s formation due to Cr(VI) exposure. The antioxidant enzymes maintain intracellular balance, and our results determine the activity of these anti-oxidant markers. Cr(VI) exposure led to the increase in the activities of LPO and PCC with declined levels of SOD, GST, CAT, GSH, and TT levels compared to the control. Nevertheless, with the treatment of CoQ10, BCA, and PHL, the levels of these anti-oxidant markers were restored to normal levels and even higher, reflecting the ameliorative effects of the selected natural compounds.
A significant reduction was seen in LPO levels with the CoQ10 (p < 0.05) treatment, and also with all three combined nutraceuticals co-treatment (p < 0.001) as compared to the Cr(VI) intoxicated mice (Fig. 1).
Biochemical estimation of lipid peroxidation (LPO) levels (Gp1: vehicle control; Gp2: Cr(VI); Gp3: Cr(VI) + CoQ10; Gp4: Cr(VI) + BCA; Gp5: Cr(VI) + PHL; Gp6: Cr(VI) + CoQ10 + BCA + PHL. Values are expressed as the mean ± SE; * represents significant deviation from Cr(VI) exposed values as *p < 0.05, ***p < 0.001
The PCC also declined significantly in CoQ10 (p < 0.01), BCA (p < 0.01), and PHL (p < 0.05) co-treatment groups, respectively. However, the reduction in the PCC levels was found more significant in the combined treatment group (p < 0.01) as compared to the Cr(VI) exposed mice (Fig. 2).
Biochemical estimation of protein carbonyl assay (PCC) levels (Gp1: vehicle control; Gp2: Cr(VI); Gp3: Cr(VI) + CoQ10; Gp4: Cr(VI) + BCA; Gp5: Cr(VI) + PHL; Gp6: Cr(VI) + CoQ10 + BCA + PHL. Values are expressed as the mean ± SE; * represents significant deviation from Cr(VI)-exposed values as *p < 0.05, **p < 0.01
Alternatively, the enzymatic activities of SOD, GST, and CAT were found to be reduced in the Cr(VI) treatment group. However, with the selected natural compound treatment, it was restored to the normal levels but was found significant only with PHL and combined treated group (CoQ10, BCA, and PHL) with respect to the SOD levels as compared to the Cr(VI)-administered mice (p < 0.05) (Fig. 3).
Biochemical estimation of superoxide dismutase (SOD) levels (Gp1: vehicle control; Gp2: Cr(VI); Gp3: Cr(VI) + CoQ10; Gp4: Cr(VI) + BCA; Gp5: Cr(VI) + PHL; Gp6: Cr(VI) + CoQ10 + BCA + PHL. Values are expressed as the mean ± SE; * represents significant deviation from Cr(VI)-exposed values as *p < 0.05
We have also assessed the effect of selected natural compounds on restoring the GST activity, although it was not found significant as compared to the Cr(VI)-exposed group (Fig. 4).
Similar results were seen in the CAT activity but was found significant only with the combined-treated group (CoQ10, BCA, and PHL) as compared to the Cr(VI)-administered mice (p < 0.05) (Fig. 5).
A similar trend was observed with the GSH levels, where it was restored significantly to the basal levels after co-treatment with the combined bioactive compounds as compared to the Cr(VI)-intoxicated mice (p < 0.01) (Fig. 6).
Biochemical estimation of reduced glutathione (GSH) levels (Gp1: vehicle control; Gp2: Cr(VI); Gp3: Cr(VI) + CoQ10; Gp4: Cr(VI) + BCA; Gp5: Cr(VI) + PHL; Gp6: Cr(VI) + CoQ10 + BCA + PHL. Values are expressed as the mean ± SE; * represents significant deviation from Cr(VI)-exposed values as **p < 0.01
Whereas in the total thiols estimations, the total thiol levels were found to increase with the CoQ10, BCA, and PHL co-treatment groups (p < 0.05) respectively; however, the value was highly significant in the combined group of antioxidants as compared to the Cr(VI)-administered group (p < 0.01) (Fig. 7).
Biochemical estimation of total thiols (TT) levels (Gp1: vehicle control; Gp2: Cr(VI); Gp3: Cr(VI) + CoQ10; Gp4: Cr(VI) + BCA; Gp5: Cr(VI) + PHL; Gp6: Cr(VI) + CoQ10 + BCA + PHL. Values are expressed as the mean ± SE; * represents significant deviation from Cr(VI)-exposed values as *p < 0.05, **p < 0.01
Effect of Cr(VI) and Natural Compounds on Cholinesterase Enzyme Activity
The activity of cholinesterase enzymes were observed in the brain tissue homogenates of the mice among the different experimental groups. Our results showed the devaluation in the AChE and BChE levels of the Cr(VI)- exposed mice compared to the untreated control. Nonetheless, the cholinesterase activity was restored to the basal levels with selected natural compounds CoQ10, BCA, and PHL co-treatments. However, a significant rise in the values of the cholinesterase enzymes was seen only in the combined co-treatment group as compared to the Cr(VI)- exposed mice (p < 0.05) (Fig. 8).
