Abstract
Green evolutionary products such as biologically fabricated nanoparticles (NPs) pose a hazard to aquatic creatures. Herein, biogenic silver nanoparticles (AgNPs) were synthesized by the reaction between ionic silver (AgNO3) and aqueous onion peel extract (Allium cepa L). The synthesized biogenic AgNPs were characterized with UV–Visible spectrophotometer, XRD, FT-IR, and TEM with EDS analysis; then, their toxicity was assessed on common carp fish (Cyprinus carpio) using biomarkers of haematological alterations, oxidative stress, histological changes, differential gene expression patterns, and bioaccumulation. The 96 h lethal toxicity was analysed with various concentrations (2, 4, 6, 8, and 10 mg/l) of biogenic AgNPs. Based on 96 h LC50, sublethal concentrations (1/15th, 1/10th, and 1/5th) were given to C. carpio for 28 days. At the end of experiment, the bioaccumulations of Ag content were accumulated mainly in the gills, followed by the liver and muscle. At an interval of 7 days, the haematological alterations showed significance (p < 0.05) and elevation of antioxidant defence mechanism reveals the toxicity of biogenic synthesized AgNPs. Adverse effects on oxidative stress were probably related to the histopathological damage of its vital organs like gill, liver, and muscle. Finally, the fish treated with biogenic synthesized AgNPs were significantly (p < 0.05) downregulates the oxidative stress genes such as Cu–Zn SOD, CAT, GPx1a, GST-α, CYP1A, and Nrf-2 expression patterns. The present study provides evidence of biogenic synthesized AgNPs influence on the aquatic life through induction of oxidative stress.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
An increasing number of technologies are employed for manufacturing the engineered nanomaterials in many scientific and industrial sectors. Nowadays, nanoparticles (NPs) have applications in textiles, electronics, paints, cosmetics, and pharmaceuticals industries [1,2,3,4]. NPs are commonly used for their unique physico-chemical properties, especially metal oxide nanoparticles (MNPs) [5, 6]. Particularly noble metals such as silver, gold, platinum, and palladium have received much attention among scientists due to their high surface ratio and innovative applications [7,8,9]. In recent years, silver nanoparticles (AgNPs) have a new scope in functionalized surfactant and biocompatible [10, 11]. Ag has been used for medical purposes since the beginning of the last century [12,13,14].
Techniques have been developed and adapted as valuable tools to monitor pollution in aquatic environment for the last two decades [15, 16]. Usually, aquatic organisms are quickly and constantly exposed to toxic substances from natural or dissolved anthropogenic sources [17, 18]. The nanomaterials (AgNPs, ZnONPs, CuONPs, TiO2NPs, Fe3O4NPs, and NiNPs) contribute a great deal of aquatic pollution that poses threat to aquatic organisms [19,20,21,22,23,24,25]. Bioaccumulation and biomagnification capacity of nanomaterials (Ag, Cu, Zn, and CdCl2) have longer effects on aquatic species like planktonic crustacean (Daphnia magna) [26], ragworm (Nereis diversicolor) [24], mussel (Mytilus galloprovincialis) [27], fishes (Clarias gariepinus, Oreochromis niloticus, Oncorhynchus mykiss, Danio rerio, Carassius auratus) [28,29,30,31,32,33]. Increasing use of nanomaterials decreases the population of aquatic organisms mainly fishes. According to their intimate dependence on the environment and comparing with other species, fishes are more vulnerable to nanotoxic stress [34, 35]. Heavy metal/nanotoxicants accumulate in fish through biomagnifications and cause changes in their biological mechanisms [28, 36,37,38,39].
Fabrication of NPs can be achieved through different synthesis methods. Conventional (physical and chemical) approaches are the most widespread methods for the synthesis of NPs. However, in chemical approach, the use of toxic chemicals in the synthesis methods is inevitable. Since noble metal NPs are widely exposed to environment, there is a growing need to develop eco-friendly synthesis methods, which do not use toxic chemicals. Biogenic approach of developing AgNPs could reduce the environmental impact and generate minimal waste. Usually, AgNPs are applied in electronics and storage devices, biomedical, etc. [40, 41]. AgNPs are synthesized by several biological sources like bacteria, fungi, algae, and plants with the absence of hazardous materials [22, 42,43,44]. The biogenic AgNPs would be a least toxic and also an eco-friendly approach to inhibit the microbial contaminations in aquaculture environment. In addition, biogenic AgNPs are highly stable during large-scale production of fish feed and have significant antibacterial effects [45]. Biogenic synthesis of AgNPs is cost-effective and suitable for large-scale production in controlled environmental condition to their stability, shape, and size [46, 47]. In recent years, to promote environmental sustainability, nontoxic precursors are used in the development of nanomaterials [48]. Biogenic nanoparticles are the most admired inorganic NPs utilized as an efficient antibacterial, antifungal, antiviral, anti-inflammatory agents, and food additives [49]. Biogenic nanomaterials are nontoxic that increase growth and immune responses in various fishes like Catla catla, Labeo rohita, Cirrhinus mrigala, and Oreochromis niloticus and which plays a crucial role in aquaculture operations [50,51,52,53,54]. Several studies showed that the toxicity of conventional AgNPs depending on particle size, capping or coating agent, binding to DNA, residues of Ag+ ions, and release of reactive oxygen species (ROS) [55,56,57,58,59]. Through various pathways, AgNPs cause severe impacts on living organisms [60, 61]. The dispensable release of AgNPs in the aquatic ecosystem eventually may modify the physico-chemical and biological characteristics of aquatic system resulting in an environmental imbalance [35, 62,63,64].
Despite their number of applications, detailed information on the toxic effect and mechanical action of biogenic AgNPs on fish is limited. Therefore, the current study was to examine the toxicological impacts of biogenic AgNPs on freshwater common carp (Cyprinus carpio). This study further investigates the total accumulation, haematological parameters, and changes in antioxidant enzymes, oxidative stress genes, and histology in fish C. Carpio treated with sublethal concentrations of biogenic AgNPs.
Materials and Methods
Synthesis and Characterization of AgNPs
Using aqueous onion peel extract (Allium cepa L.), AgNPs were synthesized as described in our previous study [65]. Briefly, 5 g of A. cepa peel was cleaned and boiled in 100 ml of double distilled water (dd H2O) at 60 °C for 20 min in 250 ml of Erlenmeyer flask. Ten millilitres (10 ml) of resulting filtrate was mixed drop wise with 100 ml of (0.001 M) AgNO3 solution. The darkish brown colour indicates the formation of AgNPs, which were characterized using UV–Vis spectrophotometer, X-ray diffraction (XRD), fourier transform infrared (FT-IR), and transmission electron microscopy (TEM) with energy dispersive spectrum (EDS).
Animal Maintenance
Freshwater fish Cyprinus carpio was procured from Nathan Fish Farm, Thanjavur, Tamil Nadu, India. The mean body weight and length of the fish were 5.34 ± 0.53 g and 6.06 ± 0.69 cm, respectively. Fish were kept in 2000 l circular water tanks in the Aquarium Facility at Department of Animal Science, Bharathidasan University. The water was changed routinely and its temperature, pH, and dissolved oxygen (DO) were maintained at 29 ± 2 °C, 7.0 ± 0.1, and 6.5 ± 0.5 mg/l, respectively. Fish were allowed to acclimatize for 7 days and fed with commercial pellet feed at ad libitum.
Acute Toxicity Test
After the acclimatization period, fish were randomly divided into six groups in plastic troughs and each group contains 10 fish (Triplicate setup) for the determination of LC50. The acute study was conducted for 96 h according to OECD 203: Fish Acute Toxicity Test [66]. A series of AgNPs suspension (0, 2, 4, 6, 8, and 10 mg/l) (100 W, 53 kHz at 30 min, Misonix Ultrasonicator, USA) was exposed to each group separately. The NPs exposed to water were changed every 24 h to ensure and maintain the concentration of NPs. The experimental fish were fed with 10% of body weight of commercial feed. According to Finney [67], the LC50 values were obtained using probit analysis (SPSS ver. 16.0, IBM, Chicago, IL, USA).
Sublethal Toxicity
For sublethal toxicity, C. carpio fish were separated into four groups; each group consists of 90 fish (3 replicates). The first group was left as control. The suspension of 1/15th, 1/10th, and 1/5th of 96 h LC50 concentrations were prepared by dispersing (100 W, 53 kHz at 30 min, Misonix Ultrasonicator, USA) of AgNPs and exposed to remaining groups (2–4). The total experiment was carried out for 28 days. Optimal parameters were maintained similar to the acclimatization period. Renewal method was carried out daily with the same concentrations of NPs after 30 min of feeding.
Fish Sampling
AgNPs exposed fish were collected for sampling with an interval of every 7 days. Fish were anesthetized by hypothermia method (non-chemical method); then, the blood sample was withdrawn from the dorsal aorta, and the vital organs like the gill, liver, and muscle were dissected out and stored for further analysis at −80 °C.
Analysis of Bioaccumulation
The accumulation of Ag content in the gill, liver, and muscles of fish C. carpio was estimated using slightly modified method of Zhang [68]. Briefly, at the end of the exposure, fish tissue samples (0.5–1 g) were digested by the triple acid digestion method with the mixture of concentrated HNO3, HCl, and H2O2 in the ratio of 3:3:1. Finally, the total suspension (25 ml) was made with dd H2O and stored in polypropylene tubes. The bioaccumulation of Ag in the target tissues was estimated using ICP-OES (Manufacturer: PerkinElmer; Model: Optima 5300 DV, USA; wavelength range 165–782 nm; RF generator 40 MHz; Detection limit: upto ppb level using SCD detector). The obtained results were expressed in µg/g of the tissue analysed.
Haematological Parameters
The blood sample was drawn with 1 ml syringe (30-gauge) at dorsal aorta with an interval of 7 days and immediately transferred into collection tubes (containing EDTA) and subjected to analyse the haematological parameters. Haemoglobin, haematocrit, red blood cells, and white blood cells were evaluated in Mindray BC-2800Vet® automated haematology analyser. The mean cell volume (MCV), mean cell haemoglobin (MCH), and mean cell haemoglobin concentration (MCHC) were calculated by the method of Dacie and Lewis[69].
