Abstract
Lead (Pb) is found in almost all phases in environment and biological systems. Pb stimulated oxidative stress is a state that involves the generation of free radicals beyond the permissible limits, which can deplete the antioxidant reserves and can result in oxidative stress, thus hampering the ability of the biological system to reverse the result. Exposure of rats to Pb (25 mg/kg body weight) for 8 weeks caused an increase in Pb levels in blood and brain. Activity of delta-aminolevulinic acid dehydratase (δ-ALAD) and antioxidant enzymes such as Superoxide dismutase (SOD) and Catalase (CAT) decreased in the blood of Pb-treated group with a concomitant increase in the level of lipid peroxidation (LPO) and no significant change in the level of reduced glutathione (GSH) level was found. Interestingly, co-treatment of Pb-treated rats with curcumin (30 mg/kg body weight) and quercetin (30 mg/kg body weight) for 8 weeks caused a significant decrease in Pb levels of blood and all brain regions versus those treated with Pb alone. A significant improvement in levels of MDA, δ-ALAD, SOD and CAT activities was observed in rats simultaneously treated with curcumin or Quercetin or both with lead. Therefore, the ameliorative impact of curcumin and Quercetin might be due to their antioxidant property hence were able to counter the oxidative stress generated by Pb. These results suggest that combination of curcumin and Quercetin could be utilized as a possible supplement with the relevant therapeutics in the suitable management of Pb toxicity
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lead (Pb) is a well-known heavy metal that continues to be one of the most problematic ecotoxicant found in almost all phases of environment and biological systems due to its natural origin and as a result of massive industrial use [1]. The enhanced anthropogenic activities and vehicular emissions like atmospheric dust, leaded paint, polluted food, automobile exhaust play a key role in contribution of Pb exposure and mainly responsible for increase in the Pb level in human body through inhalation, ingestion and dermal contact [2]. Upon Absorption, Pb replaces other useful divalent metal ions that may involved in key physiological functions of the body’s organs and affects almost all organs of the body but more frequently the central nervous system and impaired cognitive behavior [3, 4]. Ionic mechanism of action for Pb mainly arises due to its ability to substitute other bivalent cations like Ca2+, Mg2+, Fe2+ and monovalent cations like Na+ (though bivalent cations are more readily substituted), affecting various fundamental biological processes of the body [5]. Pb affects almost all organs of the body but compared to other organ systems, the developing nervous system appears to be the most sensitive and chief target for Pb-induced toxicity [6, 7]. It has been reported that, Pb has almost no safe threshold value; even < 10 µg/dl blood Pb level can induce adverse health problems specially in children where Pb severely affected the haematological, renal as well as hepatic functions of the children [8]. There is evidence to suggest that low Pb level exposure significantly affects IQ along with behavior, concentration, ability and attentiveness of the child and may leads to peripheral artery disease, hypertension and renal problems [9]. At higher levels, Pb can cause permanent brain damage and even death [10]. Pb-stimulated oxidative stress is a state that involves the generation of free radicals like Hydroperoxides (HO2•), singlet oxygen (O2–1) and hydrogen peroxide (H2O2); beyond the permissible limits, these reactive oxygen species (ROS) can deplete the antioxidant reserves of the body and, thus, may hamper the ability of the biological system to reverse the result [11] Primarily effects of Pb-induced Oxidative stress include damage to cell membrane,nucleic acid, as well as various antioxidant enzymes and markers of Pb-toxicity such as δ-ALAD, CAT, SOD, GPx, and glucose-6-phosphate dehydrogenase (G6PD) and also affects the pool of nonenzymatic antioxidant molecules such as thiols including GSH of animals and human systems [12]. Apart from targeting the sulfhydryl groups, Pb can also replace the zinc ions that serve as important co-factors for δ-ALAD and other enzymes possessing sulfhydryl groups accessible to this metal ion, hence it has ability to inhibit activities/functions of all those enzymes/proteins including δ-ALAD, thus, hamper the activity of these enzymes [3]. δ-ALAD, also called as porphobilinogen synthase is the second enzyme in the heme biosynthetic pathway which catalyses the condensation of two molecules of δ-ALA to form one molecule of porphobilinogen. About 99% of the Pb in blood is present in erythrocytes due to their high affinity for Pb, from where Pb can be transported to different organs of the body via blood [13]. In contrast, it has been reported that half the Pb in erythrocyte hemolysate obtained from a Pb-exposed worker was found to be bound to protein fractions with high molecular weight including δ-ALAD, which had highest affinity for Pb among erythrocyte components in vivo and in vitro [14].