DNA Isolation and Its Quantitation
The brains from the different experimental groups of mice were selected for the isolation of genomic DNA. Isolated DNA was then run on the agarose gel (1%) to visualize the genomic DNA bands for various treatments (Fig. 9). The gel picture could reveal the changes in the DNA yield among different treated groups. It was observed that in the Cr(VI)-treated mice (group 2), DNA yield decreased compared to the other groups and showed degradation. The yield of DNA increased amid all the protected groups, especially in the combined co-treatment group (Fig. 9).
Cell Damage Due to Cr(VI) and Its Alleviation by Natural Compounds
DNA damage in the blood cells of the mice was evaluated in all the experimental groups. The head DNA% was found to be declined significantly in the Cr(VI)-exposed mice compared to the untreated control (p < 0.001). With the different protected groups, the head DNA% rose to the basal status, although it was found significant only with the CoQ10 group (p < 0.01) and Cr + CoQ10 + BCA + PHL-treated group respectively (p < 0.001) as compared to the Cr(VI)-intoxicated group, alleviating the Cr(VI)-induced toxicity (Fig. 10a).
COMET (DNA damage) assay in blood cells (Gp1: vehicle control; Gp2: Cr(VI); Gp3: Cr(VI) + CoQ10; Gp4: Cr(VI) + BCA; Gp5: Cr(VI) + PHL; Gp6: Cr(VI) + CoQ10 + BCA + PHL. Values are expressed as the mean ± SE; * represents significant deviation from Cr(VI) exposed values as **p < 0.01, ***p < 0.001. a Head DNA %, b Tail DNA %, c Olive Tail Moment (%)
Alternatively, tail DNA% was significantly higher in the Cr(VI)-exposed mice than in control depicting higher cell damage (p < 0.001). However, a significant decrease in the tail DNA % was found with Cr + Q10 (p < 0.01) and Cr + CoQ10 + BCA + PHL (p < 0.001), respectively, compared to the Cr(VI)-exposed mice rendering the protective effects of the combined anti-oxidant treatment (Fig. 10b).
Similarly, the % of the olive tail moment was seen to be significantly higher in the Cr(VI)-intoxicated mice as compared to the untreated control (p < 0.001). However, it was reduced significantly with the various anti-oxidant treatments, viz., CoQ10, BCA, and PHL (p < 0.01) as compared to the Cr(VI)-exposed mice. Moreover, it was found that with the combined anti-oxidant treatment, the reduction in the % of the olive tail moment was further decreased in a highly significant manner (p < 0.001) as compared to the Cr(VI)-exposed group (Fig. 10c).
Histopathological Studies
Figure 11 represents the hematoxylin and eosin–stained brain sections. The cerebrum sections of control mice did not show any significant histopathological changes (Fig. 11a). In Cr(VI)-treated group, the brain revealed perivascular lymphocytic cuffing (Fig. 11b). Mice treated with Cr + CoQ10, Cr + BCA, and Cr + PHL showed mild cellular aggregation and presented normal histological architecture (Fig. 11c, d, and e). However, some inflammatory characteristics due to focal perivascular mononuclear cell infiltration in the brain may be noted due to the Cr(VI)-induced tissue response. In Fig. 11f, Cr + CoQ10 + BCA + PHL mice group showed nearly normal histological appearance with reduced lesions.
Alterations in the Nrf2, HO-1, and NQO-1 Gene Expressions
The alterations in the gene expressions of Nrf2/HO-1/NQO1 were observed to illustrate the Cr(VI)-induced neurotoxicity, and the curative effects of the natural compounds. Compared to the vehicle control, the Nrf2, HO-1, and NQO1 basal levels were lowered in the Cr(VI)-exposed mice (Fig. 12a, b and c). With the different natural bioactive compounds, the expressions of Nrf2, HO-1, and NQO1 were restored to the basal level and even more after 30 days of treatment. Nonetheless, it was found significant only with the combined anti-oxidant treatment group in Nrf2 (p < 0.05), HO-1 (p < 0.01), and NQO1 (p < 0.05), respectively, compared to the Cr(VI)-intoxicated mice (Fig. 12a, b and c).
Nrf2, HO-1, and NQO1 gene expressions (Gp1: vehicle control; Gp2: Cr(VI); Gp3: Cr(VI) + CoQ10; Gp4: Cr(VI) + BCA; Gp5: Cr(VI) + PHL; Gp6: Cr(VI) + CoQ10 + BCA + PHL. Values are expressed as the mean ± SE; * represents significant deviation from Cr(VI)-exposed values as *p < 0.05, **p < 0.01. a NRF2 gene expression, b HO-1 gene expression, c NQO1 gene expression
Discussion
In the current scenario of ongoing development across the boundaries, heavy metal contamination is becoming a deliberate threat to the environment accompanying several health risk factors [39]. One of the major environmental toxins of global concern is Cr(VI), affecting human physiology with expanded neurobehavioral ailments [56]. Our experimental result statistics advocate the remedial effects of the CoQ10 and the polyphenolic compounds BCA and PHL in negating the Cr(VI)-induced neurotoxicity in the Swiss albino mice.