Measurement of Oxidative Biomarkers
For evaluating the oxidative stress damage, the gill, liver, and muscle tissues were homogenized with cold phosphate buffer (pH 7.4) and centrifuged at 11,200 × g for 10 min and the filtrate was stored at −80 °C until used. Lowry [70] method was adopted to estimate the total tissue protein with BSA as standard. Superoxide dismutase (SOD) activity was analysed by the method of Marklund and Marklund [71]. The measured activity was expressed as unit/mg protein. The activity of catalase (CAT) enzyme was measured by the method of Claiborne [72]. One unit of catalase was defined as 1 μMol of H2O2 consumed/mg protein/min. Rotruck et al. [73] method was followed to identify the glutathione peroxidase (GPx) activity and expressed as μMol consumed/mg protein/min. Habig et al. [74] was used to determine the glutathione-S-transferase (GST) activity and expressed as unit/mg protein. Glutathione (GSH) was analysed by the method of Moron et al. [75] and expressed as μg/mg protein.
Histoarchitecture Analysis
For the analysis of AgNPs toxicity in vivo, the histopathological examinations were done in selected tissues of C. carpio (gill, liver, and muscle) fixed in 10% formalin. The paraffin-embedded tissues were fixed and stained (haematoxylin and eosin). Morphological alterations were observed under a microscope attached with a camera (DM750, Leica Microsystems, Germany).
RNA Extraction and cDNA Synthesis
Total RNA was extracted from liver tissue (~50 mg) of experimental fish using RNAiso Plus Kit (Takara Clontech, India). The purity and concentration of RNA were checked by BioDrop µLITE spectrophotometer. Then, the cDNA were synthesized from the obtained RNA using RevertAid First Strand cDNA Synthesis Kit (Thermo Scientific). Total volume of reverse transcription reaction mixture contains 5 × reaction buffer (4 µl), 10 mM dNTP mix (2 µl), RiboLock RNase inhibitor (1 µl), and RevertAid H minus (1 µl) with 800 ng of RNA. Reverse transcription was performed by the following thermal conditions 25 °C for 5 min, 42 °C for 60 min, and 70 °C for 5 min (Eppendorf MasterCycler® Gradient, Hamburg, Germany). The synthesized cDNA was stored at −20 °C.
Real-Time Quantitative PCR Analysis
RT-qPCR was performed by using a single-step real-time PCR machine (LightCycler® 96, Roche Life Science, USA) with 1X SYBR Green (Takara), 0.5 μl of each primer (Table 1), and 1 μl synthesized cDNA. The RT-qPCR conditions were initial denaturation (95 °C for 5 min), 45 cycles of 3 step amplification (95 °C for 10 s, 48/52 °C for 30 s, and 72 °C for 10 s). Table 1 shows the annealing temperatures of each primer. β-actin served as the internal control to normalize the RNA level. Three technical repeats and experimental replicates were performed for each gene. Threshold cycle (Ct) values were used to quantify the gene expression by 2−∆∆CT method [76].
Statistical Analysis
The data obtained were showed as mean ± standard deviation (SD) for each group. The differences among groups were analysed statistically at p < 0.05 against the control group using one-way Analysis of variance (ANOVA) followed by Duncan’s multiple range (DMRT) as post hoc test (SPSS).
Results and Discussion
Characterization of Biologically Synthesized AgNPs
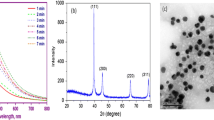
AgNPs optical property was studied by using UV–Vis spectroscopy (Fig. 1a). Herein, the synthesis of AgNPs may be due to the phytochemical constituents present in the peel of onion. It has been already reported that the onion wastes (Allium cepa L.) contain dietary fibre and bioactive compounds [77]. During synthesis, the formation of AgNPs was observed by the colour change due to the excitation of surface plasmon resonance (SPR) in the visible region ranging from 350 to 700 nm. The wavelength region has a typical SPR absorption band of synthesized AgNPs at a wavelength of maxima (λ max) at around 466 nm. The previous study biosynthesized AgNPs showed SPR around at 460 nm [78]. The results were an agreement with earlier study conducted in Dimocarpus Longan Lour. peel extract synthesized AgNPs [79].
The XRD pattern of biogenic AgNPs was indexed completely with the results supporting that the prepared material exhibited and confirmed by JCPDS card no: 03–0921 with a face-centred cubic (fcc) structure of silver (Fig. 1b). The planes of 1 1 1, 2 0 0, 2 2 0, and 3 1 1 were located the 2θ at 38.07°, 46.18°, 64.32°, and 77.35°, respectively, and confirmed the face-centred cubic (fcc) structure of AgNPs, which might have resulted from the bioactive compounds in the onion peel extract. The average particle size of the biogenic synthesized AgNPs was calculated at 33 nm with the Scherrer equation. D = Kʎ/βcosθ, whereas D is particle size, ʎ is X-ray wavelength (0.15426 nm), β is full width at half maximum (FWHM), and θ is Bragg’s angle. In comparison to the previous report [50], the AgNPs particles are slightly smaller, with an XRD modal diameter of 12 nm.
The AgNPs synthesized using brown skin of onion peel extract, which contains different phytochemical constituents such as dietary fibre, phenolics, and flavonoids [77]. These phytochemicals play an important role in the formation and stabilization of AgNPs. The FT-IR spectral peaks at 3429-and 1637 cm−1 assigned to the deformative vibration of water molecules (Fig. 1c). The weak peaks at 2922, 1259, 1546, and 1044 cm−1, corresponding to the stretching vibration of methyl [80], germinal methyl [81], amide I groups proteins [82], and C = O stretching [83]. Characteristics peaks at 801 and 545 cm−1 were attributed to Ag–O stretching vibration [84]. From this functional group analysis, which revealed the presence of flavonoids, phenolics, methyl, amide groups, and proteins was present in the biogenic AgNPs.
TEM micrograph with EDS was given in Fig. 1d. The size of the synthesized NPs was 8–50 nm and exhibited with the agglomerated spherical silver nanoparticles [85]. Ganesh Kumar et al. [86], reported, that the morphology of the NPs were depending on the concentration of reducing agents, which gives the structure to the NPs. The EDS analysis reveals a strong signal at 3 keV (Fig. 1e), which was generally exhibited by metallic Ag nanocrystals due to SPR, the peaks at 0.3 and 0.5 keV showed the un-reacted precursors of AgNO3 and biomolecules [87].
Acute Toxicity of AgNPs
The toxicity of different NPs has been widely studied and used in the fields of biomedical and pharmaceuticals. Due to the increasing production and application of NPs, there is a growing likelihood of occupational and possibility of environmental exposure [88]. NPs in the aquatic ecosystem encounter various changes, such as dissolution, aggregation, oxidation, and bioavailability that cause toxicity to the aquatic species [89]. Nowadays, traditional and biological NPs exhibit attractive characteristics and can trigger several risk factors [90]. The determined LC50 values were used to fix the sublethal concentrations to investigate the chronic effect of biogenic synthesized nanomaterials. No mortality was noted in the control groups; however, the biogenic nanomaterial-treated fish groups showed mortality in the increasing concentrations and 100% mortality was recorded at the maximum tested concentrations (10 mg/l) at 96 h. The 96 h acute exposure of AgNPs synthesized from A. cepa was toxic to C. carpio at the LC50 of 2.76 mg/l. In the earlier study, exposure of AgNPs synthesized using blood serum of sheep showed LC50 at 0.61 mg/l to common carp, which is higher toxicity concentration than the present study [91]. Liaqat et al. [92] analysed 96 h LC50 concentration of AgNPs synthesized using Halymenia porphyraeformis in C. carpio was found to be 0.331 mg/l, which is more toxic than the LC50 value shown in this present investigation. On the other hand, commercially available colloidal AgNPs showed highest mortality at the 48 h LC50 concentration of 0.5 mg/l [93]. Ramachandran et al. [94] reported that the 96 h LC50 of biologically synthesized AgNPs were toxic to adult zebrafish at 24.5 µg/l. A study conducted by Krishnaraj et al. [95] has shown the highest toxicity even on the lowest concentration of AgNPs synthesized using aqueous extract of Malva crispa leaves at 142.2 µg/l to adult zebrafish. Similarly, another study has revealed that fish exposed to AgNPs synthesized using Psidium guajava in zebrafish were found to be 400 µg/l [96]. Aquatic toxicity evaluation may provide insights to the relative sensitivity of different species to AgNPs, which may also provide suitable data on the adverse effect of NPs on aquatic environment, as these species hold important positions in aquatic ecosystems. The toxicity difference in the different species reveals the sensitivity of species distributions. This study indicates that the toxic nature of NPs was not only dependent on the species difference but also depends on the concentration and physio-chemical properties of NPs [97,98,99,100,101].
Sublethal Toxicity Analysis
Following the LC50 determination, 28 days chronic toxicity was analysed. The sublethal concentrations of 1/15th, 1/10th, and 1/5th of the LC50 (2.36 mg/l) of AgNPs (0.184, 0.276, and 0.552 mg/l) were used. No mortality was observed in control and AgNPs treated groups during the experiment.
