Abstract
A number of environmental chemicals are known to cause neurotoxicity to exposed organisms. Chromium (Cr), one of the major elements in earth’s crust, is a priority environmental chemical depending on its valence state, and limited information is available on its neurotoxic potential. We, therefore, explored the neurotoxic potential of environmentally present trivalent- (Cr(III)) and hexavalent-Cr (Cr(VI)) on tested brain cell types in a genetically tractable organism Drosophila melanogaster along with its organismal response. Third instar larvae of w 1118 were fed environmentally relevant concentrations (5.0–20.0 μg/ml) of Cr(III)- or Cr(VI)-salt-mixed food for 24 and 48 h, and their exposure effects were examined in different brain cells of exposed organism. A significant reduction in the number of neuronal cells was observed in organism that were fed Cr(VI)- but not Cr(III)-salt-mixed food. Interestingly, glial cells were not affected by Cr(III) or Cr(VI) exposure. The tested cholinergic and dopaminergic neuronal cells were affected by Cr(VI) only with the later by 20.0 μg/ml Cr(VI) exposure after 48 h. The locomotor activity was significantly affected by Cr(VI) in exposed organism. Concomitantly, a significant increase in the level of reactive oxygen species (ROS) coupled with increased oxidative stress led to apoptotic cell death in the tested brain cells of Cr(VI)-exposed Drosophila, which were reversed by vitamin C supplementation. Conclusively, the present study provides evidence of environmental Cr(VI)-induced adversities on the brain of exposed Drosophila along with behavioral deficit which would likely to have relevance in humans and recommends Drosophila as a model for neurotoxicity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Prevalence of neurological adversities associated with environmental chemical exposure is a concern nowadays. Effect of environmental chemicals on neuronal health may eventually affect an organism in multiple ways including dysregulation of other organ systems such as digestive and respiratory system, muscle movement, etc. [1–3] and various disorders such as loss of memory, vision, uncontrolled muscle movement [4, 5], etc. In this context, a number of environmental chemicals such as pesticides, herbicides, heavy metals, etc., are reported to cause adverse neuronal health effects by inducing oxidative stress [4–6].
Among environmental chemicals, chromium (Cr), a heavy metal, is a priority environmental chemical depending on its oxidation state. It has diverse uses in industrial sectors right from chrome plating to leather industries. While hexavalent form of Cr (Cr(VI)) is reported as a human class I carcinogen, its trivalent form (Cr(III)) is required for glucose metabolism and is taken as a food supplement [7]. Reduction of Cr(VI) to Cr(III) is a multistep process by which various types of free radicals such as superoxide (O2 ·−), hydroxyl (.OH), hydrogen peroxide (H2O2) [8], and reactive Cr intermediates (Cr(V), Cr(IV)) are generated. The Cr intermediates themselves are oxidants and form DNA adducts and various types of DNA damage [9, 10]. In this context, Cr(VI) is reported to inhibit various anti-oxidative enzymes such as thioredoxin (Trx), peroxiredoxins (Prx), thioredoxin reductase (TrxR) [11], catalase (CAT) [12], etc. Trx/Prx system is reported to have a role in the brain since their overexpression in neurons results in increased locomotor activity and longevity in Drosophila with suppressed neurotoxicity [13].
Much of the earlier studies on Cr(VI) were focused on its effect on lung and skin with limited studies on brain [14–18]. For example, intraperitoneal administration of Cr(VI) (2.0 mg/kg b.w.) to rabbits resulted in alterations in their brain [15]. Later, it was reported that this kind of alteration was accompanied by the marked degeneration of the cerebral cortex [16]. Moshtaghie et al. [17] have reported that Cr(VI) inhibits catecholamines and acetylcholinesterase (AchE) levels in the cerebellum, mid-brain, and brain cortex of rat in a concentration-dependent manner. In another study, Cr(VI) was shown to induce oxidative damage in the brain of rats mainly in their cerebrum and cerebellum due to the inhibition of AchE activity [18]. In matured cultured cerebellar granule neuronal cells, Cr(VI) was shown to induce oxidative stress that further led to apoptosis and rosmarinic acid, an anti-oxidant, which was shown to rescue the exposed cells from death [14]. On a positive note, Cr(III) supplementation in early Alzheimer’s disease has been shown to improve cognitive function in older humans [19]. Conversely, Cr(III) is reported to act as a genotoxic agent by forming DNA adducts and induces mutation in exposed organism/cells [10, 20]. However, available reports lack information about environmental Cr(VI)- and Cr(III)-induced adversities on different cell types of the brain in an organism wherein cross talk is likely to occur in in vivo condition as a part of the complex circuitry.
In order to have an insight on environmental Cr(VI)- and Cr(III)-induced effects on different cells of brain, Drosophila melanogaster was used as a tractable in vivo model organism due to its well-studied genetics and developmental biology, and ∼70 % of its genes share homology with that in higher organisms including humans [21]. The model raises limited ethical concern and is recommended by the European Centre for the Validation of Alternative Methods (ECVAM) in line with reduce, replace, and refine (3Rs) [22] for toxicological studies and testing [23, 24]. In recent years, several human neurodegenerative diseases have been modeled in this organism to understand the pathophysiological pathways towards the development of therapeutics [5, 25].
The present study, therefore, aims to examine environmental Cr(III)- and Cr(VI)-induced effects on Drosophila larval brain by targeting different brain cell types by using cell-specific markers.
Materials and Methods
Fly Strains
A D. melanogaster mutant, w 1118, was used for the study since the same fly stock has been extensively utilized as a genetic background strain to generate thousands of transposon insertion lines, deficiency, and RNAi lines. Brain cell-specific driver lines: Elav-GAL4 (pan neuronal driver), ChAT-GAL4 (cholinergic neuronal driver), Repo-GAL4 (glial cell driver), UAS-GFP (reporter line which expresses green fluorescence protein), and Ddc-GAL4:UAS-GFP (driver line having the promoter of DOPA decarboxylase gene, a marker of dopaminergic neurons, tagged with GFP) were also used. All flies and their different developmental stages were maintained at 24 ± 1 °C on standard Drosophila food medium consisting of agar, corn flour, sugar, yeast, nepagin, and propionic acid. Additional yeast supplement was provided for the healthy growth of the organisms.
Treatment Schedule
Analytical grade potassium dichromate (K2Cr2O7) and chromium chloride (CrCl3.6H2O) (HIMEDIA Laboratories Ltd., Mumbai, India) were used for the study. Based on previously published reports [26–28], three different concentrations of Cr(VI) (5.0, 10.0, and 20.0 μg/ml in terms of Cr), which have environmental relevance and two exposure periods, i.e., 24 and 48 h were chosen. In parallel, the same concentrations for Cr(III) were used. Ascorbic acid (vitamin C; 15.0 μg/ml; Sisco Research Laboratories Pvt. Ltd., Mumbai, India) was used as an anti-oxidant. Larvae of 80 ± 1 and 56 ± 1 h were fed on Cr(III)- or Cr(VI)-salt-contaminated food for the above-mentioned periods. The control group received normal food.
Total Chromium Estimation
Quantitative estimation of total Cr was performed in the brain ganglia of Drosophila larvae that were fed food contaminated with Cr(VI)- or Cr(III)-salt (5.0, 10.0, and 20.0 μg/ml in terms of Cr) for 48 h. Dissected out brain ganglia (∼0.5 gm) from Drosophila larvae were incubated overnight with 5.0 ml HNO3/HClO4 (6:1) mixture and then heated at 100 °C on a hot plate. The obtained residue after heating was dissolved in 5.0 ml of 1 % 1 N HNO3 and analyzed for Cr. The concentration of Cr was estimated using an Atomic Absorption Spectrometer (ZEEnit 700 P, Flame and Graphite Furnace, Analytic Jena AG, Konrad-Zuse-Strasse, Germany).
Immunohistochemical Staining
Brain ganglia isolated from the larvae of control and treated groups (20 larvae/group) were placed in 1× phosphate-buffered saline (PBS; 17.5 mM NaCl, 8.41 mM Na2HPO4, 1.86 mM NaH2PO4, pH ∼7.4). They were first fixed in 4 % paraformaldehyde (PFA) for 20 min and then washed three times with 0.1 % PBST (1× PBS + 0.1 % TritonX100) for 5 min each. The fixed tissues were first incubated with 4 % bovine serum albumin (BSA) for 1 h to prevent non-specific binding of antibody followed by their incubation overnight with different primary antibodies, viz., anti-Elav (pan neuronal cell marker protein; anti-rat, 1:100; Developmental Studies Hybridoma Bank, IA, USA), anti-Repo (glial cell marker protein) and anti-ChAT (cholinergic neuronal cell marker protein; anti-mouse, 1:100; Developmental Studies Hybridoma Bank, IA, USA), and anti-TH (dopaminergic neuronal cell marker protein; anti-rabbit, 1:100; a kind gift from Dr. W. Neckameyer, St. Louis University, Missouri, USA), in 4 % BSA at 4 °C. After washing with 1× PBS, the brain ganglia were incubated with Alexa-488 conjugated secondary antibodies (anti-mouse, anti-rat, and anti-rabbit, 1:200; Invitrogen, Eugene, Oregon, USA) in 4 % BSA for 2 h at 25 ± 2 °C. Finally, after washing, brain ganglia were mounted on slides by using Vectashield mounting medium containing nuclear stain DAPI (Vector Laboratories, Burlingame, CA, USA). They were visualized under a Leica TCS-SPE confocal microscope (Nussloch, Germany) and captured images were processed using Adobe Photoshop 7.0 software. The intensity of the signal was analyzed by ImageJ software (NIH) and average intensity of 20 images of brain cells from each group by subtracting their negative control was recorded.