Following 30 days of treatment, we found that the bodyweight of the Cr(VI)-exposed group slightly declined as compared to the sham control and with other co-treatment groups. However, with the combined treatment of CoQ10, BCA, and PHL, the bodyweight of the experimental mice was restored significantly to the normal levels as compared to the Cr(VI). In contrary to the individual treatment of the selected natural compounds, there was a gain in the bodyweight of the experimental mice, although it was found non-significant with respect to sham control, the Cr(VI) exposed group, and also among each other. Our results are consistent with the previous findings on rabbits [34], rats, and mice [44], which showed significant declination in the bodyweight as a result to the exposure of different doses of Cr(VI). The brain is a soft tissue that is accessible to free radical generation because of a higher quantity of polyunsaturated fatty lipids than a pool of non-enzymatic and enzymatic antioxidants. This may lead to a generation of oxidative stress, causing damage to brain tissues and neurological abnormalities. Several reports are suggestive of the generation of ROS due to the Cr(VI) administration in experimental models [7, 23]. The increased ROS results from the formation of free radicals such as superoxide, hydroxyl, and singlet O2 via the Haber–Weiss mechanism. During the reduction of Cr, the intermediates formed are assumed to react with H2O2 leading to the formation of HO radical [24]. The resultant attacks the DNA, membrane lipids, and proteins leading to disruption of cellular defense mechanism.
Our study assessed the MDA levels as a marker of LPO, circulating in brain homogenates of mice across the groups. The data revealed the rise of MDA in Cr(VI)-exposed mice compared to control, indicating the oxidative burst. Prior study is in concordance with the enhanced MDA levels due to Cr(VI) administration in a dose-dependent manner in Wistar rats [42]. However, with the administration of the CoQ10, BCA, and PHL individually and in combination, the MDA levels rose back to the normal levels stating the therapeutic effects of these anti-oxidants, although it was found significant with CoQ10 and the combined anti-oxidant co-treatment respectively. It appears from our results that CoQ10 was better in bringing down MDA level as compared to the other natural compounds viz., BCA and PHL respectively. Similar results were seen in the previous study, where the MDA levels rose with Cr(VI) administration and declined significantly with selenium supplementation [49].
PCC, another marker for detecting oxidative stress, was carried out during our study. During ROS production, the protein carbonyl group’s content increases, leading to the formation of aldehydes and ketones as their final products [50]. Our findings showed that PCC levels in brain homogenates increased in Cr(VI)- exposed mice compared to the vehicle control. However, with the selected anti-oxidant co-treatment, viz., CoQ10, BCA, and PHL, individually and in combination, the PCC levels were restored significantly compared to the Cr(VI)-treated mice. Based on this analysis, it appeared that both of the selected anti-oxidants viz., CoQ10 and BCA, were found superior compared to the PHL in restoring the PCC levels as result of Cr(VI) exposure. Our findings are in accordance with the previous study [51], where mice were exposed to Cr(VI) for 90 days, which resulted in the increased PCC levels in the intestinal tissues.
Anti-oxidant enzyme SOD is considered to avert the macromolecules from oxidative injury present in a biological system, thereby removing free radicals such as H2O2 formed due to the dismutation of superoxide anion. This H2O2 is further degraded by the enzymes CAT and GPx. Normally, H2O2 is degraded by GPx, but in oxidative stress condition where the amount of H2O2 increases and the activity of CAT concomitantly rises to degrade the concentration of H2O2 [35]. Our results represent the decrease in the activity of SOD and CAT in the mice exposed to Cr(VI), thus introspecting the intensity of the oxidative injury. Nonetheless, there was an upsurge seen in the activity of SOD and CAT in the brain of mice treated individually with CoQ10, BCA, and PHL. However, the SOD was found significant only with PHL and with the combination group of antioxidants, whereas CAT levels were found significant in the combined anti-oxidant group compared to the Cr(VI)- intoxicated mice. Out of the selected anti-oxidants, PHL was found better in curtailing oxidative stress in terms of restoring the SOD and CAT levels in comparison with the other anti-oxidants (CoQ10 and BCA). This restoration of the SOD and CAT activity indicates selected natural compounds’ potential in crossing the blood–brain barrier, thereby counteracting the Cr(VI)-associated neurotoxicity. Earlier reports have also shown the decreased SOD activity in the rats [31] and chickens brains [20] exposed to the Cr(VI); however, with the treatment with caffeic acid phenethylester and selenium, respectively, the SOD activity was restored in the protected groups. Similar results were reported in the previous study where SOD and CAT activity was reduced on exposure to the Cr(VI). However, the activities were reversed with the taurine pre-treatment [10].
Another enzymatic antioxidant, GST, is imperative in maintaining the cellular homeostasis cycle. It helps in detoxification by catalyzing the union of reduced GSH to xenobiotic compounds. In our study, the GST levels asserted the drift of reduction in the Cr(VI)-exposed mice compared to control. Our result was analogous to the study performed in Cr(VI)-exposed fish for 3 months [12]. Furthermore, in our study, the results are noticeable as the GST levels were restored in CoQ10, BCA, and PHL-treated mice; although, it was not found significant as compared to Cr(VI) treatment group. However, looking at the GST levels, the CoQ10 was found promising among other two anti-oxidants in counteracting the oxidative stress due to Cr(VI) exposure.