Curcumin (CUR), also called diferuloylmethane, is the main natural polyphenol found in the rhizome of Curcuma longa (turmeric), a member of family Zingiberaceae. CUR acts as an antioxidant due to the presence of beta-diketone group in its chemical structure [15, 16]. Quercetin (QUE) is a polyphenolic compound ubiquitously distributed and found in common vegetables and fruits such as onions, broccoli and apples. Previous studies have demonstrated that QUE contains many good biological properties for human health including antioxidant, anti-inflammation and anticancer activities [17]. The presence of multiple hydroxyl groups and conjugated electrons system in its chemical structure account for the antioxidant and metal-chelating properties of QUE. Recently, it has been reported that QUE can pass through the blood brain barrier (BBB) in situ models of Alzheimer’s disease and afford significant protection to the neuronal cells from the oxidative stress-induced neurodegeneration [18]. Based on the antioxidant and anti-inflammatory actions of CUR and QUE, it was hypothesized that both CUR and QUE might mitigate the oxidative stress in rats induced by Pb. The present study aimed to determine the effects of CUR and QUE on Pb-induced oxidative stress in rats and to assess the levels of antioxidant enzymes in Pb-treated rats pre- and post-treatment with CUR and QUE. In this study there is aim to know which one of these two molecules (CUR or QUE) will show better antioxidant property against Pb-induced oxidative stress in rats at same concentration. The present study also aimed to determine Pb levels in the hippocampus, cerebellum and frontal cortex region of brain.
Materials and Methods
Chemicals and Reagents
CUR and QUE were purchased from SRL Company, Mumbai, India. All chemical substances used in this study were of analytical grade. All reagents administered to animals were prepared fresh.
Animals Treatment
In this study, 56 Adult male Sprague Dawley Rats (10 weeks old), weighed between 250 and 300 g were selected and obtained from CSIR-Central Drug Research Institute (CDRI, Lucknow India), and were acclimatized for 1 week before being used in experiments.. The animals randomly divided into seven group including control group (8 per group) were housed in stainless steel cages in an air-conditioned room with temperature maintained at 25 ± 2 °C, and maintained in 12:12 light:dark cycle and given access to food and water ad libitum in at the animal house of King George’s Medical University (KGMU), Lucknow, India. Protocols for care and maintenance of the rats were strictly followed as per approval by the Institutional Animal Ethics Committee (IAEC Approval N0.77/IAEC/2017) of KGMU, Lucknow, India. Treatment was given to all animals in each group (control and experimental groups) as follows:
Experimental Protocol
Group A (Control): Animals were not treated with any drugs or injections, and were administered distilled water (placebo) for 8 weeks.
Group B (Pb treated): Rats in this group were exposed to Pb at a concentration of 25 mg/kg body weight in the form of an aqueous solution of lead acetate and administered orally for 8 weeks.
Group C (CUR treated): Rats in this group received CUR at a concentration of 30 mg/kg body weight orally for 8 weeks.
Group D (QUE treated): Rats in this group received QUE at a concentration of 30 mg/kg body weight orally for 8 weeks.
Group E (Pb + CUR treated): Rats in this group were administered CUR at a concentration of 30 mg/kg body weight in addition to lead acetate at a concentration of 25 mg/ kg body weight orally for 8 weeks.
Group F (Pb + QUE treated): Rats in this group were administered QUE at a concentration of 30 mg/kg body weight in addition to lead acetate at a concentration of 25 mg/ kg body weight orally for 8 weeks.
Group G (Pb + CUR + QUE treated): Rats in this group were simultaneously administered CUR and QUE at concentrations of 30 mg/kg body weight, respectively, along with lead acetate at a concentration of 25 mg/kg body weight orally for 8 weeks.