Bioaccumulation Assay
ICP-OES is a powerful multi-element analyser used to quantify the toxic metals in fish and other aquatic organisms even at subparts per billion because of its high sensitivity [102,103,104]. Total accumulation level of Ag at the end of 28th day in gill, liver, and muscle of C. carpio fish exposed to biogenic AgNPs was shown in Table 2. The biotransformation in the present study observed that the accumulation in all organs was dose-dependent. According to Iversen et al. [105] report, the accumulation of NPs was based on the composition, size, surface charge, and surface coating. Generally, metal uptake is depending upon water which pumped through gills. Significantly accumulated Ag content was observed in the gill, liver, and muscle when comparing with control fish. In this study, the accumulation was observed in the following order of gill > liver > muscle in AgNPs exposed C. carpio fish. Generally, gills are in direct contact with aquatic environment and are physiologically complex and vulnerable structures that makes them easily target organ for waterborne toxicants [106]. In addition, AgNPs can accumulate and bind with gills by transportation process, which affects the ability of fish respiration to hypoxic (low oxygen) conditions, and passes through blood stream to liver tissue leading to oxidative stress. Generally, fish have various transportation systems to maintain their required mineral levels for a wide range of metabolic pathways, either by regulating the absorption of minerals through their diet [1]. Results of another study confirmed the highest accumulation of Zn was observed in gills after exposure of ZnONPs [107, 108]. These findings are comparable with previous studies that have shown the bioaccumulation of Ag in various vital organs of O. mykiss [109, 110]. The study of possible uptake of TiO2NPs showed that the gill, intestine, and brain have higher level of Ti content, and the least amount of Ti was accumulated in the muscle of the goldfish (C. auratus) [33]. Liver is known to be one of the vital organs in fish and have detoxification process when exposed to contamination via excretion. Thus, a lower content of Ag elimination in the liver of common carp may be due to the binding of the metallothionein-like proteins produced in these tissues and its eventual detoxification and storage in them; hence, suggesting that Ag is excreted gradually and slowly in the liver tissues in comparison to the gills [111]. The accumulation of NPs in fish could occur due to the exposure of NPs for longer times [112]. This was most pronounced in fish treated with higher concentrations of AgNPs [113, 114]. The influence of different metals induces toxicity and it might have been caused by the structural variabilities of the particles, potential adsorption by tissues, and physiological and toxicological responses by organisms. Moreover, the accumulation of nanomaterials in common carp was depending on the target organ, concentration, and duration of exposure [106].
Haematological Analysis
Haemoglobin (Hb) and haematocrit (Hct) content in control and AgNPs treated fish were illustrated in Fig. 2a, b. Hb and Hct content in the AgNPs treated fish was significantly decreased (p < 0.05) on all sampling days except the 14th day, which showed the higher content of Hb at 0.184 mg/l compared to control. Exposure of AgNPs on O. niloticus significantly decreased the values of erythrocyte count, Hct and Hb compared to the control groups [115]. Reduced values of haematological parameters were observed by Dhanapakiam and Ramasamy [116] after exposing Cu for 30 days in C. carpio were due to the destruction of erythrocytes. Similar to the present findings, Alkaladi et al. [117] and Rather et al. [52] found a decreased value of Hct in fish exposed to varying sublethal levels of ZnONPs and AgNPs, respectively. Similar observations were also reported in C. catla treated with zinc and cadmium [118], arsenic [119], and O. niloticus treated with cadmium [28]. A decline in the Hct level suggests anaemia or destruction of the erythrocyte membrane, resulting in haemodilution [120,121,122].
The erythrocyte count of control and fish exposed to sublethal concentrations of AgNPs were illustrated in Fig. 2c. The RBC in the AgNPs treated fish was decreased significantly (p < 0.05) when comparing with control. This phenomenon was more evident in fish treated with higher sublethal concentrations of AgNPs. Reduction in RBC may also be due to a decline in haematopoiesis [123, 124]. Exposure of NPs causes respiratory dysfunction through gill damage and eventually alters the RBC values [125]. Farmen et al. [126] have reported that the RBC count and haemoglobin values significantly decreased, resulting in macrocytic anaemia in Atlantic salmon exposed to AgNPs. A similar reduction of RBC was reported in Oreochromis mossambicus treated with Cu [127], O. mykiss treated with CuNPs [128], L. rohita treated with waterborne Fe2O3 NPs [129], and ZnONPs treated O. niloticus [117].
In the present investigation, significant increase (p < 0.05) in white blood cells (WBC) count was observed in the AgNPs -treated fish than control (Fig. 2d). In the higher sublethal concentration (0.276 and 0.552 mg/l), the WBC count was decreased significantly, whereas the lower concentration (0.184 mg/l) showed a maximum increase. Due to the nano-toxicity, the WBCs respond immediately to the medium alteration. This shows that the fish can build a defensive mechanism to withstand nanotoxic stress. The increase in WBC may be due to induced proliferation as a result of the nano-toxicity of pluripotential hematopoietic cells, which, in effect and may result from decreased circulation between differentiated cells [130, 131]. In this study, increased WBC counts suggest the stress of the fish caused by nano-toxicants, which may have caused hypoxia and gill damages.
The MCV, MCH, and MCHC levels of AgNPs treated fish were illustrated in Fig. 2e–g, respectively. RBC index level in the treated fish was significantly altered (p < 0.05) throughout the experiment. This was most pronounced in fish exposed to the sublethal concentrations of AgNPs. The present results confirmed the alteration of haematological indices as a non-specific immune response to NPs toxicity. Similar results were found in C. mrigala exposed to AgNPs [52] and O. niloticus exposed to ZnONPs [117]. Exposure of nanomaterials may disrupt the iron absorption in intestine or hematopoietic tissues and increases the destruction rate of RBC due to osmoregulatory dysfunction [132].
Oxidative Biomarkers
The activity of antioxidant enzyme SOD in the gills of AgNPs was shown in Fig. 3a. AgNPs significantly altered (p < 0.05) the SOD levels in the gill tissues of AgNPs exposed groups comparing control. In contrast, 7th day treatment showed a similar response except for 0.184 mg/l of AgNPs treated fish gill. On 14thth day of AgNPs exposure significantly enhanced the enzyme activity at 0.552 mg/l compared with control and other tested concentrations. On 21stst day exposure, there was a significant decrease in AgNPs exposed groups. On 28th day, levels of SOD were decreased in 0.276 and 0.552 mg/l. The liver and muscle SOD activity were increased significantly throughout the study period compared with the control (Fig. 3b and c). C. carpio exposed to 0.552 mg/l AgNPs showed a significant increase in muscle SOD activity up to 21st day and significantly decreased on 28th day. Lower and intermediate concentrations of AgNPs induced a significant alteration. These results indicated the generation of O•−2 in the tissues of C. carpio and demonstrated that AgNPs induced oxidative stress after chronic exposure. In another study, similar findings were reported by Pirsaheb et al. [133], a significant decrease in activity of SOD was observed in gills of C. carpio exposed to metal-doped TiO2NPs and the mechanism behind this, is the metallic nature of NPs and the presence of transition metals encourages the production of ROS leading to oxidative stress. For maintaining cell homeostasis and preventing oxidative stress, SOD catalyses superoxide anion radical dismutation by forming less-reactive molecular oxygen [134]. A similar response was observed in O. mossambicus exposed to AgNPs [135] and juvenile C. auratus exposed to fullerene C60 [136].
Antioxidant enzyme activity of C. carpio exposed to sublethal concentrations of AgNPs. a Gill SOD. b Liver SOD. c Muscle SOD. d Gill CAT. e Liver CAT. f Muscle CAT. g Gill GPx. h Liver GPx. i Muscle GPx. j Gill GST. k Liver GST. l Muscle GST. m Gill GSH. n Liver GSH. o Muscle GSH. Each value represents the mean ± SD; n = 3. Different letters above the bars show a significant difference among the groups at p < 0.05
CAT activity in the gill was found to be increased in AgNPs treated groups comparing the control, whereas the gill CAT activity was decreased significantly on 14th day (0.552 mg/l of AgNPs) (Fig. 3d). In this present study, the liver and muscle of AgNPs treated fish showed significantly decreased CAT activity throughout the study period compared with control (Fig. 3e and f). Excessive productions of ROS from SOD catalytic activity inhibits the CAT function [137,138,139,140]. Several studies have also demonstrated a biphasic tendency in CAT activity in fish after treating with increasing levels of TiO2 in O. niloticus and C. Carpio [141,142,143].
Comparing with control, significant increase (p < 0.05) in gill GPx activity was observed throughout the experiment period except on 14thth day (Fig. 3g). GPx activity in the liver decreased significantly in all the concentrations of AgNPs after 7th day exposure compared with control fish (Fig. 3h). AgNPs induced a biphasic trend elevation of GPx in the muscle on all sampling days compared with its control (Fig. 3i). A maximum elevation of muscle GPx was observed on 28th day experiment in (0.552 mg/l) of AgNPs. Reduced intracellular GSH levels or toxicity of nanomaterials on enzyme correlate with the reduction or induction of GPx activity. Likewise, Wu and Zhou [144] found significant alternations in GPx activity of fish Oryzias latipes exposed to AgNPs. Elimination of hydroperoxides by GPx through the reduction of GSH alters the function [145]. In response to CuONPs exposure, GPx activity was stimulated in the liver and gill of O. niloticus to counteract hydroperoxides [31].
The GST activity in the gill was significantly increased on all the sampling days except on 7th day of treatment, and significantly decreased activity was observed on 14th day experiment (0.552 mg/l) (Fig. 3j). Induced activity of GST is an adaptive mechanism for neutralizing the impacts of nanomaterials. Lee et al. [146] observed increased activity of GST in tissues of C. carpio treated with various concentrations of AgNPs and suggested that GST enzyme is induced to protect the fish against the NPs toxicity. The increase in GST activity in gills could be a good marker for compensatory tissue response against to toxicant exposure. The antioxidant enzyme activity of GST in the liver tissues exposed to AgNPs was shown in Fig. 3k. AgNPs exposure significantly decreased the liver GST activity throughout the experiment period except for the 7th day compared with its control. Significantly decreased (p < 0.05) muscle GST activity was observed throughout the experiment (Fig. 3l). Stimulated activity of GST in Brycon amazonicus fish indicates its significance against ROS [147]. Similar induced activity of GST was reported by Chae et al. [148] after studying the effects of AgNPs in Japanese medaka (Oryzias latipes). Increased activity of GST reveals ROS generation due to the existence of nanomaterials in fish [149]. The altered antioxidant defense mechanism exhibits imbalanced oxidant and antioxidant levels in the tissues [150, 151].