Assessment of Apoptosis
Preparation of Cell Suspension
Single cell preparation was done essentially following the method of Howell and Taylor [29] with minor modifications. In brief, brain ganglia were dissected out from the control and treated larvae (20 nos/group) of Drosophila in Poels’ salt solution (PSS; 14.72 mM NaCl, 42.0 mM KCl, 7.89 mM CaCl2·2H2O, 7.34 mM KHCO3, 20.81 mM MgSO4·7H2O, pH 7.0) [30]. Dissected tissues were taken in a microcentrifuge tube containing PSS. They were treated with 400 μl of collagenase (0.5 mg/ml in 1× PBS; Type VIII, Sigma, USA) for 15 min at 24 ± 1 °C with gentle intermittent mixing. Dissociated and unclamped cells were collected after collagenase-treated tissues were passed through a 60-μm nylon mesh. Thereafter, these cells were washed gently with 1× PBS, pH 7.4 three times.
Trypan Blue Dye Exclusion Test
Cell viability and their number was measured by trypan blue dye exclusion test essentially following the method of Phillips [31]. Briefly, to 10.0 μl of single cell suspension of brain tissue, 10.0 μl trypan blue (0.02 % in 1× PBS, pH 7.4) solution was added and immediately scored for trypan blue-positive cells using a hemocytometer.
Apoptosis Estimation by Flowcytometry
The percentage of apoptosis was determined by monitoring the translocation of phosphatidylserine to the cell surface using an Annexin V-FITC apoptosis detection kit (Sigma, UK) essentially following the manufacturer’s instructions. Cells were analyzed for Annexin V-FITC and propidium iodide (PI) double staining by a Becton Dickerson Flow Cytometer (BD Biosciences, New Jersey, USA). The FITC signal was detected at 518 nm and PI was detected at 620 nm. The data were analyzed using cell quest software (Mac OS 8.6) and 10,000 events were counted per sample.
Determination of DEVDase Activity
-
(a)
Biochemical method: DEVDase activity was measured in the larval brain ganglia using caspase-3 colorimetric assay kit according to the manufacturer’s instructions. In brief, brain ganglia from control and treated larvae (40 nos/group) were homogenized in 1× PBS (pH 7.4) containing 0.15 M KCl. Thereafter, it was mixed with 1× assay buffer and the caspase-3 substrate. The reaction mixture was incubated at 37 °C for 1.5 h. Spectrophotometric detection of the chromophore p-nitroaniline (pNA) at 405 nm was measured on a plate reader (Molecular Devices, LLC, CA, USA), and the caspase activity was calculated in terms of micromoles of pNA released per minute per milligram of protein.
-
(b)
In vivo imaging method: cleaved caspase level in different brain cells was studied by immunohistochemical staining using different brain cell-specific marker antibodies (anti-Elav, anti-Repo, anti-ChAT) along with anti-cleaved caspase-3 antibody (a marker for apoptosis; anti-rabbit, 1:100; Cell Signaling Technology, MA, USA) by confocal microscopy (methodology stated in “Immunohistochemical staining”). For dopaminergic neurons, brain ganglia from Ddc-GAL4:UAS-GFP larvae (control and treated) were immunostained with anti-cleaved caspase-3 antibody (incubation step and secondary antibody staining same as above in “Immunohistochemical staining”). Finally, brain ganglia were visualized under a Leica TCS-SPE confocal microscope (Nussloch, Germany) and captured images were processed using Adobe Photoshop 7.0 software.
Determination of Mitochondrial Membrane Potential (ΔΨ)
-
(a)
Biochemical method: depolarization of mitochondrial membranes was analyzed by a fluorochrome, 5,5′,6,6′tetrachloro-1,1′,3,3′-tetraethyl benzimidazolyl carbocyanine iodide (JC-1, Invitrogen, USA). In polarized mitochondria, JC-1 forms aggregates and emits red fluorescence. When mitochondria get depolarized, JC-1 changes into monomeric form and emits green fluorescence. Briefly, single cell suspension of brain ganglia from control and treated groups were incubated with 10.0 μg/ml JC-1 dye for 10 min at 24 ± 1 °C following the method reported earlier [32]. After washing the cells in 1× PBS, fluorescence was measured on a plate reader (Molecular Devices, LLC, CA, USA) at an excitation/emission wavelength of 530/590 nm (red) and 485/538 nm (green), respectively, to determine the shift in the fluorescence spectra. The fluorescence intensity was normalized to the number of cells. Mitochondrial depolarization was indicated by a shift from red to green fluorescence intensity ratio.
-
(b)
In vivo imaging method: in vivo imaging was performed by confocal microscopy following the method of Hwang et al. [33]. Briefly, the brain ganglia from control and treated groups were stained with 20.0 μg/ml JC-1 dye for 10 min at 24 ± 1 °C in dark. After two washings with 1× PBS for 5 min each, tissues were mounted in 1× PBS. Finally, brain ganglia were visualized under a Leica TCS-SPE confocal microscope (Nussloch, Germany) and captured images were processed using Adobe Photoshop 7.0 software.
Climbing Assay
The locomotor performance was assayed as described previously by Feany and Bender [34]. One hundred adult male flies (20 flies/vial, 5 vials/group) per group were subjected to the assay. Drosophila larvae of 56 ± 1 h were fed different concentrations of Cr(VI)-salt-mixed (5–20 μg/ml) Drosophila food till 48 h and left on normal food till their emergence. Five-day-old flies were used for the said experiment [35]. In brief, flies were allowed to get acclimatized with their environment for 1 min after gentle tapping to the bottom of a vertical plastic tube (18 × 2 cm). The number of flies that crossed the 15-cm line within 30 s from the time they were tapped to the bottom of the vials was scored. Locomotor performance was measured in terms of percentage of flies that crossed the 15-cm line among the total number of flies per experiment. For each experiment, a performance index (PI) was calculated using values for flies that moved above 15 cm (ntop) and that remained below 15 cm (nbot) after 30 s and the total number of flies (ntot) and formulae 1/2[(ntot + ntop + nbot)/ntot]. Results were expressed as mean ± SD of the scores obtained from three independent experiments.
Jumping Assay
For examining neuromuscular activity, jumping activity assay was performed. The treatment schedule similar to the climbing assay was followed. The threshold for the jumping response appears to be related to the speed of locomotor activity. For this, flies, one at a time, were transferred to a vial marked 1–10 cm. Distance jumped by the fly was recorded from the bottom of the vial, and the average number of jumps in five replicates was taken as the jumping activity essentially following the method reported previously [35]. One hundred flies per group with five replicates for each group were used.
Assay of Oxidative Stress Parameters
In order to measure oxidative stress endpoints, different reactive oxygen species (ROS) along with activities of superoxide dismutase (SOD), CAT, TrxR and glutathione (GSH) content, protein carbonylation (PC), and lipid peroxidation (LPO) product were assayed in 10 % homogenate of brain ganglia from control and treated larvae. In brief, brain ganglia from control and treated groups (80 larvae/group) were homogenized in cold 1× PBS (pH 7.4) containing 0.15 M KCl followed by centrifugation at 12,000×g for 10 min. The supernatant obtained was used for various assays.
Measurement of ROS and Reactive Nitrogen Species Levels
The levels of total ROS was measured in the homogenate of larval brain ganglia from control and Cr(VI)-salt fed groups using 2′,7′-dihydrofluorescein diacetate (H2DCFDA; Invitrogen, USA) following the method reported earlier [36] with minor modifications. Briefly, to 10 % brain ganglia homogenate, the dye was added to a final concentration of 10 μM and incubated for 1 h in the dark at 24 ± 1 °C. The mixture was then placed on a microplate reader (Molecular Devices, LLC, CA, USA) for fluorescence quantification at an excitation/emission wavelength of 495/519 nm. The mean fluorescence intensity was used for the estimation of ROS level in each sample. Three samples from each group were analyzed in triplicates.
H2DCFDA was used as a measure for general index of cellular oxidative stress [37]. Since H2DCFDA can be oxidized by H2O2 as well as other oxidants (·OH, ONOO−) [37], the fluorescence measurement against ROS level may include the possible involvement of other oxidant species. Cr(VI) was reported to generate a wide spectrum of free radicals such as ·OH, ONOO−, and H2O2 (reviewed by Jomova and Valko [38]) and therefore, dihydrorhodamine 123 (DHR) and dihydroethidium (DHE) dyes were used for the estimation of ONOO− and O2 ·− levels, respectively.