Lead Acetate, CUR and QUE Supplementation
Lead acetate (SRL) was solubilized in Milli-Q water and given to rats orally at a dose of 25 mg/kg body weight. QUE was solubilized in 50% ethanol for oral administration at a dose of 30 mg/kg body weight. Curcumin was solubilized in ethyl oleate and was given orally at a dose of 30 mg/kg body weight. CUR was protected from light during the time of the experiment.
Sampling and Blood Collection
After 8 weeks of treatment for each group as per the above regimen, followed by 24 h of resting and 12–14 h overnight fasting, the animals were anaesthetized by chloroform and cardiac blood samples were collected post 24 h of the last dose of treatment in heparin-coated vials for various biochemical parameters. Blood samples were taken for estimation of Pb, MDA, GSH levels, as well as status of various antioxidant enzymes SOD, CAT, δ-ALAD, etc. Seven rats from each treatment group were sacrificed by cervical decapitation and brains were immediately excised, cleaned, weighed and dissected into the hippocampal, cerebellar and prefrontal-cerebral cortex regions as reported previously [19].These brain tissues of all groups were used to estimate the Pb level.
Biochemical Parameters
Measurement of Pb Levels in Blood and Brain Tissue
Pb levels in blood, hippocampal, cerebellar and prefrontal cortex regions of the brain were estimated as described by Gupta & Gill [20]. The absorbance was read at 283 nm using the graphite furnace atomic absorption spectrophotometer. A calibration curve was drawn by adding known amounts of Pb standard to calculate Pb levels in the blood and brain and data were expressed as μg/dl in blood and μg/g wet tissue of brain.
Estimation of δ-ALAD
δ–ALAD was assayed in blood by the method of Granick et al. [21]. Blood sample was incubated with δ–ALA in the presence and absence of dithiothreitol (DTT). DTT helps in the complete restoration of the enzyme activity by providing 2 -SH groups. The reaction was stopped with TCA-HgCl2 mixture and the color was developed by Ehrlich’s reagent. Enzyme activity was calculated using the molar absorption coefficient (6.1 × 104 M−1 cm−1) of the final Ehrlich color salt at 553 nm and the results expressed as nmol porphobilinogen /mL RBC/h.
Estimation of Lipid Peroxidation (LPO) in RBCs
Lipid peroxidation in RBCs was estimated according to the method described by Stocks and Dormandy (1971) [22]. LPO was estimated in erythrocytes as malondialdehyde (MDA) which was formed by Thiobarbituric acid (TBA) reaction.
Assay of reduced glutathione (GSH) level
The assay is based on method described by Ellman GL. (1959) [23]. The oxidation of reduced GSH by 5,5-dithiobis-2-nitrobenzoic acid (DTNB), also called Ellman’s reagent, producing GSSG and 2-nitro-5-thiobenzoic acid (TNB), a colored ion with maximal absorbance at 412 nm.
Assay of Superoxide Dismutase (SOD) and Catalase (CAT) Activity
The activities of SOD and CAT were determined spectrophotometrically bases on method describe by Misra and Fridovich (2000), and Sinha AK. (1972), respectively [24, 25]
Statistical Analysis
Data was expressed as mean ± SE. ANOVA (one-way analysis of variance) was carried out by using SPSS-20. Difference between control and experimental groups having p < 0.01 were considered as being significant.
Results
Decrease in Pb Concentration of Blood and Brain with CUR and QUE Treatment
The Pb concentration was about 28.63 µg/dl in blood of rats of Group B. Co-treatment with CUR (Group E) and QUE (Group F) decreased blood Pb concentration to about 16.57 and 19.93 µg/dl, respectively whereas co-treatment with both CUR and QUE (Group G) decreased blood Pb concentration to about 13.43 µg/dl (Fig. 1).