Reduced GSH acts as a catalyst with –SH groups in exchanging disulphides by scavenging free radicals. Our study concluded that brain glutathione levels was flawed by Cr(VI) intoxication as could be seen by the reduction in the GSH level. However, the GSH levels were restored to normal levels with the different bioactive compounds, although it was found significant only with the combined anti-oxidant co-treatment group. While comparing the antioxidant activity of the selected compounds, BCA was found better in restoring the GSH activity. The statistics were similar to the past findings in the chicken’s brain, where GSH levels got reduced with Cr(VI) exposure and restored with selenium treatment [20].
Also, some proteins remain in the reduced form in the cytoplasm to maintain the redox balance in the cellular microenvironment. Under oxidative injury, some proteins behave as redox switches by balancing the mechanism of other proteins to maintain cellular signaling. Excessive oxidation leads to depletion of protein thiols that results in cellular imbalance and ultimately to the cell death. Our study revealed a decrease in total thiol in the brain of Cr(VI)-exposed mice compared to the vehicle control. However, with the selected natural compound treatment, the total thiols levels were significantly restored to the basal levels, although it was found highly significant with the combined natural compound co-treatment group. In this assay, it was found that all the three selected compounds were equally effective individually in restoring total thiols levels as compared to the Cr(VI) exposure.
The results are in accordance with the previous findings, where the total thiol level declined in the Cr(VI)-exposed mice [52]. Similar conclusions could be drawn from the study carried out in the chrome electroplating workers regarding Cr(VI) exposure in altering the oxidative stress markers [60].
To appraise the function of the brain, AChE and BChE activities were carried out in the different experimental groups. Both are necessary for cholinergic and biochemical neurotransmissions. We found that the activity of AChE and BChE got reduced in the Cr(VI)-intoxicated mice as compared to the control. In addition, the levels got elevated with different anti-oxidant treatment groups, viz., CoQ10, BCA, and PHL, respectively. However, only the combined treatment group showed significant elevation in AChE and BChE activities compared to the Cr(VI)-exposed mice. Although BCA and CoQ10 was found promising amid the selected antioxidants in restoring the AChE and BChE activities respectively. It is believed that the metals bind to the anionic site of cholinesterases (ChEs) and inhibit cholinesterase (ChE) activity, thus averting acetylcholine (ACh) conjugation to ChE [16] for the neurotransmission. The results reflected in our study are similar to the findings of the other studies, where the AChE/BChE levels were decreased with the administration of Cr(VI) in rats [31, 49] and with co-exposure to the arsenic and Cr(VI) in mice [53].
The present study emphasizes on the damaged DNA as a repercussion of the induced oxidative injury due to Cr(VI) exposure. The single-strand breakage recurrence was lowest in the Cr(VI)-intoxicated mice represented as head DNA% along with escalation in tail DNA % and olive tail moment % as compared to the vehicle control. The Cr(VI) post-reduction process involves several intermediates states that invade the macromolecules leading to DNA crosslinks, DNA damage, or Cr-DNA adducts that leads to the cellular damage and could be a possible reason for the significant increase of tail DNA% in Cr(VI)-exposed mice as reported earlier [38]. Simultaneously, with the co-treatment of CoQ10, BCA, and PHL individually, the tail DNA% and the olive tail moment decreased with the upsurge in the head DNA%, and a significant difference was observed with combined doses of the natural compounds in comparison to the Cr(VI)-exposed group. On the other hand, CoQ10 was found potent in preventing the head DNA and tail DNA % damage amid all the bioactive compounds, whereas in olive tail moment %, BCA appeared to be a better antioxidant with respect to the other compounds.
The brain tissue examination was performed under the microscope on H&E-stained slides to depict the abnormalities caused due to Cr(VI) administration. The results seemed to be consistent with the biochemical parameters performed. The cellular compartment was distorted with infiltration of the neuroglia in the Cr(VI)-exposed mouse brain. The cerebrum sections of the untreated mice did not present any histopathological abnormalities. While perivascular lymphocytic cuffing was observed in the Cr(VI)-treated group. The different bioactive dietary compound treatments reversed the abnormalities in the cerebrum, and normal differentiation in the cells was seen in the different treatment groups. The investigation revealed the efficacy of the selected natural bioactive compounds in counteracting the neurotoxicity due to Cr(VI) exposure.