Measurement of ONOO− Level
ONOO− level in Drosophila larval brain ganglia was monitored by using DHR (Invitrogen, USA) as described previously [39]. The conversion of non-fluorescent DHR to rhodamine, a fluorescent product after its oxidation with ONOO−, was measured at an excitation/emission wavelength of 485/535 nm using a microplate reader (Molecular Devices, LLC, CA, USA). Briefly, 10 % brain ganglia homogenate from control and treated groups were incubated in 20 μM DHR (dissolved in 1× PBS) for 10 min at 24 ± 1 °C. The mean fluorescence intensity was used as a measure of ONOO− generation. Three samples from each group were analyzed in triplicates.
Measurement of O2 ·− Level
The level of superoxide was measured in the brain ganglia with DHE (10 μM; Invitrogen, USA) as described previously [40]. Briefly, 10 % brain ganglia homogenate from control and treated groups were incubated in 10 μM DHE (dissolved in 1× PBS) for 30 min at 24 ± 1 °C. Oxidation of DHE (excitation/emission 535/610 nm; blue fluorescence) into ethidium (E+; excitation/emission 370/420 nm; red fluorescence) was measured using a microplate reader (Molecular Devices, LLC, CA, USA). The ratio of E+/DHE fluorescence intensity was calculated as an index of O2 ·− generation.
In vivo detection of O2 ·− level was performed by using DHE dye following the method as described previously [41]. Briefly, brain ganglia from control and treated groups (Elav-GAL4>UAS-GFP, Repo-GAL4>UAS-GFP, ChAT-GAL4>UAS-GFP, Ddc-GAL4:UAS-GFP) were dissected out in Schneider’s insect medium (HiMedia Laboratories Pvt. Ltd., Mumbai, India), and they were incubated with 30 μM DHE in dark at 24 ± 1 °C for 10 min. After washing with 1× PBS, tissues were imaged under a Leica TCS-SPE confocal microscope (Nussloch, Germany). At least ten brain ganglia from each group were analyzed by using ImageJ software (NIH).
Measurement of Anti-oxidant Defense Enzymes
SOD (Superoxide/Superoxideoxidoreductase EC 1.15.1.1) Activity Assay
For the determination of SOD activity, biochemical assay was performed following the method described earlier [42] with minor modification [24]. The reaction mixture consists of 25.0 μl of 10 % brain ganglia homogenate, 1.2 ml (0.052 M) sodium pyrophosphate buffer (pH 8.3), 1.175 ml of doubled distilled water, 0.1 ml of 186 μM phenazinemethosulphate, and 0.3 ml of 300 μM nitrobluetetrazolium. In order to start the reaction, 0.2 ml of 780 μM reduced nicotinamide adenine dinucleotide was added and the reaction was stopped by the addition of 1.0 ml glacial acetic acid. Finally, the colored product was extracted using 4.0 ml of n-butanol. Enzyme concentration required for the 50 % inhibition of chromogen production (optical density at 560 nm) was considered as 1 unit of enzyme activity, and results were expressed as specific activity in units per minute per milligram of protein. Standards and tissue homogenates were assayed in triplicate.
CAT (H2O2/H2O2 Oxidoreductase EC 1.11.1.6) Activity Assay
CAT activity was measured by following its ability to split H2O2 within 1 min of incubation time. The reaction mixture consists of 25.0 μl of 10 % brain ganglia homogenate, 1.0 ml (0.01 M) sodium phosphate buffer (pH 7.0), 0.475 ml of doubled distilled water, and 0.5 ml of (0.2 M) H2O2. The reaction was then stopped by adding dichromate/acetic acid reagent, and the remaining H2O2 was determined by measuring chromic acetate at 570 nm, which is formed by reduction of dichromate/acetic acid in the presence of H2O2 as described earlier [43]. The results were expressed as micromoles of H2O2 per minute per milligram of protein. Standards and tissue homogenates were assayed in triplicate.
TrxR Activity Assay
The activity of TrxR, a substitute for Drosophila glutathione reductase (GR), was measured following a published method [44] with minor modifications. Briefly, to a 10 % homogenate of brain ganglia, potassium phosphate and ethylenediamine tetraacetic acid (EDTA; pH 7.4) was added. After an oxidation step in the presence of reduced nicotinamide adenine dinucleotide (NADPH) and 5,5-dithiobis(2-nitrobenzoate) (DTNB), TrxR activity in the sample was assessed at an absorbance of 412 nm. The results were expressed as nanomoles of NADPH oxidized per minute per milligram of protein using a molar extinction coefficient of 13.6 mM−1 cm−1.
GSH Content
GSH content was estimated using Ellman’s reagent by following the method by Ellman [45]. Reduced GSH was used as an external standard. Briefly, 0.5 ml of 10 % brain ganglia homogenate and 0.5 ml of 5 % Trichloro acetic acid (TCA) were mixed and centrifuged at 2500×g for 10 min. The obtained supernatant was mixed with 0.3 ml of 0.2 M phosphate buffer (pH 8.0) and 2.5 ml of 0.01 % DTNB. The reaction was monitored at 412 nm using a spectrophotometer (SPECORD®210 PLUS, Analytic Jena AG, Konrad-Zuse-Strasse, Germany). GSH content was calculated using the standard GSH curve and expressed as nmoles/mg protein.
PC Content
PC content was determined essentially following the method of Levine et al. [46] with minor modifications. In brief, two equal aliquots (0.1 ml) of 10 % brain ganglia homogenate were taken, one was treated with an equal volume of 2,4-dinitrophenyl hydrazine (DNPH; 10.0 mM dissolved in 2.0 M HCl; test sample) and the other with 2.0 M HCl (blank). After 1 h incubation, 0.2 ml of 20.0 % TCA was added for precipitation and for subsequent extraction with ethanol:ethylacetate mixture (1:1) three times. The pellets obtained were then dissolved in 1.0 ml of 6.0 M guanidine hydrochloride at 37 °C. The absorbance of the DNPH treated sample vs the HCl blank was determined at 370 nm on a spectrophotometer (SPECORD®210 PLUS, Analytic Jena AG, Konrad-Zuse-Strasse, Germany). PC content was expressed in terms of nmoles DNPH incorporated/mg protein based on molar absorption coefficient of 22,000 M−1 cm−1.
Assay for LPO
Generation of malondialdehyde (MDA) as an intermediate during LPO was measured using tetraethoxypropane as an external standard [47]. The assay mixture was prepared using 25.0 μl of 10 % brain ganglia homogenate, 0.15 ml of 10 % SDS, 0.825 ml of doubled distilled water, and 1.0 ml of 20 % acetic acid (pH 3.5) and kept at 25 ± 2 °C for 5 min. Then 1.0 ml of 0.8 % thiobarbituric acid (TBA) was added. The mixture was kept in boiling water for 1 h. Finally, the color was extracted using 3 ml of n-butanol and MDA content was expressed in terms of nanomoles of MDA formed per hour per milligram of protein.
Assay for Acetylcholinesterase Activity
AchE activity was measured essentially following the method of Ellman et al. [48] with minor modifications. In brief, larval brain ganglia (50 nos.) or adult brain (50 nos.) from control and treated groups were homogenized in 50 mM N-(2-hydroxyethyl)piperazine-N′-(2-ethane sulfonic acid) sodium salt buffer containing a protease inhibitor. The supernatant, obtained after centrifugation (10,000×g for 15 min), was mixed with phosphate buffer, DTNB, and acetyl thiocholine iodide. AchE catalyzes the hydrolysis of acetylthiocholine to acetate and thiocholine which on reaction with DTNB gives a yellow color product. Its absorbance was measured at 412 nm for 5 min at intervals of 30 s. AchE activity was expressed in terms of millimoles of substrate hydrolyzed per minute per milligram of protein.
Protein Estimation
Protein content in control and treated samples was estimated by the method of Lowry et al. [49] using BSA as the standard.
Statistical Analysis
Different parameters were monitored in control and exposed organisms. Analysis of variance was carried out to find out the significant differences in means considering each end point as dependent variable and concentrations (5.0, 10.0, and 20.0 μg/ml) as the independent variable. Prior to analysis, normality assumption of the data and homogeneity of variance among different groups were tested and data were found to hold the above two assumptions. The results of the different treatment groups were compared with control using two-way analysis of variance (ANOVA) followed by Bonferroni post-tests to compare replicate means by row. A value of p < 0.05 was considered statistically significant. Graphpad Prism 5.0 software (San Diego, CA, USA) was used for analysis.
Results
Estimation of Cr Uptake in the Brain of Exposed Drosophila Larvae
To examine the effect of environmental Cr(VI) or Cr(III) exposures through food on the brain tissue of Drosophila, total Cr estimation was performed in control and treated groups. A concentration-dependent increase in Cr burden was observed in the brain of Drosophila that were fed food mixed with Cr(VI)-salt (a maximum increase of ∼294 % in Cr burden at 20.0 μg/ml Cr(VI) after 48 h in comparison with control). On the other hand, burden of Cr in the brain of the organisms that were fed Cr(III)-salt contaminated food was significantly higher at 10.0 and 20.0 μg/ml of Cr(III) in comparison with respective control. However, the same was ∼125 and ∼237 % less when compared with similar tested concentration of Cr(VI) (Fig. 1).