Effect of Pb, CUR and QUE and their co-treatment on Pb concentration in blood of rats. Values are mean ± SE (n = 8). Significant change at P < 0.001 in B with respect to values in control group A. Significant change at P < 0.001 in E, F, G with respect to values in group B. Group A-Control; Group B-Lead acetate (Pb) only; Group C-Curcumin (CUR) only; Group D-Quercetin (QUE) only; Group E-Pb + CUR; Group F-Pb + QUE; Group G- Pb + CUR + QUE
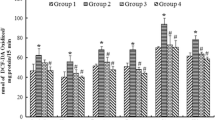
The levels of Pb were significantly increased in hippocampus (7.06 fold), pre-frontal cortex (28.10fold), cerebellum (8.36 fold) of rats treated with Pb (Group B) as compared to controls (Group A) (Fig. 2a,b,c). An increase of Pb concentration about 6.87 fold was also observed in the blood. A significant decrease in the levels of Pb in blood and hippocampal, pre-frontal cortex and cerebellar regions of brain was observed in Group E rats co-treated with Pb and CUR (42.1%, 10.8%, 10.9%, 28.0%, respectively) or Group F rats treated with Pb and QUE (30.3%, 14.5%, 13.8%, 14.5%, respectively) or both i.e. Group G rats (53.0%, 29.1%, 43.1%, 54.0%, respectively) as compared to rats treated with Pb alone (Group B). No significant changes in the level of Pb in the brain regions and blood were observed in rats treated with CUR or QUE alone (Groups C and D, respectively) as compared to the control group (Group A).
Effect of Pb, CUR and QUE and their co-treatment on Pb concentration in brain regions a -Hippocampus, b-Prefrontal Cortex and c-Cerebellum of rats. Values are mean ± SE (n = 8). Significant change at P < 0.001 in B with respect to values in control group A. Significant change at P < 0.001 in E, F, G with respect to values in group B
Restoration of δ-ALAD Activity With CUR and QUE Treatment
There was a significant decrease in δ-ALAD activity of blood (53.4%) in Pb-treated rats as compared to the control group (Group A) (Fig. 3). Co-treatment with CUR, QUE or both significantly restored the activity of δ-ALAD in blood to the tune of 49.1%, 26.20% and 67.4%, respectively versus rats treated with lead alone (Group B). No significant alteration in δ-ALAD activity was observed in blood of rats belonging to Groups C and D versus Group A (Fig. 3).
Effect of Pb, Curcumin and Quercetin and their co-treatment on the activity of δ -aminolevulinic acid dehydratase (δ-ALAD) in blood of rats. Values are mean ± SE (n = 8). Significant change at P < 0.001 in B with respect to values incontrol group A. Significant change at P < 0.001 in E, F, G with respect to values in group B
Restoration of CAT and SOD Activity With CUR and QUE Treatment
The activities of SOD and CAT underwent a significant decrease (40.4% and 60.9%, respectively) in the blood of Pb-treated rats as compared to control group (Group A) (Fig. 4a, b). A significant increase in the activities of SOD and CAT was found in blood of rats belonging to groups E (15.8%, 73.1%, respectively), F (11.6%, 52.0%, respectively) and G (50.3%, 128.5%, respectively) versus Group B. No significant change in SOD and catalase activities was observed in blood of rats treated with CUR or QUE alone (Groups C and D) versus controls (Group A) (Fig. 4a, b).
Restoration of GSH levels with CUR and QUE treatment
GSH was found to be significantly decreased in blood (35.6%) of Pb-treated rats (Group B) versus controls (Group A) (Fig. 5). However, the increase in GSH levels was not found to statistically significantly in the blood of rats belonging to Groups E (17.2%), F (10.0%), or G (39.9%) versus Group B. No significant alteration in GSH level was observed in blood of the rats treated with CUR or QUE alone (Groups C and D) versus controls (Group A) (Fig. 5).
Decrease in Lipid peroxidation (LPO) With CUR and QUE Treatment
MDA level was found to be significantly increased in blood (103.6%) of Pb-treated rats (Group B) versus controls (Group A) (Fig. 6). Co-treatment with CUR (Group E), QUE (Group F) or both (Group G) significantly decreased LPO to the tune of 22.1%, 16.5% and 46.9%, respectively versus rats treated with Pb alone (Group B). No significant alteration in MDA levels was observed in blood of the rats treated with CUR or QUE alone (Groups C and D) versus controls (Group A) (Fig. 6).