In addition to the biochemical parameters, we have studied the expressions of the oxidative injury responsive genes in the experimental setup. Under oxidative stress conditions, Nrf2 is the key transcription protein factor that governs the anti-oxidant defense mechanism. Under normal conditions, it is conjugated with Kelch-like ECH-associated protein 1 (Keap 1) in the cytosol [22]. This interaction is interrupted when oxidants invade the body causing free radical generation, resulting in the translocation of Nrf2 into the nucleus, thereby increasing the anti-oxidant enzyme transcription viz., HO-1 and NQO1 [28]. In our study, we found that Cr(VI) administration decreased the expressions of Nrf2, HO-1, and NQO-1 as compared with the vehicle control. However, with the various anti-oxidant treatments, the expression of nuclear proteins such as Nrf2, HO-1, and NQO1 increased and restored almost to the normal expression levels in bringing the cellular equilibrium. However, it was found significant with the combined treatment with respect to Nrf2/HO-1/NQO1 gene expressions as compared to the Cr(VI) exposure. It was observed that the relative expressions of CoQ10, PHL, BCA were found better in Nrf2, HO-1, and NQO1 gene analysis respectively, amid all individual antioxidant treatment groups. In some instances, such as in Nrf2 gene expression, CoQ10 seemed better than the combined treatment group of the selected antioxidants, but there was no significant difference with respect to the Cr(VI)-exposed group. This slight increase in CoQ10 activity can be regarded as an adaptive response to Cr(VI)-induced oxidative stress initially and may subside subsequently.
Our findings reflect the curative effects of the natural compounds against Cr(VI)-induced neurotoxicity by upregulation of the stress-responsive genes. These results may illustrate that the stimulation of Nrf2 may cause a decline in ROS production with an escalation in the expression of HO-1 and NQO1 genes that are downstream to the Nrf2 promoter, thereby improving the anti-oxidant defense system and maintaining cellular homeostasis. Similar findings were observed in the study conducted in Kunming mice experimental model, where pre-treatment with N-acetyl cysteine significantly increased Nrf2 and HO-1 expressions against Cr(VI)-induced oxidative stress [28]. Several other studies have also demonstrated that chronic exposure to xenobiotics can induce oxidative stress along with inflammation and apoptosis by various pathways [27, 58].
Our study provides an insight into the protective effects of CoQ10 BCA, and PHL on neurological abnormalities in Cr(VI)-exposed mice. These anti-oxidants may potentially render protection against several neurodegenerative diseases associated with these heavy metal exposure, particularly Cr(VI). Our data present a lead in the Cr(VI)-induced disease model manifestation and throw a light on the therapeutic effects of these selected natural compounds through dietary supplements in a day-to-day life. Further studies are warranted in this direction to fully understand the underlying mechanism in abrogating heavy metal–associated neurotoxicity.
Conclusion
Cr(VI) administration to mice induced oxidative alterations in the brain, decreased cholinesterase’s activity, and increased DNA fragmentation, and histological alterations in the brain along with dysregulation of Nrf2/HO-1/NQO1 gene expressions. Co-treatment with the selected natural bioactive compounds reversed the above effects in a major way in rendering the neuroprotection. Neurological disorders are often associated with the modified neuronal proteins and subsidiary functions, due to free radical–mediated pathological changes in the neurons. A combination of these antioxidants could be a better strategy for managing neurodegenerative diseases with the available dietary nutraceuticals that are in everybody’s reach and could further be used in clinical settings as a potential combination therapy.
Data Availability
All data generated or analyzed during this study are included in this article. Data will be available on request. Correspondence and requests for materials should be addressed to Gyanendra Singh.
References
Aebi H (1974) Catalase. Academic Press, Methods Of Enzymatic Analysis. https://doi.org/10.1016/B978-0-12-091302-2.50032-3