A maximum elevation of gill GSH was observed on 14th day (0.276 mg/l) and a minimum of decreased activity was found on the same day at 0.552 mg/l concentration (Fig. 3m). The changes of liver GSH activities in the AgNPs exposed groups were shown in Fig. 3n. It was found that GSH activity was varied with the exposure concentration and different times in this study. The change in the levels of GSH is inter-related with the activity of GST. According to Reddy et al. [152], increased glutathione reductase activity is a result of pro-oxidant system which forms reduced GSH by recycling oxidized GSH. Increased activity of GSH level was noted in fish (Lateolabrax japonicus) after treated with benzo[a]pyrene by Jifa et al. [153]. In muscle, the AgNPs treatment significantly decreased the elevation of GSH activity throughout the experiment period compared with the control group (Fig. 3o). AgNPs exposure depleted the glutathione activity in cells associated with increasing ROS levels [154, 155]. The ability of NPs can induce the toxicity to fish by several factors such as their concentration, the exposure period, accessibility to the target site, and distribution in organism’s tissues and kind of species [156].
Histological Observations
Histopathological analysis of fish tissues has been used as a tool that is essential for evaluating the quality of aquatic water bodies [157,158,159]. Gills are vital organs used for many physiological functions [160]. Typical gills of the untreated fish indicated a neat architecture of primary and secondary lamellae, mucous cells, pillar cells, epithelial cells, and central venous sinus, which could be differentiated easily (Fig. 4a–d). The AgNPs treated (0.184 and 0.276 mg/l) fish gill showed lamellar blood sinus constricts, leukocyte infiltration of epithelium and fusion of lamellae were noticed (Fig. 4e–l), whereas 0.552 mg/l of AgNPs treated fish showed severely damaged primary and secondary lamellae, complete fusion of lamellae, epithelial necrosis, epithelial desquamation, aneurysm, epithelial lifting, hyperplasia, thickening of primary lamellae, and curved lamellae (Fig. 4m–p). Damages in the gill are a chain-like process induced by toxicants, leading to respiratory disease [161]. Histopathological changes in fish gills when exposed to a toxicant lead to toxicity by direct and indirect. Necrosis and degeneration of lamellae were directly affected by toxicants, whereas indirect mode is based on the epithelial lifting, hyperplasia [162]. Alterations, such as epithelial hyperplasia, fusion of lamellae, shortening of lamellae, and aneurysm, can be considered as adaptive responses of fish when exposed to toxicants [163]. In addition, the aneurism in the gills represents the blood-filled and swelling blood vessel may lead to disturbances in blood flow in the gills, increase risk of disagreement and result in severe haemorrhage and bleeding or death [164]. Lamellar fusion, hypertrophy, hyperplasia, and thickening of lamellae are considered as defense responses, widely studied in fish, and can prevent the toxicants from the bloodstream. In the present study, the severity of histo-morphological changes in the gill of common carp was concentration dependent, so that the fish treated with higher concentration of AgNPs showed more intense damages. Most important observed damages were fusion of secondary lamellae, shortening of lamellae, hyperplasia, and aneurism. These histo-morphological differences were defense mechanisms against toxic substances to protect from further damages [108].
Light microscopic structural analysis of gill tissue of freshwater fish C. carpio exposed to AgNPs. (× 40, H & E). a, b, c, and d are control gills of 7th, 14th, 21stst, and 28th days, respectively, showing MC — mucus cells, PL — primary lamellae, SL — secondary lamellae, EC — epithelial cells, PC — pillar cells, and red star mark — central venous sinus. The other photo plates (e-p) showing the alterations of AgNPs treated gill tissue structures such as CF — complete fusion, ED — epithelial desquamation, EN – epithelial necrosis, FL — fusion of lamellae, NSC — naked supporting cartilage, H/HP — hyperplasia, SSL — shortening of secondary lamellae, FCL — fully collapsed lamellae, LI — leukocyte infiltration of epithelium, LBSC — lamellar blood sinus constricts, A — aneurism, SPL — splitting of primary lamellae, DSL — disintegrating secondary lamellae, FC — complete fusion of lamellae, CL — curved lamellae, EL — epithelial lifting, black star mark — thickening of primary lamellae, D — debris, and CCSL — completely collapsed secondary lamellae. Scale bars are 50 μm
Exposure of fish to nanomaterials contributes to enter into the digestive tract and respiratory organs then distributed to all over the body through blood circulation and deposited eventually in the liver, which is considered as an essential metabolic organ that detoxifies toxic matters [165,166,167,168,169]. Histological examination of the control liver showed the normal hepatocytes with sinusoids and nucleus (Fig. 5a–d). Our results showed that waterborne AgNPs (0.184 mg/l) induced blood congestion, karyolysed hepatocytes, infiltrating erythrocytes in the liver (Fig. 5e–h). Moreover, cloudy degradation of hepatocytes, kupffer cells, karyolysis, necrotic pancreatic tissue, and nuclear alteration were noticed in the liver of 0.276 mg/l AgNPs treated fish (Fig. 5i–l). However, 0.552 mg/l AgNPs treated liver has different structural characters like karyolysis, kupffer cells, dilated sinusoids, necrosis, cytoplasmic vacuolation, and aggregation of melanoacrgophages (Fig. 5m–p). An induced proliferation of endoplasmic membranes may cause alterations in the liver, biotransformation, and metabolism of intracellular enzymes [160, 170, 171]. Among the changes, erythrocyte infiltration is an initial immunological fish defense system because of damages caused by toxicants [172]. Similar alterations were reported by Rodrigues et al. [173] in O. mykiss and Cunha and de Brito-Gitirana [174] in D. rerio.
Light microscopic structural analysis of liver tissue of freshwater fish C. carpio exposed to AgNPs. (× 40, H & E).a, b, c, and d are control gills of 7th, 14th, 21stst and 28th days, respectively, showing H — hepatocytes, N — nucleus, and S — sinusoids. The other photo plates (e–p) showing the alterations in the liver tissue structures such as HC — hepatocyte congestion, CDH — cloudy degradation of hepatocytes, KC — kupffer cells, KL/K — karyolysis, V — cytoplasmic vacuolation, BC — blood congestion, PN — pancreatic nucleus, KH — karyolysed hepatocytes, PNZ — pancreatic necrotic zone, NPT — necrotic pancreatic tissue, CBV — congestion of blood vessels, IE — infiltrating erythrocytes, NA — nuclear alteration, KAC — karyolysed aciner cell, MA — melano-macrophage aggregates, DS — dilated sinusoids, N — necrosis, and EN — enlarged nucleus. Scale bars are 50 μm
In this study, the standard histological structure of the muscle shows an elegant assembly of bundles of muscle fibres, sarcolemma, nucleus, and connective tissue. The fibres appeared irregular with nuclei as black dots in section. The entire muscle mass was covered in a dense collagenous tissue sheath called epimysium. As the name implies, they also had many cross striations (Fig. 6a–d). Fish exposed to AgNPs (0.184 mg/l) revealed several tissue alterations, namely, degradation of muscle bundle, pyknotic nuclei, and fragmented fibre (Fig. 6e–h). Several alterations in muscle cytoplasm and degeneration of muscle bundle may be related to oxidative stress in the target tissue caused by generated ROS through nanomaterials exposure [175, 176]. However, vacuolar degradation in muscle, necrosis in muscle fibre, bend muscle fibre, necrotic zone, and degeneration endomysium were also observed in 0.279 mg/l AgNPs treated fish (Fig. 6i–l). Similar changes were detected in 0.552 mg/l AgNPs treated fish muscle with increased eosinophilia of the cytoplasm, intracellular space, lesion of striated muscle, shrinkage of muscle fibre, and tissue debris (Fig. 6m–p). Histological changes might have occurred as a toxicity response to the increased accumulation of toxicants in the muscle tissues. Maharajan et al. [177] reported cytoplasmic degeneration, damaged epithelium, hydropic swelling of hepatocytes, blood congestion, nuclear pyknosis, cytoplasmic vacuolation, and nuclear degeneration, accumulation of dark granules, and cellular necrosis were the responses of copper exposure to Asian sea bass (Lates calcarifer).
Light microscopic structural analysis of muscle tissue of freshwater fish C. carpio exposed to AgNPs. (× 40, H & E). a, b, c, and d are control gills of 7th, 14th, 21st and 28thth days, respectively, showing MF — muscle fibre, S — sarcolemma, CT — connective tissue, N — nucleus, MB — muscle bundle, SM — striated muscle. The other photo plates (e–p) showing the alterations in the liver tissue structures such as MFN — muscle fibre necrosis, FF — fragmented fibre, DMB — degradation of muscle bundle, PN — pyknotic nuclei, VDM — vacuolar degradation in muscle, N — necrosis, NMF — necrotic muscle fibre, NZ — necrotic zone, CD — cellular debris, BMF — bend muscle fibre, DMT — degradation of muscle tissue, DMF — degradation of muscle fibre, IS — intracellular space, DE — degenerating endomysium, BMF — bend muscle fibre, ICE & P — increased eosinophilia in the cytoplasm and pyknotic nuclei, SMF — shrinkage of muscle fibre, and LSM — lesion of striated muscle. Scale bars are 50 μm
Expression of Oxidative Stress Response Genes
It is well established that the induction of oxidative damage is an underlying mechanism of nanomaterials induced toxicity in the biological systems. The release of free radicals changes various signalling pathways causing apoptosis. The present study investigated the changes in the transcript level of genes (Cu–Zn SOD, CAT, GPx1a, GST-α, CYP1A, and Nrf-2) after the exposure of AgNPs, by adopting RTqPCR method. The expression of Cu–Zn SOD was found to be downregulated in fish exposed to AgNPs (Fig. 7a). Cu–Zn SOD is an important antioxidant defense system of all living organisms [178]. AgNPs significantly decreased the Cu–Zn SOD gene expression levels after 28 days of exposure. When the oxidant insult is produced, tissues can counteract and activate the Cu–Zn SOD gene expression more smoothly. Transcript levels of the CAT gene were downregulated in all the treatment conditions of AgNPs exposure (Fig. 7b). In the present study, AgNPs exposure downregulated the Cu–Zn SOD and CAT gene expression, which might be partially disturbed by other signalling factors in fish liver tissue [179].