Discussion
A number of previous studies have revealed that Pb-exposure even at very low doses is extremely hazardous and dangerous to living organisms including human beings as well as animals, resulting in a variety of neurological consequences [26, 27]. The ionic mechanism contributes principally to oxidative stress, as Pb, after replacing Ca2+, is able to cross the blood brain barrier (BBB) where it gets deposited in brain regions like cerebellum, hippocampus and prefrontal cortex causing oxidative stress. Oxidative-stress induced damage to these parts of brain are found to be associated with behavioral abnormalities, learning impairment, decreased hearing, neuromuscular weakness and impaired cognitive functions like forgetfulness, irritability, poor attention span in humans and in experimental animals [28, 29]. Pb can cross across cell membranes and enter different cells of the body through different types Ca2+ channels at an appreciable rate thereby causing significant disruption of Ca2+ homeostasis by mimicking the action of Ca2+ [30]. Initiation of oxidative stress by Pb is accrued due to production of hydroxyl radicals during oxidation of various biomolecules including proteins, lipids and nucleic acids along with a parallel compromise in the activity of antioxidant defense system comprising of SOD, CAT, GPx, MDA level, etc. [31,32,33]. Upon inhibition of δ-ALAD by Pb, ALA accumulates which is an endogenous source of free radical generation in the body and, thus, leads to overproduction of superoxide anions and hydrogen peroxide (ROS) as a result of auto-oxidation of ALA [34].
CUR (diferuloylmethane), an active ingredient of turmeric, is a natural product with multiple biological activities and numerous potential therapeutic applications. In the present study, CUR displayed antioxidant and free radical scavenging properties by restoring the activities of antioxidant enzymes SOD, CAT and ALAD and causing a significant decline in LPO with a concomitant decrease in Pb levels in all the studied brain regions of Pb-treated rats versus those treated with lead alone. The obtained results have been found to be in agreement with those reported by earlier [35]. These results also support earlier hypothesis that CUR is an effective chelating agent that may reduce Pb load in the body by chelating Pb thereby decreasing the oxidative stress caused by this heavy metals as well as restoring the activity of the endogenous antioxidant enzymes [36]. Thus, CUR treatment may be beneficial, because of its antioxidant role in the body as well as its ability to cross the blood–brain barrier where it acts as a neuro-protectant [37].
Previous studies have also demonstrated that like CUR, QUE which is a polyphenolic compound found in many vegetables and fruits, also possesses biological properties including antioxidant, anti-inflammatory and anticancer. Like CUR, QUE can pass through the blood–brain barrier of in situ models resulting in a significant decrease in MDA levels and increase in the activity of antioxidant enzymes viz. SOD, CAT, and GPx [17, 38]. In the present study, QUE decreased lipid peroxidation, and restored the activity of CAT and SOD in Pb-treated rats.
However, the Restoration of δ-ALAD activity is found in study groups administered with Pb & CUR or Pb & QUE. It suggests the protective effect of CUR and QUE against Pb induced alterations in hematological parameters may be attributed to the chelating properties of CUR and QUE that help in removing metals from the binding site of δ-ALAD. This suggests that both CUR and QUE alter the kinetics of Pb toxicity and reduce body Pb burden, thus confirming the beneficial effect of dietary CUR and QUE in the prevention of Pb toxicity. These findings however, require more detailed investigations using chronic in vivo exposure. CUR and QUE at doses of 30 mg/kg body weight for eight weeks were effective in reducing the generation of ROS and also in restoring the activities of antioxidant enzymes that were disturbed as a result of Pb-treatment. It can, thus, be concluded from the present study that δ-ALAD may be a sensitive biochemical indicator for lead exposure and that combined administration of CUR and QUE may be a good treatment option for Pb-induced toxicity.