Aldhahri RS, Alghamdi BS, Alzahrani NA, Bahaidrah KA, Alsufiani HM, Mansouri RA and Ashraf GM (2022) Biochanin A Improves Memory Decline And Brain Pathology In Cuprizone-Induced Mouse Model Of Multiple Sclerosis. Behav Sci (Basel) 12
Atici S, Cinel L, Cinel I, Doruk N, Aktekin M, Akca A, Camdeviren H, Oral U (2004) Opioid Neurotoxicity: Comparison Of Morphine And Tramadol In An Experimental Rat Model. Int J Neurosci 114:1001–1011
Atsdr U (2012) Toxicological Profile For Chromium. Us Department Of Health And Human Services, Public Health Service. https://wwwn.Cdc.Gov/Tsp/Toxprofiles/Toxprofiles.Aspx
Bagchi D, Balmoori J, Bagchi M, Ye X, Williams CB, Stohs SJ (2002) Comparative Effects Of Tcdd, Endrin, Naphthalene And Chromium (Vi) On Oxidative Stress And Tissue Damage In The Liver And Brain Tissues Of Mice. Toxicology 175:73–82
Bagchi D, Stohs SJ, Downs BW, Bagchi M, Preuss HG (2002) Cytotoxicity And Oxidative Mechanisms Of Different Forms Of Chromium. Toxicology 180:5–22
Bagchi D, Vuchetich PJ, Bagchi M, Hassoun EA, Tran MX, Tang L, Stohs SJ (1997) Induction Of Oxidative Stress By Chronic Administration Of Sodium Dichromate [Chromium Vi] And Cadmium Chloride [Cadmium Ii] To Rats. Free Radic Biol Med 22:471–478
Bailey MM, Boohaker JG, Jernigan PL, Townsend MB, Sturdivant J, Rasco JF, Vincent JB, Hood RD (2008) Effects Of Pre- And Postnatal Exposure To Chromium Picolinate Or Picolinic Acid On Neurological Development In Cd-1 Mice. Biol Trace Elem Res 124:70–82
Barceloux DG (1999) Chromium. J Toxicol Clin Toxicol 37:173–194
Bosgelmez Ii, Soylemezoglu T, Guvendik G (2008) The Protective And Antidotal Effects Of Taurine On Hexavalent Chromium-Induced Oxidative Stress In Mice Liver Tissue. Biol Trace Elem Res 125:46–58
Buege JA, Aust SD (1978) Microsomal Lipid Peroxidation. Methods Enzymol 52:302–310
Chen H, Mu L, Cao J, Mu J, Klerks PL, Luo Y, Guo Z, Xie L (2016) Accumulation And Effects Of Cr(Vi) In Japanese Medaka (Oryzias Latipes) During Chronic Dissolved And Dietary Exposures. Aquat Toxicol 176:208–216
Dashti A, Soodi M, Amani N (2016) Cr (Vi) Induced Oxidative Stress And Toxicity In Cultured Cerebellar Granule Neurons At Different Stages Of Development And Protective Effect Of Rosmarinic Acid. Environ Toxicol 31:269–277
Ellman GL (1959) Tissue Sulfhydryl Groups. Arch Biochem Biophys 82:70–77
Ellman GL, Courtney KD, Andres V Jr, Feather-Stone RM (1961) A New And Rapid Colorimetric Determination Of Acetylcholinesterase Activity. Biochem Pharmacol 7:88–95
Guilhermino L, Soares AM, Carvalho AP, Lopes MC (1998) Correlation Between Whole Blood Cholinesterase Activity And Cerebral Cortex Cholinesterase Activity In Rats Treated With Parathion. Chemosphere 37:1385–1393
Guo M, Lu H, Qin J, Qu S, Wang W, Guo Y, Liao W, Song M, Chen J, Wang Y (2019) Biochanin A Provides Neuroprotection Against Cerebral Ischemia/Reperfusion Injury By Nrf2-Mediated Inhibition Of Oxidative Stress And Inflammation Signaling Pathway In Rats. Med Sci Monit 25:8975–8983
Habig WH, Pabst MJ, Jakoby WB (1974) Glutathione S-Transferases. The First Enzymatic Step In Mercapturic Acid Formation. J Biol Chem 249:7130–7139
Han B, Li S, Lv Y, Yang D, Li J, Yang Q, Wu P, Lv Z, Zhang Z (2019) Dietary Melatonin Attenuates Chromium-Induced Lung Injury Via Activating The Sirt1/Pgc-1alpha/Nrf2 Pathway. Food Funct 10:5555–5565
Hao P, Zhu Y, Wang S, Wan H, Chen P, Wang Y, Cheng Z, Liu Y, Liu J (2017) Selenium Administration Alleviates Toxicity Of Chromium(Vi) In The Chicken Brain. Biol Trace Elem Res 178:127–135
Hu ML (1994) Measurement Of Protein Thiol Groups And Glutathione In Plasma. Methods Enzymol 233:380–385
Itoh K, Wakabayashi N, Katoh Y, Ishii T, Igarashi K, Engel JD, Yamamoto M (1999) Keap1 Represses Nuclear Activation Of Antioxidant Responsive Elements By Nrf2 Through Binding To The Amino-Terminal Neh2 Domain. Genes Dev 13:76–86
Jin Y, Zhang S, Tao R, Huang J, He X, Qu L, Fu Z (2016) Oral Exposure Of Mice To Cadmium (Ii), Chromium (Vi) And Their Mixture Induce Oxidative- And Endoplasmic Reticulum-Stress Mediated Apoptosis In The Livers. Environ Toxicol 31:693–705
Kadiiska MB, Xiang QH, Mason RP (1994) In Vivo Free Radical Generation By Chromium(Vi): An Electron Spin Resonance Spin-Trapping Investigation. Chem Res Toxicol 7:800–805
Levine RL, Berlett BS, Moskovitz J, Mosoni L, Stadtman ER (1999) Methionine Residues May Protect Proteins From Critical Oxidative Damage. Mech Ageing Dev 107:323–332