Level of Cr in the brain ganglia of Cr(VI)- or Cr(III)-salt-mixed food fed D. melanogaster (w 1118) larvae. Graphical representation of total Cr present in the brain ganglia of w 1118 larvae after they were fed Cr(VI)- or Cr(III)-salt-mixed food for 48 h. Note a significantly higher burden of Cr in the brain ganglia of organism that were fed Cr(VI)-salt as compared with that in Cr(III)-salt fed organism after 48 h. Significance ascribed as *p < 0.05; **p < 0.01; ***p < 0.001 vs control; # p < 0.05; ## p < 0.01; ### p < 0.001 vs Cr(III)
Effect of Cr(VI) and Cr(III) on Brain Cell Marker Proteins in Exposed Drosophila Larvae
Figure 2a shows representative confocal images from the optical sections of the brain ganglia from control and Cr(VI)- or Cr(III)-salt-mixed food fed larvae. Elav being a pan neuronal marker protein would provide the status of neuronal cells [50]. Immunostaining of the brain ganglia with Elav antibody showed significantly (p < 0.001) weak fluorescence in the neuronal cells of Drosophila larvae that were fed Cr(VI)-salt mixed food in a concentration- and time-dependent manner; whereas, no appreciable change in Elav immunostaining was observed in the brain ganglia of larvae that were fed Cr(III)-salt-mixed food even at its highest tested concentration after 48 h (Fig. 2a, b).
Decreased Elav fluorescence in the neuronal cells of the brain ganglia of Cr(VI)- but not in Cr(III)-salt-mixed food fed w 1118 larvae. Representative confocal images of Elav (green) antibody-stained brain ganglia from control and in organism that were fed Cr(VI)-salt-mixed food for 24 and 48 h or Cr(III)-salt-mixed food for 48 h (a). Bar, 100 μm. For each group, 20 brain hemispheres were examined. Graphical representation of fluorescence intensity measured by ImageJ for the detection of Elav (b). Significance ascribed as *p < 0.05; **p < 0.01; ***p < 0.001 vs control; #p < 0.05; ##p < 0.01; ###p < 0.001 vs Cr(III)
The other major cell type of the brain is glial cells that provide support and nutrition to the neurons. However, these cells were not found to be significantly (p > 0.05) affected when larvae were fed Cr(VI)- or Cr(III)-salt-mixed food. This was evidenced by comparable immunofluorescence of glia among control and treated groups after their staining with anti-Repo antibody (Fig. 3a, b).
Repo immunostaining of glial cells in the brain ganglia of Cr(VI)- or Cr(III)-salt-mixed food fed w 1118 larvae. Representative confocal images of Repo (green) antibody-stained brain ganglia from control and in organism that were fed Cr(VI)-salt-mixed food for 24 and 48 h or Cr(III)-salt-mixed food for 48 h (a). Bar, 100 μm. Graphical representation of fluorescence intensity measured by ImageJ for the detection of Repo (b). Significance ascribed as *p < 0.05; **p < 0.01; ***p < 0.001 vs control; #p < 0.05; ##p < 0.01; ###p < 0.001 vs Cr(III)
Subsequently, the status of cholinergic or dopaminergic cells were assessed in control and exposed organism by immunostaining their brain with anti-ChAT and anti-TH antibodies. AchE activity was also measured. A significantly (p < 0.001) weak ChAT fluorescence was observed in the brain ganglia of organisms that were fed Cr(VI)-salt-mixed food in a concentration-dependent manner (Fig. 4a, b) along with a concentration- and time-dependent decrease (∼4.5-fold) in AchE activity (Fig. 4c). Both these end points were insignificantly (p > 0.05) changed in organisms that were fed Cr(III)-salt-mixed food (Fig. 4a–c).
Decreased ChAT fluorescence and AchE activity in the brain ganglia of Cr(VI)- but not in Cr(III)-salt-mixed food fed w 1118 larvae. Representative confocal images of ChAT (green) antibody stained brain ganglia from control and in organism that were fed Cr(VI)-salt mixed food for 24 and 48 h or Cr(III)-salt-mixed food for 48 h (a). Bar, 100 μm. Graphical representation of fluorescence intensity measured by ImageJ for the detection of ChAT (b). A histogram showing decreased AchE activity in the brain ganglia of Cr(VI)- but not in Cr(III)-exposed organism (c). Significance ascribed as *p < 0.05; **p < 0.01; ***p < 0.001 vs control; #p < 0.05; ##p < 0.01; ###p < 0.001 vs Cr(III)
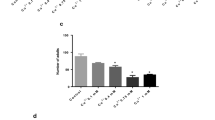
The larval dopaminergic neuronal system consists of 8-dorso-medial (DM) and 13-dorso-lateral (DL) cells [51]. Immunostaining of brain ganglia with anti-TH antibody revealed a significant (p < 0.05) reduction in the number of TH-positive cells in organisms that were fed 20.0 μg/ml Cr(VI)-salt-mixed food for 48 h (Fig. 5a, b). However, at lower concentrations of Cr(VI) (5.0 and 10.0 μg/ml) and at the highest concentration of Cr(III), no significant (p < 0.05) reduction in the number of TH-positive cells was observed in organisms that were fed Cr(VI)- or Cr(III)-salt-mixed food even for 48 h in comparison with control (Fig. 5a, b).
Reduced number of dopaminergic neurons in the brain ganglia of Cr(VI)- but not in Cr(III)-salt-mixed food fed w 1118 larvae. Representative confocal images of TH (green) antibody-stained brain ganglia from control and in organism that were fed Cr(VI)-salt mixed food for 24 and 48 h or Cr(III)-salt-mixed food for 48 h (a). Bar, 100 μm. Quantitative representation of average number of dorso-lateral (DL; indicated by yellow arrow) and dorso-medial (DM; indicated by white arrow) neurons in dopaminergic clusters of control, Cr(VI)- and Cr(III)-exposed organism wherein cells were affected at 20.0 μg/ml Cr(VI) after 48 h (b). Significance ascribed as *p < 0.05 vs control; #p < 0.05 vs Cr(III)
Enhanced Levels of ROS and RNS in the Brain of Cr(VI)-Exposed Drosophila Larvae
Since an excess of Cr in the body is expected to generate free radicals, levels of different species of radicals were measured in organisms that were fed Cr(VI)-salt-mixed food. A significant (p < 0.001) increase in the level of DHE, H2DCFDA, and DHR fluorescence intensities was observed in the brain ganglia of Cr(VI)-salt-mixed food fed w 1118 larvae as compared with control with a maximum increase in DCF (∼3.4-fold; Fig. 6a), DHR (∼2.7-fold; Fig. 6b), and E+/DHE (∼1.6-fold; Fig. 6c) fluorescence at 20.0 μg/ml Cr(VI) after 48 h. No significant change in the level of the above radicals was observed in organisms that were fed Cr(III)-salt-mixed food (data not shown). That difference in free radicals levels are indeed taking place in exposed organism as compared with that in control was further confirmed in vivo by DHE staining of different brain calls tagged with GFP. Both dopaminergic and cholinergic neuronal cells exhibited higher fluorescence intensity as compared with respective control and to that in glial cells (Fig. 7a–d).
Increased levels of free radicals and oxidative stress in the brain ganglia of Cr(VI)-salt mixed food fed w 1118 larvae. Graphical representation of total ROS (a), ONOO− (b), and O2 ·− levels (c) in the brain from control and in organism that were fed Cr(VI)-salt-mixed food for 24 and 48 h by measuring fluorescence using H2DCFDA, DHR, and DHE dyes, respectively. Data represent mean ± SD (n = 3; 80 larvae in each replicate). Graphical representation of superoxide dismutase (SOD), catalase (CAT), and thioredoxin reductase (TrxR) activities (d–f) and glutathione ( GSH) (g), malondialdehyde (MDA) (h), and protein carbonyl (PC) content (i), respectively, in the brain ganglia from control and in organism that were fed Cr(VI)-salt-mixed food for 24 and 48 h. Statistically significant differences ascribed as *p < 0.05; **p < 0.01; ***p < 0.001 vs control
Increased in vivo O2 ·− levels in the brain ganglia of Cr(VI)-salt-mixed food fed w 1118 larvae. Representative confocal images of DHE-stained brain ganglia from control and in organism that were fed 20.0 μg/ml Cr(VI)-salt-mixed food for 48 h. Extreme right represents overlay images of DHE (blue) staining, its oxidation product E+ (red) in GFP-tagged different brain cell types viz., Elav-GAL4>UAS-GFP (neuronal cells) (a), Ddc-GAL4:UAS-GFP (dopaminergic cells) (b), ChAT-GAL4>UAS-GFP (cholinergic cells) (c), and Repo-GAL4>UAS-GFP (glial cells) (d). Note that higher red fluorescence in different brain cell types except in glial cells show higher ROS levels in them
Increased Oxidative Stress in the Brain of Cr(VI)-Exposed Drosophila Larvae
A time- and concentration-dependent significant decrease in SOD activity in the brain ganglia of w 1118 larvae was observed that were fed Cr(VI)-salt-mixed food (a maximum decrease of ∼155 % in SOD activity was observed in 20.0 μg/ml Cr(VI)-salt-mixed food fed group after 48 h) (Fig. 6d). A similar trend was observed for CAT (∼27 %) (Fig. 6e), TrxR (∼60 %) (Fig. 6f) activities, and GSH (∼120 %) (Fig. 6g) content in the brain ganglia of larvae that were fed 20.0 μg/ml Cr(VI)-salt-mixed food. Concomitant with an inhibition in all the above endpoints, a concentration- and time-dependent (p < 0.001) significant increase in MDA (∼338 %) and PC (∼112 %) content in the brain ganglia of exposed w 1118 larvae was observed (Fig. 6h, i).