The normalization of the altered oxidative stress indices and antioxidant enzymes with administration of Pb with CUR or QUE or both (CUR and QUE), reaffirmed the antioxidant properties of CUR and QUE. In addition to its antioxidant role, CUR and QUE has been postulated as one of the elements that can prevent lead accumulation within the tissues and its subsequent toxicity. It might therefore be reasoned that CUR and QUE protects against Pb-induced oxidative stress by two mechanisms; by improving the antioxidant defense systems and by abrogating the generation of reactive species through reduction of lead accumulation in tissues.
Though both CUR and QUE were found to be effective against Pb-induced oxidative stress in rats, CUR was found to be more active than QUE in the parameters assessed in the present study viz. decrease in Pb concentration in blood, restoration of δ-ALAD, CAT, SOD and GSH levels and decrease in lipid peroxidation. However, a combination of both CUR and QUE was found to be more effective than either of them alone in improvement of the above parameters (Figs. 1, 2, 3, 4, 5 and 6).
Conclusion
Our findings clearly indicate that Pb induces production of ROS and at the same time reduces the levels of antioxidant enzymes leading to oxidative stress. The results also suggest that CUR and QUE restored the activities of antioxidant enzymes by successfully chelating Pb in blood as well as soft tissues of brain. It is concluded that CUR and QUE act by reducing accumulation of Pb in the soft tissue of brain or possibly in other organs and through improving Pb-induced hematological and biochemical abnormalities and are effective in ameliorating lead toxicity. There is therefore dietary CUR and QUE intake in Pb polluted environment is recommended. Thus, the antioxidant and chelating properties of CUR and QUE might possibly make these molecules as promising drug candidates against Pb-induced oxidative stress if studied and explored further in vitro and in vivo.
Availability of data and material (data transparency)
All the data has been provided with the manuscript.
Code availability (software application or custom code)
Not Applicable.
Consent to participate
Not Applicable.
References
Khodamoradi N, Komaki A, Salehi I, Shahidi S, Sarihi A. Effect of vitamin E on lead exposure- induced learning and memory impairment in rats. Physiol Behav. 2015;144:90–4. https://doi.org/10.1016/j.physbeh.2015.03.015.
Singh N, Kumar A, Gupta VK, Sharma B. Biochemical and Molecular Bases of Lead induced toxicity in mammalian systems and possible mitigations. Chem Res toxicol. 2018. https://doi.org/10.1021/acs.chemrestox.8b00193.
Sharma B, Singh S, Siddiqui NJ. Biomedical implications of heavy metals induced imbalances in redox systems. Biomed Res Int. 2014;2014:640–54. https://doi.org/10.1155/10.1155/2014/640754.
Links JM, Schwartz BS, Simon D, Roche KB, Stewart WF. Characterization of toxicokinetics and toxicodynamics with linear systems theory: application to lead-associated cognitive decline. Environ Health Perspect. 2001;109:361–8. https://doi.org/10.1289/ehp.01109361.
Lidsky TI, Schneider JS. Lead neurotoxicity in children: basic mechanisms and clinical correlates. Brain. 2003;126:5–19. https://doi.org/10.1093/brain/awg014.
Cory-Slechta DA. Legacy of lead exposure: consequences for the central nervous system. Otolaryngol Head Neck Surg. 1996;114:224–6. https://doi.org/10.1016/S0194-59989670171-7.
Lidsky TI, Schneider JS. Adverse effects of childhood lead poisoning: The clinical neuropsychological perspective. Environ Res. 2006;100:284–93. https://doi.org/10.1016/j.envres.2005.03.002.
Rawat PS, Singh S, Zahid M, Mehrotra S. An integrated assessment of lead exposure in children: correlation with biochemical and haematological indices. J Trace Elem Med Biol. 2021;68:1–7. https://doi.org/10.1016/j.jtemb.2021.126835.
Goyer RA. Results of lead research: prenatal exposure and neurological consequences. Environ Health Perspect. 1996;104:1050–4. https://doi.org/10.1289/ehp.961041050.
Cleveland LM, Minter ML, Cobb KA, Scott AA, German VF. Lead hazards for pregnant women and children: Part 1: immigrants and the poor shoulder most of the burden of lead exposure in this country. Part 1 of a two-part article details how exposure happens, whom it affects, and the harm it can do. Am J Nurs. 2008;108:40–9. https://doi.org/10.1097/01.NAJ.0000337736.76730.66.