Li J, Yu Z, Han B, Li S, Lv Y, Wang X, Yang Q, Wu P, Liao Y, Qu B, Zhang Z (2022) Activation Of The Gpx4/Tlr4 Signaling Pathway Participates In The Alleviation Of Selenium Yeast On Deltamethrin-Provoked Cerebrum Injury In Quails. Mol Neurobiol 59:2946–2961
Li S, Wu P, Han B, Yang Q, Wang X, Li J, Deng N, Han B, Liao Y, Liu Y, Zhang Z (2022) Deltamethrin Induces Apoptosis In Cerebrum Neurons Of Quail Via Promoting Endoplasmic Reticulum Stress And Mitochondrial Dysfunction. Environ Toxicol 37:2033–2043
Li X, He S, Zhou J, Yu X, Li L, Liu Y, Li W (2021) Cr (Vi) Induces Abnormalities In Glucose And Lipid Metabolism Through Ros/Nrf2 Signaling. Ecotoxicol Environ Saf 219:112320
Liu Y, Zhang L, Liang J (2015) Activation Of The Nrf2 Defense Pathway Contributes To Neuroprotective Effects Of Phloretin On Oxidative Stress Injury After Cerebral Ischemia/Reperfusion In Rats. J Neurol Sci 351:88–92
Lu J, Jiang H, Liu B, Baiyun R, Li S, Lv Y, Li D, Qiao S, Tan X, Zhang Z (2018) Grape Seed Procyanidin Extract Protects Against Pb-Induced Lung Toxicity By Activating The Ampk/Nrf2/P62 Signaling Axis. Food Chem Toxicol 116:59–69
Mahmoud AM, Abd El-Twab SM (2017) Caffeic Acid Phenethyl Ester Protects The Brain Against Hexavalent Chromium Toxicity By Enhancing Endogenous Antioxidants And Modulating The Jak/Stat Signaling Pathway. Biomed Pharmacother 91:303–311
Majumdar A, Nirwane A, Kamble R (2014) New Evidences Of Neurotoxicity Of Aroclor 1254 In Mice Brain: Potential Of Coenzyme Q10 In Abating The Detrimental Outcomes. Environ Health Toxicol 29:E2014001
Marklund S, Marklund G (1974) Involvement Of The Superoxide Anion Radical In The Autoxidation Of Pyrogallol And A Convenient Assay For Superoxide Dismutase. Eur J Biochem 47:469–474
Mary Momo CM, Ferdinand N, Omer Bebe NK, Alexane Marquise MN, Augustave K, Bertin Narcisse V, Herve T and Joseph T (2019) Oxidative Effects Of Potassium Dichromate On Biochemical, Hematological Characteristics, And Hormonal Levels In Rabbit Doe (Oryctolagus Cuniculus). Vet Sci 6
Michiels C, Raes M, Toussaint O, Remacle J (1994) Importance Of Se-Glutathione Peroxidase, Catalase, And Cu/Zn-Sod For Cell Survival Against Oxidative Stress. Free Radic Biol Med 17:235–248
Nyariki JN, Ochola LA, Jillani NE, Nyamweya NO, Amwayi PE, Yole DS, Azonvide L, Isaac AO (2019) Oral Administration Of Coenzyme Q10 Protects Mice Against Oxidative Stress And Neuro-Inflammation During Experimental Cerebral Malaria. Parasitol Int 71:106–120
O’flaherty EJ, Kerger BD, Hays SM, Paustenbach DJ (2001) A Physiologically Based Model For The Ingestion Of Chromium(Iii) And Chromium(Vi) By Humans. Toxicol Sci 60:196–213
Patlolla AK, Barnes C, Yedjou C, Velma VR, Tchounwou PB (2009) Oxidative Stress, Dna Damage, And Antioxidant Enzyme Activity Induced By Hexavalent Chromium In Sprague-Dawley Rats. Environ Toxicol 24:66–73
Pratush A, Kumar A, Hu Z (2018) Adverse Effect Of Heavy Metals (As, Pb, Hg, And Cr) On Health And Their Bioremediation Strategies: A Review. Int Microbiol 21:97–106
Prince PD, Rodriguez Lanzi C, Fraga CG, Galleano M (2019) Dietary (-)-Epicatechin Affects Nf-Kappab Activation And Nadph Oxidases In The Kidney Cortex Of High-Fructose-Fed Rats. Food Funct 10:26–32
Ray RR (2016) Adverse Hematological Effects Of Hexavalent Chromium: An Overview. Interdiscip Toxicol 9:55–65
Salama A, Hegazy R, Hassan A (2016) Intranasal Chromium Induces Acute Brain And Lung Injuries In Rats: Assessment Of Different Potential Hazardous Effects Of Environmental And Occupational Exposure To Chromium And Introduction Of A Novel Pharmacological And Toxicological Animal Model. PLoS ONE 11:E0168688
Sanchez-Diaz G, Escobar F, Badland H, Arias-Merino G, Posada De La Paz M, and Alonso-Ferreira V (2018) Geographic Analysis Of Motor Neuron Disease Mortality And Heavy Metals Released To Rivers In Spain. Int J Environ Res Public Health 15
Shipkowski KA, Sheth CM, Smith MJ, Hooth MJ, White KL Jr, Germolec DR (2017) Assessment Of Immunotoxicity In Female Fischer 344/N And Sprague Dawley Rats And Female B6c3f1 Mice Exposed To Hexavalent Chromium Via The Drinking Water. J Immunotoxicol 14:215–227
Singh G, Thaker R, Sharma A, Parmar D (2021) Therapeutic Effects Of Biochanin A, Phloretin, And Epigallocatechin-3-Gallate In Reducing Oxidative Stress In Arsenic-Intoxicated Mice. Environ Sci Pollut Res Int 28:20517–20536