Increased DEVDase Activity in the Brain of Cr(VI)-Exposed Drosophila Larvae
Since apoptosis was reported to be the primary mode of cell death due to Cr(VI) exposure to mouse skin epidermal cells (JB6 C141 cells) [52], DEVDase (caspase-3 like) activity was measured in the brain ganglia of control and in organisms that were fed Cr(VI)- or Cr(III)-salt-mixed food. A significant increase in the DEVDase activity was observed in organism given Cr(VI)-salt-mixed food as compared with control with a maximum increase in activity (∼420 %) in 20.0 μg/ml Cr(VI)-exposed organism after 48 h (Fig. 8a). Parallel to the DEVDase activity, immunofluorescence analysis using cleaved caspase-3 antibody also revealed activation of caspase-3 in dopaminergic and cholinergic neurons but not significantly in glial cells of larvae that were given Cr(VI)-salt-mixed food (Fig. 9a–d). Unlike the above, no significant increase in the DEVDase activity was observed in the group that were fed Cr(III)-salt-mixed food in comparison with control (data not shown).
Increased apoptosis in the brain ganglia of Cr(VI)-salt-mixed food fed w 1118 larvae. Graphical representation of DEVDase (caspase-3-like) activity in the brain ganglia of w 1118 larvae after they were fed Cr(VI)-salt mixed food for 24 and 48 h (a). Graphical representation of dissipation of mitochondrial membrane potential in the brain ganglia of w 1118 larvae after they were fed Cr(VI)-salt-mixed food for 24 and 48 h using JC1 dye representing a shift from red to green fluorescence intensity (b). Data represent mean ± SD (n = 3; 40 larvae/replicate). Significance ascribed as *p < 0.05; **p < 0.01; ***p < 0.001 vs control. Representative confocal images of brain ganglia showing JC1 staining in the brain from control and in organism that were fed Cr(VI)-salt-mixed food with extreme right panel representing overlay of red and green fluorescence (c). Bar, 100 μm
Increased cleaved caspase-3 in different brain cells of Cr(VI)-salt-mixed food fed w 1118 larvae. Representative confocal images of brain ganglia showing levels of cleaved caspase-3 from control and in organism that were fed 20.0 μg/ml Cr(VI)-salt-mixed food for 48 h. Extreme right panel represents overlay images of DAPI (blue) and brain cell marker proteins (red) such as Elav (a), ChAT (b), and Repo (d) and cleaved caspase-3 (green) antibody stained tissue. Representative confocal images of GFP-tagged dopaminergic neurons (green) and cleaved caspase-3 (red) antibody stained tissue and overlaying images (c). Note that the cleaved caspase-3 was localized in neuronal cells, cholinergic neurons, and dopaminergic neurons but not in glial cells indicating apoptosis in these cells
Increased Mitochondrial Depolarization in the Brain of Cr(VI)-Exposed Drosophila Larvae
Parallel to the detectable increase in DEVDase activity in w 1118 larvae that were fed Cr(VI)-salt-mixed food at higher dietary concentrations of the test chemical, a significant increase in the mitochondrial membrane depolarization as evident by a shift from red to green fluorescence was observed in the brain ganglia of exposed organism in comparison with control with maximum shift from red to green fluorescence (∼201 %) at 20.0 μg/ml Cr(VI) after 48 h (Fig. 8b). Parallel to the above, immunofluorescence analysis also revealed shift from red to green fluorescence in the brain ganglia of Cr(VI)-salt mixed food fed organism (Fig. 8c).
Decreased ROS/RNS as well as Apoptotic Cell Death in the Brain of Vitamin C and Cr(VI)-Exposed Drosophila Larvae
If oxidative stress is responsible for the cellular adversities in tested brain cells of organisms that were fed Cr(VI)-salt-mixed food, reversal of the same was tested by adding a known anti-oxidant, vitamin C in Drosophila food medium. Larvae feeding the anti-oxidant along with Cr(VI)-salt-mixed food, showed lesser levels of ROS and reactive nitrogen species (RNS) in their brain in comparison with that observed in the organisms that were fed Cr(VI)-salt-mixed food only (A significant decrease in total ROS levels (∼20 %), O2 ·− (∼23 %), as well as in RNS (∼60 %) in the brain cells of organisms that were given vitamin C + Cr(VI)-salt -mixed food in comparison with that in Cr(VI)-salt-only treated organism) (Fig. 10a). In vivo study revealed that ROS levels were reduced both in cholinergic and dopaminergic neuronal cells in organisms that were given vitamin C + Cr(VI)-salt-mixed food (Fig. 10d). Cell death was also examined under similar experimental condition. While a significant increase in Annexin V (AV)-positive brain cells (∼541 %) was observed in organisms that were fed 20.0 μg/ml Cr(VI)-salt-mixed food for 48 h, vitamin C supplementation significantly decreased the AV-positive cell population (∼198 %) in organisms that were given Cr(VI)-salt along with vitamin C (Fig. 10b, c). Further, a trend similar to the above was observed for cleaved caspase-3 level in cholinergic and dopaminergic cells (Fig. 10e).
Decreased ROS/RNS levels, Annexin V-positive brain cells and cleaved caspase-3 level after w 1118 larvae were fed vitamin C + Cr(VI)-salt. Graphical representation of total ROS, ONOO−, and O2 ·− levels (a) in the brain from control and in organism that were fed Cr(VI)-salt-mixed food only or those fed Cr(VI)-salt-mixed food supplemented with vitamin C and those given vitamin C mixed food for 48 h by measuring the fluorescence using DCF-DA, DHR, and DHE dyes, respectively. Data represent mean ± SD (n = 3; 80 larvae/replicate). Quantitative representation of percent AV-positive cells from control and in organism that were fed Cr(VI)-salt-mixed food only or those fed Cr(VI)-salt-mixed food supplemented with vitamin C and those given vitamin C-mixed food for 48 h (b) and representative flowcytometric data of the same (c). Data represent mean ± SD (n = 3; 40 larvae/replicate). Significance ascribed as *p < 0.05; **p < 0.01; ***p < 0.001 vs control. Representative confocal images of DHE stained brain ganglia from vitamin C + Cr(VI)-exposed (20.0 μg/ml) organism after 48 h (d). Representative confocal images of brain ganglia showing cleaved caspase-3 level from vitamin C + Cr(VI)-exposed (20.0 μg/ml) w 1118 larvae after 48 h (e). Bar, 100 μm
Increased Inhibition of AchE Activity in Adult Fly Along with Decreased Organismal Response in Cr(VI)-Exposed Organism
A significant (p < 0.05) decrease in AchE activity was observed in the adult flies that emerged after the larvae were fed Cr(VI)-salt-mixed food for 48 h in a concentration-dependent manner with maximum inhibition (∼214 %) in the enzyme activity evidenced at 20.0 μg/ml of Cr(VI) (Fig. 11a).
Increased inhibition of AchE activity and decreased locomotor and jumping activity in Cr(VI)-salt-mixed food fed w 1118 larvae. Graphical representation of percent inhibition of AchE activity (a), climbing activity (b), and jumping activity (c) of flies that emerged after being fed Cr(VI)-salt-mixed food. Note a significant deficit in these activities in exposed organism. Data represent mean ± SD (n = 3). Significance ascribed as *p < 0.05; **p < 0.01; ***p < 0.001 vs control
Under similar exposure regime as stated above, a significant decrease in the climbing activity of flies was observed in a concentration-dependent manner. While ∼72 % of the control flies crossed the 15-cm line in 30 s, only ∼42 % of flies crossed the same distance after the larvae were given 20.0 μg/ml of Cr(VI)-salt-mixed food for 48 h (Fig. 11b). A trend similar to the above was observed for the jumping activity in exposed groups (Fig. 11c).
Discussion
The present study aimed to investigate the impact of environmental Cr on tested brain cells in a genetically tractable organism, D. melanogaster.