Flora SJS. Nutritional components modify metal absorption, toxic response and chelation therapy. J Nut Environ Med. 2002;12:53–67. https://doi.org/10.1080/13590840220123361.
Flora SJS, Mittal M, Mehta A. Heavy metal induced oxidative stress & its possible reversal by chelation therapy. Indian J Med Res. 2008;128:501–23.
Sivaprasad R, Nagaraj M, Varalakshmi P. Combined efficacies of lipoic acid and meso-2,3-dimercaptosuccinic acid on lead induced erythrocyte membrane lipid peroxidation and antioxidant status in rats. Hum Exp Toxicol. 2003. https://doi.org/10.1191/0960327103ht335oa.
Sakai T, Yanagihara S, Kunugi Y, Ushio K. Relationships between distribution of lead in erythrocytes in vivo and in vitro and inhibition of ALAD. Br J Ind Med. 1982;39:382–7. https://doi.org/10.1136/oem.39.4.382.
Aggarwal BB, Kumar A, Bharti AC. Anticancer potential of curcumin: Preclinical and clinical studies. Anticancer Res. 2003;23:363–98.
Sandur SK, Pandey MK, Sung B, Ahn KS, Murakami A, Sethi G, et al. Curcumin, demethoxycurcumin, bisdemethoxycurcumin, tetrahydrocurcumin and turmerones differentially regulate anti-inflammatory and anti-proliferative responses through a ROS-independent mechanism. Carcinogenesis. 2007;28:1765–73. https://doi.org/10.1093/carcin/bgm123.
Rogerio AP, Kanashiro A, Fontanari C, da Silva EVG, Lucisano-Valim YM, Soares EG, et al. Antiinflammatory activity of quercetin and isoquercitrin in experimental murine allergic asthma. Inflamm Res. 2007;56:402–8. https://doi.org/10.1007/s00011-007-7005-6.
Heo HJ, Lee CY. Protective effects of quercetin and vitamin C against oxidative stress-induced neurodegeneration. J Agric Food Chem. 2004. https://doi.org/10.1021/jf049243r.
Glowinski J, Iversen LL. Regional studies of catecholamines in the rat brain. I. The disposition of [3H]norepinephrine, [3H]dopamine and [3H]dopa in various regions of the brain. J Neurochem. 1966;13:655–9. https://doi.org/10.1111/j.1471-4159.1966.tb09873.x.
Gupta V, Gill KD. Influence of ethanol on lead distribution and biochemical changes in rats exposed to lead. Alcohol. 2000;20:9–17. https://doi.org/10.1016/s0741-8329(99)00046-4.
Granick JL, Sassa S, Granick S, Levere RD, Kappas A. Studies of lead poisoning. II. Correlation between the ratio of activated to inactivated S-aminolevuIinic acid dehydratase of whole blood and the blood lead level. Biochem Med. 1973;8:149–59. https://doi.org/10.1016/0006-2944(73)90018-5.
Stocks J, Dormandy TL. The autoxidation of human red cell lipid induced by hydrogen peroxide. Br J Haematol. 1971;20:95–111. https://doi.org/10.1111/j.1365-2141.1971.tb00790.x.
Ellman GL. Tissue sulfydryl group. Arch Biochem Biophys. 1959;82:70–7. https://doi.org/10.1016/0003-9861(59)90090-6.
Misra HP, Fridovich I. The role of superoxide anion in the autooxidation of epinephrine and simple assay for superoxide dismutase. Biol Chem. 1972;247:3170–5.
Sinha AK. Colorimetric assay of catalase. Anal Biochem. 1972;47:389–94. https://doi.org/10.1016/0003-2697(72)90132-7.
Mazumdar M, Bellinger DC, Gregas M, Abanilla K, Bacic J, Needleman HL. Low-level environmental lead exposure in childhood and adult intellectual function: a follow-up study. Environ Health. 2011;10:24–30. https://doi.org/10.1186/1476-069X-10-24.