Effect of AgNPs exposure on expression of genes involved in oxidative stress in the liver of C. carpio after 28 days exposure. a Cu–Zn SOD. b CAT. c GPx1a. d GST-α. e CYP1A. f Nrf-2. Each value represents the mean ± SD; n = 3. Different letters above the bars show a significant difference among the groups at p < 0.05
The AgNPs exposure shows a similar response between control and higher concentration in GPx1a mRNA transcript level (Fig. 7c). The tissue-specific response might partially explain the expression of GPx1a under oxidative stress conditions. Increased level of GPx1a mRNA in the liver indicates its mechanism for compensating the damages caused by nanomaterials [180]. GPx1a is a unique enzyme which catalyses membrane phospholipid hydroperoxides [181]. Therefore, it is speculated that the liver requires increased GPx1a de novo synthesis to metabolize AgNPs. However, higher level of ROS resulting from the AgNPs exposure inactivates or decreases GPx1a activity.
The transcript levels of GST-α were downregulated in AgNPs exposed fish (Fig. 7d). GST-α plays a crucial role in detoxifying various types of toxicants and protects the DNA damage from ROS-mediated oxidative stress. Lee et al. [146] observed a similar response in C. carpio when exposed to 200 µg/l of AgNPs. GST-α gene expression might be a valuable tool for evaluating oxidative stress [182, 183]. Downregulation of GST-α indicates the failure of the defence system to counteract the increased ROS generation.
The expression of CYP1A was found to be downregulated in fish exposed to AgNPs (Fig. 7e). CYP1A enzyme controls the biotransformation of several toxic substances. Nanomaterial-related elevations of the CYP1A gene may be used as biomarkers for the metal detoxification process. Mostly detoxification is occurred in the microsomal mono-oxygenase enzyme system and dependent on the heme protein, which located in liver and other organelles of fish [184]. According to Oliva et al. [185] report, the activity of CYP1A varies in different metal contaminations.
Various cell-signalling pathways regulate antioxidant enzyme gene transcription mechanism [186]. The Nrf-2 signalling is an important expression factor for antioxidant enzyme genes in higher and lower vertebrates [187, 188]. Overlapping expression of Nrf-2 was observed in fish following exposure to AgNPs (Fig. 7f); transcript levels of Nrf-2 in fish treated with 0.184 and 0.552 mg/l concentration of AgNPs were downregulated, whereas 0.276 mg/l exposure showed enhanced Nrf-2 expression in the liver. Biogenic AgNPs stimulated the H2O2 production, which increased the expression of Nrf-2 mRNA [189]. This phase 2 detoxifying enzyme removes potential materials by converting them to harmless compounds and then eliminated them from the body [190]. There are mismatch alterations between antioxidant enzymes and antioxidant gene expression levels that might be the effect of time-lag after transcription and translation. When the antioxidant mechanism could not reduce or eliminate the excessive production of ROS, it can increase the risk of oxidative stress. These impacts may degrade the enzymes or reduce the activity [191, 192].
Conclusion
Recent reports clearly stated that biogenic nanomaterials are less toxic than chemically prepared nanomaterials. However, the present study demonstrated that biogenic AgNPs showed toxicity to freshwater common carp C. Carpio in a concentration dependent manner. Bioaccumulation analysis showed a maximum accumulation of Ag in the gills compared with other organs, indicating the dose-dependent activity and availability of target tissues. A significant alteration was noticed in haematological parameters during sublethal exposure periods. Phytogenic AgNPs potentially caused oxidative stress by either stimulating or inhibiting the SOD, CAT, GPx, GST, and GSH activities of antioxidant defence systems. Histological observations reveal the damages of C. carpio tissues caused by AgNPs released into the aquatic ecosystems and may pose a risk to aquatic life. The expression levels of the antioxidant defence system of C. carpio were downregulated. It is known that the induced activities of Cu–Zn SOD, CAT, GPx1a, GST-α, CYP1A, and Nrf-2, serve as a protective mechanism to overcome the free radicals. The present work indicated that the toxicity evaluation tools might be considered as potential biomarkers for assessing the health status of fish and the data obtained in this regard provide substantial information to sustain the quality of the aquatic bodies.
Data Availability
Data and materials are available for all authors and included in this published article.
Change history
19 March 2022
A Correction to this paper has been published: https://doi.org/10.1007/s12011-022-03194-7
References
Fabrega J, Luoma SN, Tyler CR et al (2011) Silver nanoparticles: behaviour and effects in the aquatic environment. Environ Int 37:517–531. https://doi.org/10.1016/j.envint.2010.10.012
Jeevanandam J, Barhoum A, Chan YS et al (2018) Review on nanoparticles and nanostructured materials: history, sources, toxicity and regulations. Beilstein J Nanotechnol 9:1050–1074. https://doi.org/10.3762/bjnano.9.98
Shen Z, Wu A, Chen X (2017) Iron oxide nanoparticle based contrast agents for magnetic resonance imaging. Mol Pharm 14:1352–1364. https://doi.org/10.1021/acs.molpharmaceut.6b00839
Senthilkumar N, Sharma PK, Sood N, Bhalla N (2021) Designing magnetic nanoparticles for in vivo applications and understanding their fate inside human body. Coord Chem Rev 445:214082. https://doi.org/10.1016/j.ccr.2021.214082
Montes MO, Hanna SK, Lenihan HS, Keller AA (2012) Uptake, accumulation, and biotransformation of metal oxide nanoparticles by a marine suspension-feeder. J Hazard Mater 225–226:139–145. https://doi.org/10.1016/j.jhazmat.2012.05.009
Alavi M, Varma RS (2021) Phytosynthesis and modification of metal and metal oxide nanoparticles/nanocomposites for antibacterial and anticancer activities: recent advances. Sustain Chem Pharm 21:100412. https://doi.org/10.1016/j.scp.2021.100412
Giráldez-Pérez RM, Grueso E, Domínguez I et al (2021) Biocompatible DNA/5-fluorouracil-gemini surfactant-functionalized gold nanoparticles as promising vectors in lung cancer therapy. Pharmaceutics 13:423. https://doi.org/10.3390/pharmaceutics13030423
García-Fernández C, Fornaguera C, Borrós S (2020) Nanomedicine in non-small cell lung cancer: from conventional treatments to immunotherapy. Cancers (Basel) 12:1609. https://doi.org/10.3390/cancers12061609
Goudarzi F, Asadi A, Afsharpour M, Jamadi RH (2018) In vitro characterization and evaluation of the cytotoxicity effects of Nisin and Nisin-loaded PLA-PEG-PLA nanoparticles on gastrointestinal (AGS and KYSE-30), hepatic (HepG2) and blood (K562) cancer cell lines. AAPS PharmSciTech 19:1554–1566. https://doi.org/10.1208/s12249-018-0969-4
Chang K-B, Shen C-C, Hsu S et al (2021) Functionalized collagen-silver nanocomposites for evaluation of the biocompatibility and vascular differentiation capacity of mesenchymal stem cells. Colloids Surfaces A Physicochem Eng Asp 624:126814. https://doi.org/10.1016/j.colsurfa.2021.126814
Ko SW, Lee JY, Rezk AI et al (2021) In-situ cellulose-framework templates mediated monodispersed silver nanoparticles via facile UV-light photocatalytic activity for anti-microbial functionalization. Carbohydr Polym 269:118255. https://doi.org/10.1016/j.carbpol.2021.118255
Fung MC, Bowen DL (1996) Silver products for medical indications: risk-benefit assessment. J Toxicol - Clin Toxicol 34:119–126
Alexander JW (2009) History of the medical use of silver. Surg Infect (Larchmt) 10:289–292. https://doi.org/10.1089/sur.2008.9941
Barillo DJ, Marx DE (2014) Silver in medicine: a brief history BC 335 to present. Burns 40:S3–S8. https://doi.org/10.1016/j.burns.2014.09.009
Kahru A, Ivask A (2013) Mapping the dawn of nanoecotoxicological research. Acc Chem Res 46:823–833. https://doi.org/10.1021/ar3000212
Khan B, Ho KT, Burgess RM (2020) Application of biomarker tools using bivalve models toward the development of adverse outcome pathways for contaminants of emerging concern. Environ Toxicol Chem 39:1472–1484. https://doi.org/10.1002/etc.4757
Wang L, Wu WM, Bolan NS et al (2021) Environmental fate, toxicity and risk management strategies of nanoplastics in the environment: current status and future perspectives. J Hazard Mater 401:123415. https://doi.org/10.1016/j.jhazmat.2020.123415
Genchi G, Carocci A, Lauria G et al (2020) Nickel: human health and environmental toxicology. Int J Environ Res Public Health 17:679. https://doi.org/10.3390/ijerph17030679
Ağçeli GK, Hammachi H, Kodal SP et al (2020) A Novel approach to synthesize TiO2 nanoparticles: biosynthesis by using Streptomyces sp. HC1. J Inorg Organomet Polym Mater 30:3221–3229. https://doi.org/10.1007/s10904-020-01486-w