The presence of the metal in the brain of organisms that were fed Cr(VI)- or Cr(III)-salt-mixed food has relevance in causing adversities associated with it. Between the two most prevalent forms of Cr, Cr(VI) can easily enter into the cells through sulfate and chloride phosphate anionic channels due to its tetrahedral structure, activated by small thiols and ascorbate non-enzymatically [53]. However, the octahedral structure of Cr(III) negatively affects its ability to enter into the cells and higher stability of its coordinated multidentate ligand structure prevents it from binding to macromolecules of the cell [54]. Although significantly higher amount of Cr was detected in the brain of Drosophila larvae that were fed Cr(III)-salt-mixed food, it was several folds lower than the amount of Cr detected in organisms that were fed Cr(VI)-salt-mixed food. The higher adverse effects of Cr(VI) might be due to its ability to cross blood brain barrier more efficiently than Cr(III) as has been reported previously wherein Cr(VI) got deposited in human brain [55] as well as in rat pituitary glands [56]. Both Cr(VI) and Cr(III) were reported to cause genotoxicity in vitro and in vivo (reviewed by Eastmond et al. [57]). Interestingly, it was found that genotoxicity induced by Cr(III) to an extent similar to that induced by Cr(VI) was achievable when Cr(III) concentration was several folds (∼1000-fold) higher than Cr(VI) [10, 57]. In this study too, refractoriness of tested brain cells of Drosophila larvae that were fed Cr(III)-salt-mixed food (presence of ∼7–8 ng of Cr) might be due to the above stated possibility and lack of critical concentration of the test chemical therein. Conversely, organisms that were fed Cr(VI)-salt-mixed food had higher burden of Cr (∼32 ng; ∼4-fold higher than Cr(III)) which might be above the threshold limit of cellular homeostasis to evoke cellular and organismal adversities in exposed organism. Apart from the above, Cr(VI) might get converted into Cr(III) inside the cell to cause cellular toxicity which cannot be ruled out in this study.
Drosophila larval central nervous system comprises mainly neuronal and glial cells [58]. Due to higher Cr burden in the brain of exposed organism, we examined the health (marker protein level or number) of tested brain cell types in organisms that were fed Cr(VI)- or Cr(III)-salt-mixed food. ELAV, the first identified member of the ELAV family, has been shown to be expressed in all neurons with a predominantly nuclear localization [58, 59]. The elav gene function is vital for the differentiation and maintenance of neurons and is a pan neuronal marker [60]. Poor Elav immunostaining in the brain of organisms that were fed Cr(VI)-salt-mixed food in comparison with that in Cr(III)-salt-mixed food fed Drosophila larvae corroborate neuronal adversity caused by Cr(VI). Among different cell types in the brain, glial cells provide protection to the neurons with physical as well as nutritional support [61]. Though four types of glial cells and marker proteins representing them are reported [62], Repo was found to represent all types of glial cells during development [62, 63]. Interestingly, glial cells were not found to be affected by the metal as evidenced by comparable Repo immunostaining in the brain of control and in organisms that were fed Cr(VI)- or Cr(III)-salt-mixed food. In this context, one of the possible reasons for non-perturbation of glial cells due to metal exposures might be due to enriched glutathione (a major reducing agent) [64–66] in these cells to combat oxidative stress. The neuronal cells are also segregated according to the neurotransmitters they release, such as acetylcholine, dopamine, GABA, glutamate, etc. We, therefore, examined the status of cholinergic and dopaminergic neurons since they are implicated in some neurodegenerative diseases [5] and affect movement, behavioral functions, jumping, and locomotor activity [67, 68]. Cholinergic neuronal system consists of mainly choline acetyl transferase, AchE, and vesicular acetylcholine transporter by which it conducts neurotransmission [69]. Choline acetyl transferase is responsible for acetylcholine biosynthesis and AchE catalyzes the breakdown of acetylcholine for its functioning as a neurotransmitter [70]. A significant decrease in choline acetyl transferase and AchE activity in the brain of organisms that were fed Cr(VI)-salt-mixed food but not Cr(III) indicates perturbed neuronal signaling due to hexavalent form of the metal causing increased oxidative stress as evidenced by higher ROS levels in these cells. In this context, disruption in the cholinergic system in Daphnia [71], in guinea pig, and keratinocyte cells [72] due to increased oxidative stress after Cr(VI) exposure supports the above [73].
Each brain lobe consists of four clusters of dopaminergic neurons in which the lower two clusters are named as DL and upper medially located two clusters are named, as DM and they exist in adult also [51]. Like cholinergic cells, dopaminergic neuronal cells in organisms that were fed Cr(VI)-salt-mixed food were affected as evidenced by a significant reduction in the number of dopaminergic neurons (DL and DM). The Trx/Prx system maintains redox balance in the cell by scavenging ROS and also maintains dithiol-disulfide exchange of target proteins [74]. In brain, mitochondrial Trx/Prx system is responsible for the redox balance in dopaminergic neurons as was evidenced in rat dopaminergic N27 cells [75] and Cr(VI) was shown to inhibit Trx/Prx system in human broncheal epithelial cells (BEAS-2B cell line) [11]. Hu et al. [76] also reported that Prx-2 overexpression provides protection against dopaminergic neurodegeneration in MN9D DA neuronal cells by maintaining Trx in its reduced stage. Prx transcript as well as Prx and Trx associated miRNAs were found to be significantly misregulated in the gut of Cr(VI)-exposed Drosophila larvae [28, 77]. A significant decrease in TrxR activity observed in the brain of Drosophila larvae that were fed Cr(VI)-salt-mixed food indicates Cr(VI) mediated perturbed redox system vis-a-vis effect on dopaminergic neurons. Parallel to the above, increased ONOO− levels in these cells of exposed organism might cause nitration of tyrosine residue in TH leading to its inactivation and subsequently poor dopamine synthesis and alteration in neurotransmitter level [78].
Both cholinergic and dopaminergic neuronal cells are reported to control the climbing and jumping activity and are indicators of fitness of an organism [79, 80]. Alteration in the neurotransmitter level, such as, acetylcholine [35] and dopamine [5, 81], were shown to be associated with defective locomotor activity in Drosophila and in other organisms [82]. Previously, Cr(VI) exposure (98 mg kg−1 day−1 for 28 days) to rats was shown to cause deficit in motor activity along with poor balance [83]. Our observation of poor climbing and jumping activities in organisms that were fed Cr(VI)-salt-mixed food along with a significant inhibition in AchE activity both in larval and adult brain as well as reduced number of dopaminergic cells may be correlated with alteration in neurotransmitter level (both dopamine and acetylcholine).
Given that Cr(VI) can adversely affect tested brain cells and consequently locomotor behavior of exposed organism, oxidative stress seems to be the major mechanism of toxicity which was evident by an increase in the levels of ROS/RNS. The latter are the major contributors towards higher oxidative stress affecting both enzymatic and non-enzymatic components of oxidant balance in the cell. In this context, increased ONOO− might be a reason behind the decreased activity of SOD since increased ONOO− was reported to inhibit SOD activity in animals [84, 85]. That Cr can reduce various enzyme activities such as CAT and TrxR via reacting with their carbonyl and sulfhydryl groups [12] provides an explanation for increased oxidative stress in the brain of organisms that were given Cr(VI)-salt-mixed food.
Oxidative stress may lead to cell death if not balanced properly [86]. This was evidenced in this study with heaxavalent form of Cr significantly affecting cholinergic and dopaminergic neuronal cell survival. However, no significant effect on these cells was observed when organisms were fed Cr(III)-salt-mixed food. That increased oxidative stress in tested brain cells due to increased ROS/RNS levels could be one of the plausible reasons for the increased cell death in cholinergic and dopaminergic neuronal cells was tested by feeding the organism with a known anti-oxidant, vitamin C wherein reversal of apoptosis was observed. Summarizing the above, imbalanced redox system in the brain of Drosophila that were fed Cr(VI)-salt-mixed food affected the health of their brain cells leading to apoptosis.
In conclusion, the study provides evidence of perturbed health of cholinergic and dopaminergic neuronal cells due to imbalanced redox condition caused by feeding of the Cr(VI)-salt to the organism (Fig. 12) but not by Cr(III)-salt in the diet in a genetically tractable organism D. melanogaster coupled with a deficit in movement. The study signifies the adverse effect of Cr(VI) at its environmentally relevant concentrations which would likely to have relevance in humans and recommends Drosophila as a model for neurotoxicity.