Shelton JF, Geraghty EM, Tancredi DJ, Delwiche LD, Schmidt RJ, Ritz B, et al. Neurodevelopmental disorders and prenatal residential proximity to agricultural pesticides: the CHARGE study. Environ Health Perspect. 2014;122:1103–9. https://doi.org/10.1289/ehp.1307044.
Yun HJ, Kim I, Kwon S, Kang J, Om A. Protective effects of chlorella vulgaris against lead- induced oxidative stress in rat brains. J Health Sci. 2011;57:245–54.
Gandhi S, Abramov AY. Mechanism of oxidative stress in neurodegeneration. Oxid Med Cell Longev. 2012. https://doi.org/10.1155/2012/428010.
Bridges CC, Zalups RK. Molecular and ionic mimicry and the transport of toxic metals. Toxicol App Pharmacol. 2005;204:274–308. https://doi.org/10.1016/j.taap.2004.09.007.
Ahamed M, Siddiqui MK. Low level lead exposure and oxidative stress: current opinions. Clin Chim Acta. 2007;383:57–64. https://doi.org/10.1016/j.cca.2007.04.024.
Flora SJ, Gautam P, Dwivedi N. Dose-dependent effects of ethanol on lead-induced oxidative stress in rats. J Environ Pathol Toxicol Oncol. 2012;31:61–73. https://doi.org/10.1615/jenvironpatholtoxicoloncol.v31.i1.70.
Chiba M, Shinohara A, Matsushita K, Watanabe H, Ihaba Y. Indices of lead-exposure in blood and urine of lead-exposed workers and concentrations of major and trace elements and activities of SOD, GSH-Px and catalase in their blood. Tohoku J Exp Med. 1996;178:49–62. https://doi.org/10.1620/tjem.178.49.
Gautam P, Flora SJ. Oral supplementation of gossypin during lead exposure protects alteration in heme synthesis pathway and brain oxidative stress in rats. Nutrition. 2010;26:563–70. https://doi.org/10.1016/j.nut.2009.06.008.
Shukla PK, Khanna VK, Khan MY, Srimal RC. Protective effect of curcumin against lead neurotoxicity in rat. Hum Exp Toxicol. 2003;22:653–8. https://doi.org/10.1191/0960327103ht411oa.
Jeong GS, Oh GS, Pae HO, Jeong SO, Kim YC, Shin MK, et al. Comparative effects of curcuminoids on endothelial heme oxygenase-1 expression: Ortho-methoxy groups are essential to enhance hemeoxygenase activity and protection. Exp Mol Med. 2006;38:393–400. https://doi.org/10.1038/emm.2006.46.
Yang F, Lim GP, Begum AN, Ubeda OJ, Simmons MR, Ambegaokar SS, et al. Curcumin inhibits formation of amyloid beta oligomers and fibrils, binds plaques, and reduces amyloid in vivo. J Biol Chem. 2005;280:5892–901. https://doi.org/10.1074/jbc.M404751200.
Lamson DW, Brignall MS. Antioxidants and cancer III: quercetin. Altern Med Rev. 2000;5:196–208.
Acknowledgements
The authors wish to thank the Head, Department of Biochemistry, King George’s Medical University, Lucknow (UP), India, for his interest in the study. The authors are also grateful to the Council of Scientific and Industrial Research (CSIR, New Delhi) for the award of research fellowship (SRF). The technical support by Mr.Kushwant Singh is also gratefully acknowledged.
Funding
Financial support in the form of Senior/Junior Research Fellowship to from Council of Scientific and Industrial Research (CSIR, New Delhi) is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Ethics approval
The present study was approved by the Institutional Animal Ethics Committee (IAEC Approval N0.77/IAEC/2017) of KGMU, Lucknow, India.
Consent for publication
The present manuscript has been duly read by all authors and has their consent for publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zahid, M., Rawat, P.S., Singh, S. et al. Assessment of Role and Efficacy of Curcumin and Quercetin in Preventing Lead-Induced Oxidative Stress in Rats. Ind J Clin Biochem 37, 303–310 (2022). https://doi.org/10.1007/s12291-021-01001-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12291-021-01001-z