Ajmal N, Saraswat K, Bakht MA et al (2019) Cost-effective and eco-friendly synthesis of titanium dioxide (TiO2) nanoparticles using fruit’s peel agro-waste extracts: characterization, in vitro antibacterial, antioxidant activities. Green Chem Lett Rev 12:244–254. https://doi.org/10.1080/17518253.2019.1629641
Alavi M, Karimi N (2019) Ultrasound assisted-phytofabricated Fe3O4 NPs with antioxidant properties and antibacterial effects on growth, biofilm formation, and spreading ability of multidrug resistant bacteria. Artif Cells, Nanomedicine, Biotechnol 47:2405–2423. https://doi.org/10.1080/21691401.2019.1624560
Alavi M, Kennedy JF (2021) Recent advances of fabricated and modified Ag, Cu, CuO and ZnO nanoparticles by herbal secondary metabolites, cellulose and pectin polymers for antimicrobial applications. Cellulose 28:3297–3310. https://doi.org/10.1007/s10570-021-03746-5
Gaiser BK, Biswas A, Rosenkranz P et al (2011) Effects of silver and cerium dioxide micro- and nano-sized particles on Daphnia magna. J Environ Monit 13:1227–1235. https://doi.org/10.1039/c1em10060b
García-Alonso J, Khan FR, Misra SK et al (2011) Cellular internalization of silver nanoparticles in gut epithelia of the estuarine polychaete nereis diversicolor. Environ Sci Technol 45:4630–4636. https://doi.org/10.1021/es2005122
Walters CR, Cheng P, Pool E, Somerset V (2016) Effect of temperature on oxidative stress parameters and enzyme activity in tissues of Cape River crab (Potamanautes perlatus) following exposure to silver nanoparticles (AgNP). J Toxicol Environ Heal - Part A Curr Issues 79:61–70. https://doi.org/10.1080/15287394.2015.1106357
Heinlaan M, Kahru A, Kasemets K et al (2011) Changes in the Daphnia magna midgut upon ingestion of copper oxide nanoparticles: a transmission electron microscopy study. Water Res 45:179–190. https://doi.org/10.1016/j.watres.2010.08.026
Ale A, Liberatori G, Vannuccini ML et al (2019) Exposure to a nanosilver-enabled consumer product results in similar accumulation and toxicity of silver nanoparticles in the marine mussel Mytilus galloprovincialis. Aquat Toxicol 211:46–56. https://doi.org/10.1016/j.aquatox.2019.03.018
Al-Asgah NA, Abdel-Warith AWA, Younis ESM, Allam HY (2015) Haematological and biochemical parameters and tissue accumulations of cadmium in Oreochromis niloticus exposed to various concentrations of cadmium chloride. Saudi J Biol Sci 22:543–550. https://doi.org/10.1016/j.sjbs.2015.01.002
Al-Bairuty GA, Shaw BJ, Handy RD, Henry TB (2013) Histopathological effects of waterborne copper nanoparticles and copper sulphate on the organs of rainbow trout (Oncorhynchus mykiss). Aquat Toxicol 126:104–115. https://doi.org/10.1016/j.aquatox.2012.10.005
Duran S, Tuncsoy M, Ay O et al (2017) Accumulation of copper oxide nanoparticles in gill, liver and muscle tissues of Clarias gariepinus. Toxicol Lett 280:S186. https://doi.org/10.1016/j.toxlet.2017.07.522
Tunçsoy M, Duran S, Ay Ö et al (2017) Effects of copper oxide nanoparticles on antioxidant enzyme activities and on tissue accumulation of Oreochromis niloticus. Bull Environ Contam Toxicol 99:360–364. https://doi.org/10.1007/s00128-017-2129-z
Griffitt RJ, Weil R, Hyndman KA et al (2007) Exposure to copper nanoparticles causes gill injury and acute lethality in zebrafish (Danio rerio). Environ Sci Technol 41:8178–8186. https://doi.org/10.1021/es071235e
Ates M, Demir V, Adiguzel R, Arslan Z (2013) Bioaccumulation, subacute toxicity, and tissue distribution of engineered titanium dioxide nanoparticles in goldfish (Carassius auratus). J Nanomater 2013:1–6. https://doi.org/10.1155/2013/460518
Jurgelėnė Ž, Stankevičius M, Stankevičiūtė M et al (2021) Imaging of the internal chorion structure of rainbow trout Oncorhynchus mykiss live embryos and the distribution of quantum dots therein: towards a deeper understanding of potential nanotoxicity. Sci Total Environ 785:147302. https://doi.org/10.1016/j.scitotenv.2021.147302
Pérez S, la Farré M, Barceló D (2009) Analysis, behavior and ecotoxicity of carbon-based nanomaterials in the aquatic environment. TrAC - Trends Anal Chem 28:820–832. https://doi.org/10.1016/j.trac.2009.04.001
Bat L, Sezgin M, Üstün F, Şahin F (2012) Heavy metal concentrations in ten species of fishes caught in Sinop coastal waters of the Black Sea, Turkey. Turkish J Fish Aquat Sci 12:371–376. https://doi.org/10.4194/1303-2712-v12_2_24
Krishna D, Sachan HK (2021) Nano-toxicity and aquatic food chain. In: Singh P, Singh R, Verma P, Bhadouria R, Kumar A, Kaushik M. (ed) Plant-Microbes-Engineered Nano-particles (PM-ENPs) Nexus in Agro-Ecosystems. Advances in science, technology and innovation. (IEREK Interdisciplinary Series for Sustainable Development). Springer, Cham.189–198. https://doi.org/10.1007/978-3-030-66956-0_13
Mallik A, Xavier KAM, Naidu BC, Nayak BB (2021) Ecotoxicological and physiological risks of microplastics on fish and their possible mitigation measures. Sci Total Environ 779:146433. https://doi.org/10.1016/j.scitotenv.2021.146433
Zhu X, Hondroulis E, Liu W, Li CZ (2013) Biosensing approaches for rapid genotoxicity and cytotoxicity assays upon nanomaterial exposure. Small 9:1821–1830. https://doi.org/10.1002/smll.201201593
Gomes HIO, Martins CSM, Prior JAV (2021) Silver nanoparticles as carriers of anticancer drugs for efficient target treatment of cancer cells. Nanomaterials 11:964. https://doi.org/10.3390/nano11040964
Matsuda Y, Torimoto T, Kameya T et al (2013) ZnS-AgInS2 nanoparticles as a temperature sensor. Sensors Actuators, B Chem 176:505–508. https://doi.org/10.1016/j.snb.2012.09.005
Alavi M, Dehestaniathar S, Mohammadi S et al (2020) Antibacterial activities of phytofabricated ZnO and CuO NPs by Mentha pulegium leaf/flower mixture extract against antibiotic resistant bacteria. Adv Pharm Bull 11:497–504. https://doi.org/10.34172/apb.2021.057
Aljelehawy Q, Karimi N, Alavi M (2021) Comparison of antibacterial and cytotoxic activities of phytosynthesized ZnONPs by leaves extract of Daphne mucronata at different salt sources. Mater Technol 36:747–759. https://doi.org/10.1080/10667857.2020.1794280
Shankar PD, Shobana S, Karuppusamy I et al (2016) A review on the biosynthesis of metallic nanoparticles (gold and silver) using bio-components of microalgae: formation mechanism and applications. Enzyme Microb Technol 95:28–44. https://doi.org/10.1016/j.enzmictec.2016.10.015
Mani R, Vijayakumar P, Dhas S, et al (2022) Biogenic nanoscale silver particles synthesis using butter fruit pulp extract and study of antibacterial efficacy against Providencia vermicola in rohu fish. J King Saud Univ - Sci 34:101814. https://doi.org/10.1016/j.jksus.2021.101814
Li S, Shen Y, Xie A et al (2007) Green synthesis of silver nanoparticles using Capsicum annuum L extract. Green Chem 9:852. https://doi.org/10.1039/b615357g
Song JY, Kim BS (2009) Rapid biological synthesis of silver nanoparticles using plant leaf extracts. Bioprocess Biosyst Eng 32:79. https://doi.org/10.1007/s00449-008-0224-6
Singh J, Dutta T, Kim KH et al (2018) “Green” synthesis of metals and their oxide nanoparticles: applications for environmental remediation. J Nanobiotechnology 16:84. https://doi.org/10.1186/s12951-018-0408-4
Kumar B, Smita K, Cumbal L, Debut A (2017) Green synthesis of silver nanoparticles using Andean blackberry fruit extract. Saudi J Biol Sci 24:45–50. https://doi.org/10.1016/j.sjbs.2015.09.006
Antony JJ, Nivedheetha M, Siva D et al (2013) Antimicrobial activity of Leucas aspera engineered silver nanoparticles against Aeromonas hydrophila in infected Catla catla. Colloids Surfaces B Biointerfaces 109:20–24. https://doi.org/10.1016/j.colsurfb.2013.03.020
Baldissera MD, Souza CF, Zeppenfeld CC et al (2020) Dietary supplementation with nerolidol nanospheres improves growth, antioxidant status and fillet fatty acid profiles in Nile tilapia: benefits of nanotechnology for fish health and meat quality. Aquaculture 516:734635. https://doi.org/10.1016/j.aquaculture.2019.734635
Rather MA, Bhat IA, Sharma N et al (2017) Synthesis and characterization of Azadirachta indica constructed silver nanoparticles and their immunomodulatory activity in fish. Aquac Res 48:3742–3754. https://doi.org/10.1111/are.13199
Shah BR, Mraz J (2020) Advances in nanotechnology for sustainable aquaculture and fisheries. Rev Aquac 12:925–942. https://doi.org/10.1111/raq.12356
Vignesh V, Felix Anbarasi K, Karthikeyeni S et al (2013) A superficial phyto-assisted synthesis of silver nanoparticles and their assessment on hematological and biochemical parameters in Labeo rohita (Hamilton, 1822). Colloids Surfaces A Physicochem Eng Asp 439:184–192. https://doi.org/10.1016/j.colsurfa.2013.04.011
Choi JE, Kim S, Ahn JH et al (2010) Induction of oxidative stress and apoptosis by silver nanoparticles in the liver of adult zebrafish. Aquat Toxicol 100:151–159. https://doi.org/10.1016/j.aquatox.2009.12.012