Schematic illustration of Cr(VI)-induced adversities in the brain of Drosophila and its organismal consequence. Environmental Cr especially its heaxavalent form, Cr(VI) can cause increased cell death in different brain cell types [cholinergic (ChAT) and dopaminergic (TH) neuronal cells but not in glial cells]. Increased oxidative stress (increased level of ROS/RNS along with decreased SOD, CAT, and TrxR activities and GSH content and increased MDA and PC contents) is the primary mechanism of toxicity in these cells due to higher burden of Cr in the brain of exposed organism leading to cell death and deficit in organismal deficit (impaired locomotor and jumping activity)
References
Edwards FL, Tchounwou PB (2005) Environmental toxicology and health effects associated with methyl parathion exposure—a scientific review. Int J Environ Res Public Health 2(3–4):430–441
Yang D, Lauridsen H, Buels K, Chi LH, La Du J, Bruun DA, Olson JR, Tanguay RL et al (2011) Chlorpyrifos-oxon disrupts zebrafish axonal growth and motor behavior. Toxicol Sci 121(1):146–159
Dubey D, Sharma VD, Pass SE, Sawhney A, Stuve O (2014) Para-dichlorobenzene toxicity—a review of potential neurotoxic manifestations. Ther Adv Neurol Disord 7(3):177–187
Paul KC, Sinsheimer JS, Rhodes SL, Cockburn M, Bronstein J, Ritz B (2015) Organophosphate pesticide exposures, nitric oxide synthase gene variants, and gene–pesticide interactions in a case–control study of Parkinson’s disease, California (USA). Environ Health Perspect. doi:10.1289/ehp.1408976
Shukla AK, Pragya P, Chaouhan HS, Patel DK, Abdin MZ, Kar Chowdhuri D (2014) Mutation in Drosophila methuselah resists paraquat induced Parkinson-like phenotypes. Neurobiol Aging 35(10):2419, e1-241
Xu X, Weber D, Carvan MJ 3rd, Coppens R, Lamb C, Goetz S, Schaefer LA (2012) Comparison of neurobehavioral effects of methylmercury exposure in older and younger adult zebrafish (Danio rerio). Neurotoxicol 33(5):1212–1218
Balk EM, Tatsioni A, Lichtenstein AH, Lau J, Pittas AG (2007) Effect of chromium supplementation on glucose metabolism and lipids: a systematic review of randomized controlled trials. Diabetes Care 30(8):2154–2163
Wilbur S, Voytek P (1988) Centers for Disease Control and Prevention. Atlanta, Georgia, USA: 1988. Toxicological profile for chromium. Agency for Toxic Substances and Disease Registry
Mishra M, Sharma A, Negi MP, Dwivedi UN, Chowdhuri DK (2011) Tracing the tracks of genotoxicity by trivalent and hexavalent chromium in Drosophila melanogaster. Mutat Res 722(1):44–51
Fang Z, Zhao M, Zhen H, Chen L, Shi P, Huang Z (2014) Genotoxicity of tri- and hexavalent chromium compounds in vivo and their modes of action on DNA damage in vitro. PLoS One 9(8), e103194
Myers JM, Antholine WE, Myers CR (2011) The intracellular redox stress caused by hexavalent chromium is selective for proteins that have key roles in cell survival and thiol redox control. Toxicol 281(1–3):37–47
Stepniewska Z, Wolinska A, Ziomek J (2009) Response of soil catalase activity to chromium contamination. J Environ Sci 21(8):1142–1147
Umeda-Kameyama Y, Tsuda M, Ohkura C, Matsuo T, Namba Y, Ohuchi Y, Aigaki T (2007) Thioredoxin suppresses Parkin-associated endothelin receptor-like receptor-induced neurotoxicity and extends longevity in Drosophila. J Biol Chem 282(15):11180–11187
Dashti A, Maliheh SM, Amani N (2015) Evaluation of Cr (VI) induced neurotoxicity and oxidative stress in PC12 cells. Pathobiol 18(1):55–65
Mathur AK, Chandra SV, Tandon SK (1977) Comparative toxicity of trivalent and hexavalent chromium to rabbits II. Morphological changes in some organs. Toxicol Res 8(1):53–61
Carson BL, Ellis HV III, McCann JL (1986) Toxicology & biological monitoring of metals in humans, including feasibility and need. Lewis Publishers, Chelsea, pp 68–71
Moshtaghie AA, Afrang M, Mesripour M (2004) Changes in catecholamines and acetylcholinesterase levels of crebellum, mid-brain and brain cortex in chromium treated rats I. J Pharm Res 3:149–153
Soudani N, Troudi A, Amara IB, Bouaziz H, Boudawara T, Zeghal N (2012) Ameliorating effect of selenium on chromium (VI)-induced oxidative damage in the brain of adult rats. J Physiol Biochem 68(3):397–409
Krikorian R, Eliassen JC, Boespflug EL, Nash TA, Shidler MD (2010) Improved cognitive-cerebral function in older adults with chromium supplementation. Nutr Neurosci 13(3):116–122
Zhitkovich A, Voitkun V, Costa M (1996) Formation of the amino acid–DNA complexes by hexavalent and trivalent chromium in vitro: importance of trivalent chromium and the phosphate group. Biokhimiya 35(22):7275–7282
Adams MD, Celniker SE, Holt RA, Evans CA et al (2000) The genome sequence of Drosophila melanogaster. Sci 287(5461):2185–2195
Festing MF (1999) Reduction in animal use in the production and testing of biologicals. Dev Biol Stand 101:195–200
Siddique HR, Chowdhuri DK, Saxena DK, Dhawan A (2005) Validation of Drosophila melanogaster as an in vivo model for genotoxicity assessment using modified alkaline Comet assay. Mut 20(4):285–290
Gupta SC, Siddique HR, Saxena DK, Chowdhuri DK (2005) Hazardous effect of organophosphate compound, dichlorvos in transgenic Drosophila melanogaster (hsp70-lacZ): induction of hsp70, anti-oxidant enzymes and inhibition of acetylcholinesterase. Biochim Biophys Acta 1725(1):81–92
Lu B, Vogel H (2009) Drosophila models of neurodegenerative diseases. Annu Rev Pathol 4:315–342
Aravindhan R, Madhan B, Rao JR, Nair BU, Ramasami T (2004) Bioaccumulation of chromium from tannery wastewater: an approach for chrome recovery and reuse. Environ Sci Technol 38(1):300–306
Sharma P, Bihari V, Agarwal SK, Verma V, Kesavachandran CN, Pangtey BS, Mathur N, Singh KP et al (2012) Groundwater contaminated with hexavalent chromium [Cr (VI)]: a health survey and clinical examination of community inhabitants (Kanpur, India). PLoS One 7(10), e47877
Mishra M, Sharma A, Shukla AK, Pragya P, Murthy RC, de Pomerai D, Dwivedi UN, Chowdhuri DK (2013) Transcriptomic analysis provides insights on hexavalent chromium induced DNA double strand breaks and their possible repair in midgut cells of Drosophila melanogaster larvae. Mutat Res 747–748:28–39
Howell SL, Taylor KW (1968) Potassium ions and the secretion of insulin by islets of Langerhans incubated in vitro. Biochem J 108(1):17–24
Lakhotia SC, Mukherjee T (1980) Specific activation of puff 93D of Drosophila melanogaster by benzamide and the effect of benzamide treatment on the heat shock induced puffing activity. Chromosoma 81(1):125–136
Phillips HJ (1973) Dye exclusion tests for cell viability. Academic, New York
Porameesanaporn Y, Uthaisang-Tanechpongtamb W, Jarintanan F, Jongrungruangchok S, Thanomsub Wongsatayanon B (2013) Terrein induces apoptosis in HeLa human cervical carcinoma cells through p53 and ERK regulation. Oncol Rep 29(4):1600–1608
Hwang IS, Bae HK, Cheong HT (2013) Mitochondrial and DNA damage in bovine somatic cell nuclear transfer Embryos. J Vet Sci 14(3):235–240
Feany MB, Bender WW (2000) A Drosophila model of Parkinson’s disease. Nat 404(6776):394–398
Sharma A, Mishra M, Shukla AK, Kumar R, Abdin MZ, Kuamar CD (2012) Organochlorine pesticide, endosulfan induced cellular and organismal response in Drosophila melanogaster. J Hazard Mater 221–222:275–287
Wang H, Luo K, Tan LZ, Ren BG, Gu LQ, Michalopoulos G, Luo JH, Yu YP (2012) p53-induced gene 3 mediates cell death induced by glutathione peroxidase 3. J Biol Chem 287(20):16890–16902
Chen X, Zhong Z, Xu Z, Chen L, Wang Y (2010) 2′,7′-Dichlorodihydrofluorescein as a fluorescent probe for reactive oxygen species measurement: forty years of application and controversy. Free Radical Res 44(6):587–604
Jomova K, Valko M (2011) Advances in metal-induced oxidative stress and human disease. Toxicol Res 283(2–3):65–8737
Lim HS, Macfadyen RJ, Lip GY (2004) Diabetes mellitus, the renin-angiotensin-aldosterone system, and the heart. Arch Intern Med 164(16):1737–1748
Wang X, Son YO, Chang Q, Sun L, Hitron JA, Budhraja A, Zhang Z, Ke Z et al (2011) NADPH oxidase activation is required in reactive oxygen species generation and cell transformation induced by hexavalent chromium. Toxicol Sci 123(2):399–410
Owusu-Ansah E, Yavari A, Banerjee U (2008) A protocol for in vivo detection of reactive oxygen species. Nat Prot Ex. doi:10.1038/nprot.2008.23
Nishikimi M, Appaji N, Yagi K (1972) The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem Biophys Res Commun 46(2):849–854
Sinha AK (1972) Colorimetric assay of catalase. Anal Biochem 47(2):389–394
Missirlis F, Ulschmid JK, Hirosawa-Takamori M, Gronke S, Schafer U, Becker K, Phillips JP, Jackle H (2002) Mitochondrial and cytoplasmic thioredoxin reductase variants encoded by a single Drosophila gene are both essential for viability. J Biol Chem 277(13):11521–11526