Singh NP, Mccoy MT, Tice RR, Schneider EL (1988) A Simple Technique For Quantitation Of Low Levels Of Dna Damage In Individual Cells. Exp Cell Res 175:184–191
Singh P, Chowdhuri DK (2017) Environmental Presence Of Hexavalent But Not Trivalent Chromium Causes Neurotoxicity In Exposed Drosophila Melanogaster. Mol Neurobiol 54:3368–3387
Sirvio J, Soininen HS, Kutvonen R, Hyttinen JM, Helkala EL, Riekkinen PJ (1987) Acetyl- And Butyrylcholinesterase Activity In The Cerebrospinal Fluid Of Patients With Parkinson’s Disease. J Neurol Sci 81:273–279
Soudani N, Troudi A, Amara IB, Bouaziz H, Boudawara T, Zeghal N (2012) Ameliorating Effect Of Selenium On Chromium (Vi)-Induced Oxidative Damage In The Brain Of Adult Rats. J Physiol Biochem 68:397–409
Suzuki YJ, Carini M, Butterfield DA (2010) Protein Carbonylation. Antioxid Redox Signal 12:323–325
Thompson CM, Proctor DM, Haws LC, Hebert CD, Grimes SD, Shertzer HG, Kopec AK, Hixon JG, Zacharewski TR, Harris MA (2011) Investigation Of The Mode Of Action Underlying The Tumorigenic Response Induced In B6c3f1 Mice Exposed Orally To Hexavalent Chromium. Toxicol Sci 123:58–70
Travacio M, Polo JM, Llesuy S (2001) Chromium (Vi) Induces Oxidative Stress In The Mouse Brain. Toxicology 162:139–148
Tripathi S, Fhatima S, Parmar D, Singh DP, Mishra S, Mishra R and Singh G (2022) Therapeutic Effects Of Coenzymeq10, Biochanin A And Phloretin Against Arsenic And Chromium Induced Oxidative Stress In Mouse (Mus Musculus) Brain. 3 Biotech 12:1–13
Wang J, He C, Wu WY, Chen F, Wu YY, Li WZ, Chen HQ, Yin YY (2015) Biochanin A Protects Dopaminergic Neurons Against Lipopolysaccharide-Induced Damage And Oxidative Stress In A Rat Model Of Parkinson’s Disease. Pharmacol Biochem Behav 138:96–103
Wang XF, Xing ML, Shen Y, Zhu X, Xu LH (2006) Oral Administration Of Cr(Vi) Induced Oxidative Stress, Dna Damage And Apoptotic Cell Death In Mice. Toxicology 228:16–23
Wise JP Jr, Young JL, Cai J, Cai L (2022) Current Understanding Of Hexavalent Chromium [Cr(Vi)] Neurotoxicity And New Perspectives. Environ Int 158:106877
Witt KL, Stout MD, Herbert RA, Travlos GS, Kissling GE, Collins BJ, Hooth MJ (2013) Mechanistic Insights From The Ntp Studies Of Chromium. Toxicol Pathol 41:326–342
Yang X, Fang Y, Hou J, Wang X, Li J, Li S, Zheng X, Liu Y, Zhang Z (2022) The Heart As A Target For Deltamethrin Toxicity: Inhibition Of Nrf2/Ho-1 Pathway Induces Oxidative Stress And Results In Inflammation And Apoptosis. Chemosphere 300:134479
Yang X, Zhang Y, Xu H, Luo X, Yu J, Liu J, Chang RC (2016) Neuroprotection Of Coenzyme Q10 In Neurodegenerative Diseases. Curr Top Med Chem 16:858–866
Zendehdel R, Shetab-Boushehri SV, Azari MR, Hosseini V, Mohammadi H (2015) Chemometrics Models For Assessment Of Oxidative Stress Risk In Chrome-Electroplating Workers. Drug Chem Toxicol 38:174–179
Acknowledgements
The authors are thankful to the Director, ICMR-NIOH Ahmedabad, for providing the necessary infrastructure for conducting this study. The authors acknowledge the help from the Animal House Facility staff in carrying out the study. The authors are also grateful to the Zydus Research Center (ZRC), Ahmedabad, for gifting the Swiss albino mice for the study.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study objectives. Swapnil Tripathi: investigation, visualization, writing — original draft preparation. Shabrin Fhatima: investigation, visualization; Dharati Parmar: investigation, visualization; Samir Raval: investigation, visualization, resources; Gyanendra Singh: conceptualization, visualization, supervision, writing — reviewing and editing. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval
The present study on animals was conducted as per the compliance with the Institutional Animal Ethics Committee (IAEC) of the ICMR-National Institute of Occupational Health (NIOH), Ahmedabad wide approval no. IAEC/NIOH/2018–19/21/02/M.
Consent to Participate
Not applicable
Consent for Publication
Not applicable
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tripathi, S., Parmar, D., Fathima, S. et al. Coenzyme Q10, Biochanin A and Phloretin Attenuate Cr(VI)-Induced Oxidative Stress and DNA Damage by Stimulating Nrf2/HO-1 Pathway in the Experimental Model. Biol Trace Elem Res 201, 2427–2441 (2023). https://doi.org/10.1007/s12011-022-03358-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-022-03358-5