Lubick N (2008) Nanosilver toxicity: ions, nanoparticles — or both? Environ Sci Technol 42:8617. https://doi.org/10.1021/es8026314
Mulenos MR, Liu J, Lujan H et al (2020) Copper, silver, and titania nanoparticles do not release ions under anoxic conditions and release only minute ion levels under oxic conditions in water: evidence for the low toxicity of nanoparticles. Environ Chem Lett 18:1319–1328. https://doi.org/10.1007/s10311-020-00985-z
Sakka Y, Skjolding LM, Mackevica A et al (2016) Behavior and chronic toxicity of two differently stabilized silver nanoparticles to Daphnia magna. Aquat Toxicol 177:526–535. https://doi.org/10.1016/j.aquatox.2016.06.025
Shen MH, Zhou XX, Yang XY et al (2015) Exposure medium: key in identifying free Ag+ as the exclusive species of silver nanoparticles with acute toxicity to Daphnia magna. Sci Rep 5:9674. https://doi.org/10.1038/srep09674
Khoshnamvand M, Hao Z, Fadare OO et al (2020) Toxicity of biosynthesized silver nanoparticles to aquatic organisms of different trophic levels. Chemosphere 258:127346. https://doi.org/10.1016/j.chemosphere.2020.127346
Nie X, Zhu K, Zhao S et al (2020) Interaction of Ag+ with soil organic matter: elucidating the formation of silver nanoparticles. Chemosphere 243:125413. https://doi.org/10.1016/j.chemosphere.2019.125413
Oya-Silva LF, Vicari T, Rodrigo Disner G et al (2021) Tissue-specific genotoxicity and antioxidant imbalance of titanium dioxide nanoparticles (NPTiO2) and inorganic lead (PbII) in a neotropical fish species. Environ Toxicol Pharmacol 82:103551. https://doi.org/10.1016/j.etap.2020.103551
Bathi JR, Moazeni F, Upadhyayula VKK et al (2021) Behavior of engineered nanoparticles in aquatic environmental samples: current status and challenges. Sci Total Environ 793:148560. https://doi.org/10.1016/j.scitotenv.2021.148560
He X, Aker WG, Fu PP, Hwang HM (2015) Toxicity of engineered metal oxide nanomaterials mediated by nano-bio-eco-interactions: a review and perspective. Environ Sci Nano 2:564–582. https://doi.org/10.1039/C5EN00094G
Krishnasamy Sekar R, Sridhar A, Perumalsamy B et al (2020) In vitro antioxidant, antipathogenicity and cytotoxicity effect of silver nanoparticles fabricated by onion (Allium cepa L.) peel extract. Bionanoscience 10:235–248. https://doi.org/10.1007/s12668-019-00691-3
OECD (2019) Test No. 203: fish, acute toxicity test, OECD Guidelines for the testing of chemicals, Section 2, OECD Publishing, Paris. https://doi.org/10.1787/9789264069961-en
Finney DJ (1952) Probit analysis Cambridge University Press, New York. J Am Pharm Assoc (Scientific ed) 41:627. https://doi.org/10.1002/jps.3030411125
Zhang Y, Lu X, Wang N et al (2016) Heavy metals in aquatic organisms of different trophic levels and their potential human health risk in Bohai Bay, China. Environ Sci Pollut Res 23:17801–17810. https://doi.org/10.1007/s11356-016-6948-y
Dacie JV, Lewis SM (1975) Practical haematology, 5th edn. Churchill Livingstone, London
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275. https://doi.org/10.1016/0922-338X(96)89160-4
Marklund S, Marklund G (1974) Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem 47:469–474. https://doi.org/10.1111/j.1432-1033.1974.tb03714.x
Claiborne A (1985) Catalase activity. In: Greenwald RA (ed) CRC Handbook of Methods for Oxygen Radical Research. CRC Press, Boca Raton, Florida pp 283–284
Rotruck JT, Pope AL, Ganther HE et al (1973) Selenium: biochemical role as a component of glutathione peroxidase. Science 179:588–590. https://doi.org/10.1126/science.179.4073.588
Habig WH, Pabst M, Jakoby W (1974) Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem 249:7130–7139. https://doi.org/10.2307/j.ctv18b5cjk.40
Moron MS, Depierre JW, Mannervik B (1979) Levels of glutathione, glutathione reductase and glutathione S-transferase activities in rat lung and liver. BBA - Gen Subj 582:67–78. https://doi.org/10.1016/0304-4165(79)90289-7
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods 25:402–408. https://doi.org/10.1006/meth.2001.1262
Benítez V, Mollá E, Martín-Cabrejas MA et al (2011) Characterization of industrial onion wastes (Allium cepa L.): dietary fibre and bioactive compounds. Plant Foods Hum Nutr 66:48–57. https://doi.org/10.1007/s11130-011-0212-x
Ahmed MJ, Murtaza G, Mehmood A, Bhatti TM (2015) Green synthesis of silver nanoparticles using leaves extract of Skimmia laureola: characterization and antibacterial activity. Mater Lett 153:10–13. https://doi.org/10.1016/j.matlet.2015.03.143
He Y, Du Z, Ma S et al (2016) Biosynthesis, antibacterial activity and anticancer effects against prostate cancer (PC-3) cells of silver nanoparticles using Dimocarpus Longan Lour peel extract. Nanoscale Res Lett 11:300. https://doi.org/10.1186/s11671-016-1511-9
Li S, Shen Y, Xie A et al (2007) Green synthesis of silver nanoparticles using Capsicum annuum L. extract. Green Chem 9:852–885. https://doi.org/10.1039/b615357g
Tripathy A, Raichur AM, Chandrasekaran N et al (2010) Process variables in biomimetic synthesis of silver nanoparticles by aqueous extract of Azadirachta indica (Neem) leaves. J Nanoparticle Res 12:237–246. https://doi.org/10.1007/s11051-009-9602-5
Whiteman SC, Yang Y, Jones JM, Spiteri MA (2008) FTIR spectroscopic analysis of sputum: preliminary findings on a potential novel diagnostic marker for COPD. Ther Adv Respir Dis 2:23–31. https://doi.org/10.1177/1753465807087972
Jain N, Bhargava A, Majumdar S et al (2011) Extracellular biosynthesis and characterization of silver nanoparticles using Aspergillus flavus NJP08: a mechanism perspective. Nanoscale 3:635–641. https://doi.org/10.1039/c0nr00656d
Ghaseminezhad SM, Hamedi S, Shojaosadati SA (2012) Green synthesis of silver nanoparticles by a novel method: comparative study of their properties. Carbohydr Polym 89:467–472. https://doi.org/10.1016/j.carbpol.2012.03.030
Abdel-Aziz MS, Shaheen MS, El-Nekeety AA, Abdel-Wahhab MA (2014) Antioxidant and antibacterial activity of silver nanoparticles biosynthesized using Chenopodium murale leaf extract. J Saudi Chem Soc 18:356–363. https://doi.org/10.1016/j.jscs.2013.09.011
Kumar KG, Avinash BS, Rahimi-Gorji M, Alarifi IM (2019) Optical and electrical properties of Ti1-XSnXO2 nanoparticles. J Mol Liq 293:111556. https://doi.org/10.1016/j.molliq.2019.111556
Kanipandian N, Kannan S, Ramesh R et al (2014) Characterization, antioxidant and cytotoxicity evaluation of green synthesized silver nanoparticles using Cleistanthus collinus extract as surface modifier. Mater Res Bull 49:494–502. https://doi.org/10.1016/j.materresbull.2013.09.016
Judy JD, Kirby JK, Creamer C et al (2015) Effects of silver sulfide nanomaterials on mycorrhizal colonization of tomato plants and soil microbial communities in biosolid-amended soil. Environ Pollut 206:256–263. https://doi.org/10.1016/j.envpol.2015.07.002
Xia T, Kovochich M, Brant J et al (2006) Comparison of the abilities of ambient and manufactured nanoparticles to induce cellular toxicity according to an oxidative stress paradigm. Nano Lett 6:1794–1807
Kleiven M, Macken A, Oughton DH (2019) Growth inhibition in Raphidocelis subcapita – Evidence of nanospecific toxicity of silver nanoparticles. Chemosphere 221:785–792. https://doi.org/10.1016/j.chemosphere.2019.01.055
Kakakhel MA, Wu F, Feng H et al (2021) Biological synthesis of silver nanoparticles using animal blood, their preventive efficiency of bacterial species, and ecotoxicity in common carp fish. Microsc Res Tech 84:1765–1774. https://doi.org/10.1002/jemt.23733
Liaqat F, Hanif U, Bahadur S et al (2021) Comparative evaluation of the toxicological effect of silver salt (AgNO3) and silver nanoparticles on Cyprinus carpio synthesized by chemicals and marine algae using scanning electron microscopy. Microsc Res Tech 84:1531–1541. https://doi.org/10.1002/jemt.23710
Khosravi-Katuli K, Shabani A, Paknejad H, Imanpoor MR (2018) Comparative toxicity of silver nanoparticle and ionic silver in juvenile common carp (Cyprinus carpio): accumulation, physiology and histopathology. J Hazard Mater 359:373–381. https://doi.org/10.1016/j.jhazmat.2018.07.064
Ramachandran R, Krishnaraj C, Kumar VKA et al (2018) In vivo toxicity evaluation of biologically synthesized silver nanoparticles and gold nanoparticles on adult zebrafish: a comparative study. 3 Biotech 8:441. https://doi.org/10.1007/s13205-018-1457-y
Krishnaraj C, Harper SL, Il YS (2016) In vivo toxicological assessment of biologically synthesized silver nanoparticles in adult Zebrafish (Danio rerio). J Hazard Mater 301:480–491. https://doi.org/10.1016/j.jhazmat.2015.09.022
Sarkar B, Netam SP, Mahanty A et al (2014) Toxicity evaluation of chemically and plant derived silver nanoparticles on zebrafish (Danio rerio). Proc Natl Acad Sci India Sect B - Biol Sci 84:885–892. https://doi.org/10.1007/s40011-013-0298-z