Ellman GL (1959) Tissue sulfhydryl groups. Arch Biochem Biophys 82(1):70–77
Levine RL, Garland D, Oliver CN, Amici A, Climent I, Lenz AG, Ahn BW, Shaltiel S et al (1990) Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol 186:464–478
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95(2):351–358
Ellman GL, Courtney KD, Andres V Jr, Feather-Stone RM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7:88–95
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193(1):265–275
Ye Y, Lukinova N, Fortini ME (1999) Neurogenic phenotypes and altered Notch processing in Drosophila Presenilin mutants. Nat 398(6727):525–529
Selcho M, Pauls D, Han KA, Stocker RF, Thum AS (2009) The role of dopamine in Drosophila larval classical olfactory conditioning. PLoS One 4(6), e5897
Son YO, Hitron JA, Wang X, Chang Q, Pan J, Zhang Z, Liu J, Wang S et al (2010) Cr(VI) induces mitochondrial-mediated and caspase-dependent apoptosis through reactive oxygen species-mediated p53 activation in JB6 Cl41 cells. Toxicol Appl Pharmacol 245(2):226–235
Zhitkovich A (2011) Chromium in drinking water: sources, metabolism, and cancer risks. Chem Res Toxicol 24(10):1617–1629
Zhitkovich A (2005) Importance of chromium-DNA adducts in mutagenicity and toxicity of chromium(VI). Chem Res Toxicol 18(1):3–11
Clark MJ, Prentice JR, Hoggard N, Paley MN, Hadjivassiliou M, Wilkinson JM (2014) Brain structure and function in patients after metal-on-metal hip resurfacing. Am J Neuroradiol 35(9):1753–1758
Quinteros FA, Poliandri AH, Machiavelli LI, Cabilla JP, Duvilanski BH (2007) In vivo and in vitro effects of chromium VI on anterior pituitary hormone release and cell viability. Toxicol Appl Pharmacol 218(1):79–87
Eastmond DA, Macgregor JT, Slesinski RS (2008) Trivalent chromium: assessing the genotoxic risk of an essential trace element and widely used human and animal nutritional supplement. Crit Rev Toxicol 38(3):173–190
Berger C, Renner S, Luer K, Technau GM (2007) The commonly used marker ELAV is transiently expressed in neuroblasts and glial cells in the Drosophila embryonic CNS. Dev Dyn 236(12):3562–3568
Robinow S, White K (1988) The locus elav of Drosophila melanogaster is expressed in neurons at all developmental stages. Dev Biol 126(2):294–303
Yao KM, Samson ML, Reeves R, White K (1993) Gene elav of Drosophila melanogaster: a prototype for neuronal-specific RNA binding protein gene family that is conserved in flies and humans. J Neurobiol 24(6):723–739
Pereanu W, Shy D, Hartenstein V (2005) Morphogenesis and proliferation of the larval brain glia in Drosophila. Dev Biol 283(1):191–203
Stork T, Bernardos R, Freeman MR (2012) Analysis of glial cell development and function in Drosophila. Cold Spring Harb Protoc 2012(1):1–17
von Hilchen CM, Beckervordersandforth RM, Rickert C, Technau GM, Altenhein B (2008) Identity, origin, and migration of peripheral glial cells in the Drosophila embryo. Mech Dev 125(3–4):337–352
Iwata-Ichikawa E, Kondo Y, Miyazaki I, Asanuma M, Ogawa N (1999) Glial cells protect neurons against oxidative stress via transcriptional up-regulation of the glutathione synthesis. J Neurochem 72(6):2334–2344
Abdo H, Derkinderen P, Gomes P, Chevalier J, Aubert P, Masson D, Galmiche JP, Vanden Berghe P et al (2010) Enteric glial cells protect neurons from oxidative stress in part via reduced glutathione. FASEB J 24(4):1082–1094
Aiyar J, Berkovits HJ, Floyd RA, Wetterhahn KE (1991) Reaction of chromium(VI) with glutathione or with hydrogen peroxide: identification of reactive intermediates and their role in chromium(VI)-induced DNA damage. Environ Health Perspect 92:53–62
Chinta SJ, Andersen JK (2005) Dopaminergic neurons. Int J Biochem Cell Biol 37(5):942–946
Picciotto MR, Higley MJ, Mineur YS (2012) Acetylcholine as a neuromodulator: cholinergic signaling shapes nervous system function and behavior. Neuron 76(1):116–129
Wessler I, Kirkpatrick CJ (2008) Acetylcholine beyond neurons: the non-neuronal cholinergic system in humans. Br J Pharmacol 154(8):1558–1571
Oda Y (1999) Choline acetyltransferase: the structure, distribution and pathologic changes in the central nervous system. Pathol Int 49(11):921–937
Jemec A, Tisler T, Drobne D, Sepcic K, Jamnik P, Ros M (2008) Biochemical biomarkers in chronically metal-stressed daphnids. Comp Biochem Physiol C Toxicol Pharmacol 147(1):61–68
Lee YH, Su SB, Huang CC, Sheu HM, Tsai JC, Lin CH, Wang YJ, Wang BJ (2014) N-acetylcysteine attenuates hexavalent chromium-induced hypersensitivity through inhibition of cell death, ROS-related signaling and cytokine expression. PLoS One 9(9), e108317
Valko M, Morris H, Cronin MT (2005) Metals, toxicity and oxidative stress. Curr Med Chem 12(10):1161–1208
Drechsel DA, Patel M (2010) Respiration-dependent H2O2 removal in brain mitochondria via the thioredoxin/peroxiredoxin system. J Biol Chem 285(36):27850–27858
Lopert P, Day BJ, Patel M (2012) Thioredoxin reductase deficiency potentiates oxidative stress, mitochondrial dysfunction and cell death in dopaminergic cells. PLoS One 7(11), e50683
Hu X, Weng Z, Chu CT, Zhang L, Cao G, Gao Y, Signore A, Zhu J et al (2011) Peroxiredoxin-2 protects against 6-hydroxydopamine-induced dopaminergic neurodegeneration via attenuation of the apoptosis signal-regulating kinase (ASK1) signaling cascade. J Neurosci 31(1):247–261
Chandra S, Pandey A, Chowdhuri DK (2015) MiRNA profiling provides insights on adverse effects of Cr(VI) in the midgut tissues of Drosophila melanogaster. J Hazard Mater 283:558–567
Dinis-Oliveira RJ, Remiao F, Carmo H, Duarte JA, Navarro AS, Bastos ML, Carvalho F (2006) Paraquat exposure as an etiological factor of Parkinson’s disease. Neurotoxicol 27(6):1110–1122
Gilchrist GW, Huey RB, Partridge L (1997) Thermal sensitivity of Drosophila melanogaster: evolutionary responses of adults and eggs to laboratory natural selection at different temperatures. Physiol Zool 70(4):403–414
Jordan KW, Morgan TJ, MacKay TF (2006) Quantitative trait loci for locomotor behavior in Drosophila melanogaster. Genet 174(1):271–284
Ali YO, Escala W, Ruan K, Zhai RG (2011) Assaying locomotor, learning, and memory deficits in Drosophila models of neurodegeneration. J Vis Exp 49, e2504
Li SM, Collins GT, Paul NM, Grundt P, Newman AH, Xu M, Grandy DK, Woods JH et al (2010) Yawning and locomotor behavior induced by dopamine receptor agonists in mice and rats. Behav Pharmacol 21(3):171–181
Diaz-Mayans J, Laborda R, Nunez A (1986) Hexavalent chromium effects on motor activity and some metabolic aspects of Wistar albino rats. Comp Biochem Physiol C 83(1):191–195
Demicheli V, Quijano C, Alvarez B, Radi R (2007) Inactivation and nitration of human superoxide dismutase (SOD) by fluxes of nitric oxide and superoxide. Free Radic Biol Med 42(9):1359–1368
Shainkin-Kestenbaum R, Caruso C, Berlyne GM (1991) Effect of chromium on oxygen free radical metabolism, inhibition of superoxide dismutase and enhancement of 6-hydroxydopamine oxidation. J Trace Elem Electrolytes Health Dis 5(3):197–201
Rottner M, Tual-Chalot S, Mostefai HA, Andriantsitohaina R, Freyssinet JM, Martinez MC (2011) Increased oxidative stress induces apoptosis in human cystic fibrosis cells. PLoS One 6(9), e24880
Acknowledgments
The authors are thankful to the Director, CSIR-Indian Institute of Toxicology Research (CSIR-IITR) for support and Mr. R. Narayan, Mr. Ramesh Chandra, and Mr. Satguru Prasad, Technical Officer, CSIR-IITR for confocal microscopy and Cr analysis, respectively. We thank Dr. W. W. Ja, The Scripps Research Institute, Florida, USA for w 1118, Bloomington Drosophila Stock Center, USA for Elav-GAL4, ChAT-GAL4, Repo-GAL4, and UAS-GFP, Dr. S. C. Lakhotia, Banaras Hindu University, Varanasi, India for Ddc-GAL4:UAS-GFP Drosophila stock, and Dr. W. Neckameyer, St. Louis University, Missouri, USA for Drosophila TH antibody. Financial assistance to PS as SRF (20-12/2009(ii)EU-IV) from University Grants Commission (UGC), New Delhi and DKC from the Council of Scientific and Industrial Research (CSIR), New Delhi (NWP-BSC0103, UNDO) is thankfully acknowledged. IITR communication number is 3359.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Authors have declared no conflict of interest.
Rights and permissions
About this article
Cite this article
Singh, P., Chowdhuri, D.K. Environmental Presence of Hexavalent but Not Trivalent Chromium Causes Neurotoxicity in Exposed Drosophila melanogaster . Mol Neurobiol 54, 3368–3387 (2017). https://doi.org/10.1007/s12035-016-9909-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-016-9909-z