Bilberg K, Hovgaard MB, Besenbacher F, Baatrup E (2012) In vivo toxicity of silver nanoparticles and silver ions in zebrafish (Danio rerio). J Toxicol 2012:1–9. https://doi.org/10.1155/2012/293784
Girilal M, Krishnakumar V, Poornima P et al (2015) A comparative study on biologically and chemically synthesized silver nanoparticles induced heat shock proteins on fresh water fish Oreochromis niloticus. Chemosphere 139:461–468. https://doi.org/10.1016/j.chemosphere.2015.08.005
Massarsky A, Dupuis L, Taylor J et al (2013) Assessment of nanosilver toxicity during zebrafish (Danio rerio) development. Chemosphere 92:59–66. https://doi.org/10.1016/j.chemosphere.2013.02.060
Olasagasti M, Gatti AM, Capitani F et al (2014) Toxic effects of colloidal nanosilver in zebrafish embryos. J Appl Toxicol 34:562–575. https://doi.org/10.1002/jat.2975
Il KJ, Cui R, Nam S-H et al (2016) Multispecies toxicity test for silver nanoparticles to derive hazardous concentration based on species sensitivity distribution for the protection of aquatic ecosystems. Nanotoxicology 10:521–530. https://doi.org/10.3109/17435390.2015.1090028
Croteau MN, Misra SK, Luoma SN, Valsami-Jones E (2014) Bioaccumulation and toxicity of CuO nanoparticles by a freshwater invertebrate after waterborne and dietborne exposures. Environ Sci Technol 48:10929–10937. https://doi.org/10.1021/es5018703
Utembe W, Wepener V, Yu IJ, Gulumian M (2018) An assessment of applicability of existing approaches to predicting the bioaccumulation of conventional substances in nanomaterials. Environ Toxicol Chem 37:2972–2988. https://doi.org/10.1002/etc.4253
Velicogna JR, Schwertfeger DM, Jesmer AH et al (2017) The bioaccumulation of silver in Eisenia andrei exposed to silver nanoparticles and silver nitrate in soil. NanoImpact 6:11–18. https://doi.org/10.1016/j.impact.2017.03.001
Iversen TG, Skotland T, Sandvig K (2011) Endocytosis and intracellular transport of nanoparticles: present knowledge and need for future studies. Nano Today 6:176–185. https://doi.org/10.1016/j.nantod.2011.02.003
Mansouri B, Maleki A, Johari SA et al (2016) Copper bioaccumulation and depuration in common carp (Cyprinus carpio) following co-exposure to TiO2 and CuO nanoparticles. Arch Environ Contam Toxicol 71:541–552. https://doi.org/10.1007/s00244-016-0313-5
Sayadi MH, Pavlaki MD, Martins R et al (2021) Bioaccumulation and toxicokinetics of zinc oxide nanoparticles (ZnO NPs) co-exposed with graphene nanosheets (GNs) in the blackfish (Capoeta fusca). Chemosphere 269:128689. https://doi.org/10.1016/j.chemosphere.2020.128689
Mansouri B, Johari SA, Azadi NA, Sarkheil M (2018) Effects of waterborne ZnO nanoparticles and Zn2+ ions on the gills of rainbow trout (Oncorhynchus mykiss): bioaccumulation, histopathological and ultrastructural changes. Turkish J Fish Aquat Sci 18:739–746. https://doi.org/10.4194/1303-2712-v18_5_09
Ali I, Khan S, Shah K et al (2021) Microscopic analysis of plant-mediated silver nanoparticle toxicity in rainbow trout fish (Oncorhynchus mykiss). Microsc Res Tech 84:2302–2310. https://doi.org/10.1002/jemt.23785
Scown TM, Santos EM, Johnston BD et al (2010) Effects of aqueous exposure to silver nanoparticles of different sizes in rainbow trout. Toxicol Sci 115:521–534. https://doi.org/10.1093/toxsci/kfq076
Kaya H, Aydin F, Gürkan M et al (2015) Effects of zinc oxide nanoparticles on bioaccumulation and oxidative stress in different organs of tilapia (Oreochromis niloticus). Environ Toxicol Pharmacol 40:936–947. https://doi.org/10.1016/j.etap.2015.10.001
Yoo-iam M, Chaichana R, Satapanajaru T (2014) Toxicity, bioaccumulation and biomagnification of silver nanoparticles in green algae (Chlorella sp.), water flea (Moina macrocopa), blood worm (Chironomus spp.) and silver barb (Barbonymus gonionotus). Chem Speciat Bioavailab 26:257–265. https://doi.org/10.3184/095422914X14144332205573
Johari SA, Kalbassi MR, Yu IJ, Lee JH (2015) Chronic effect of waterborne silver nanoparticles on rainbow trout (Oncorhynchus mykiss): histopathology and bioaccumulation. Comp Clin Path 24:995–1007. https://doi.org/10.1007/s00580-014-2019-2
Xiao B, Wang X, Yang J et al (2020) Bioaccumulation kinetics and tissue distribution of silver nanoparticles in zebrafish: the mechanisms and influence of natural organic matter. Ecotoxicol Environ Saf 194:110454. https://doi.org/10.1016/j.ecoenv.2020.110454
Thummabancha K, Onparn N, Srisapoome P (2016) Analysis of hematologic alterations, immune responses and metallothionein gene expression in Nile tilapia (Oreochromis niloticus) exposed to silver nanoparticles. J Immunotoxicol 13:909–917. https://doi.org/10.1080/1547691X.2016.1242673
Dhanapakiam P, Ramasamy VK (2001) Toxic effects of copper and zinc mixtures on some haematological and biochemical parameters in common carp, Cyprinus carpio (Linn). J Environ Biol 22:105–111
Alkaladi A, El-Deen NAMN, Afifi M, Zinadah OAA (2015) Hematological and biochemical investigations on the effect of vitamin E and C on Oreochromis niloticus exposed to zinc oxide nanoparticles. Saudi J Biol Sci 22:556–563. https://doi.org/10.1016/j.sjbs.2015.02.012
Remyla SR, Ramesh M, Sajwan KS, Senthil Kumar K (2008) Influence of zinc on cadmium induced haematological and biochemical responses in a freshwater teleost fish Catla catla. Fish Physiol Biochem 34:169–174. https://doi.org/10.1007/s10695-007-9157-2
Lavanya S, Ramesh M, Kavitha C, Malarvizhi A (2011) Hematological, biochemical and ionoregulatory responses of Indian major carp Catla catla during chronic sublethal exposure to inorganic arsenic. Chemosphere 82:977–985. https://doi.org/10.1016/j.chemosphere.2010.10.071
Kuhn V, Diederich L, Keller TCS et al (2017) Red blood cell function and dysfunction: redox regulation, nitric oxide metabolism, anemia. antioxid redox signal 26:718–742
Levine EA, Rosen AL, Sehgal LR et al (1990) Physiologic effects of acute anemia: implications for a reduced transfusion trigger. Transfusion 30:11–14. https://doi.org/10.1046/j.1537-2995.1990.30190117621.x
Tsai AG, Acero C, Nance PR et al (2005) Elevated plasma viscosity in extreme hemodilution increases perivascular nitric oxide concentration and microvascular perfusion. Am J Physiol - Hear Circ Physiol 288:H1730–H1739. https://doi.org/10.1152/ajpheart.00998.2004
Das S, Patra A, Mandal A et al (2021) Study on impacts of direct supplementation of choline into semi-intensive fish culture system based on haematopoietic alterations. Environ Sustain Indic 9:100089. https://doi.org/10.1016/j.indic.2020.100089
Fazio F, Piccione G, Arfuso F, Faggio C (2015) Peripheral blood and head kidney haematopoietic tissue response to experimental blood loss in mullet (Mugil cephalus). Mar Biol Res 11:197–202. https://doi.org/10.1080/17451000.2014.898850
Khabbazi M, Harsij M, Hedayati SAA et al (2014) Effect of CuO nanoparticles on some hematological indices of rainbow trout Oncorhynchus mykiss and their potential toxicity. Nanomedicine J 2:67–73. https://doi.org/10.7508/nmj.2015.01.008
Farmen E, Mikkelsen HN, Evensen, et al (2012) Acute and sub-lethal effects in juvenile Atlantic salmon exposed to low μg/L concentrations of Ag nanoparticles. Aquat Toxicol 108:78–84. https://doi.org/10.1016/j.aquatox.2011.07.007
Nussey G, Van Vuren JHJ, du Preez HH (1995) Effect of copper on the haematology and osmoregulation of the Mozambique tilapia, Oreochromis mossambicus (Cichlidae). Comp Biochem Physiol Part C Comp 111:369–380. https://doi.org/10.1016/0742-8413(95)00063-1
Shaw BJ, Al-Bairuty G, Handy RD (2012) Effects of waterborne copper nanoparticles and copper sulphate on rainbow trout, (Oncorhynchus mykiss): physiology and accumulation. Aquat Toxicol 116–117:90–101. https://doi.org/10.1016/j.aquatox.2012.02.032
Remya AS, Ramesh M, Saravanan M et al (2015) Iron oxide nanoparticles to an Indian major carp, Labeo rohita: impacts on hematology, iono regulation and gill Na+/K+ ATPase activity. J King Saud Univ - Sci 27:151–160. https://doi.org/10.1016/j.jksus.2014.11.002
Ramsden CS, Smith TJ, Shaw BJ, Handy RD (2009) Dietary exposure to titanium dioxide nanoparticles in rainbow trout, (Oncorhynchus mykiss): no effect on growth, but subtle biochemical disturbances in the brain. Ecotoxicology 18:939–951. https://doi.org/10.1007/s10646-009-0357-7
Smith SD, Bolwell BJ, Rybicki LA et al (2007) Autologous hematopoietic stem cell transplantation in peripheral T-cell lymphoma using a uniform high-dose regimen. Bone Marrow Transplant 40:239–243. https://doi.org/10.1038/sj.bmt.1705712
Cogun HY, Firat Ö, Firat Ö et al (2012) Protective effect of selenium against mercury-induced toxicity on hematological and biochemical parameters of Oreochromis niloticus. J Biochem Mol Toxicol 26:117–122. https://doi.org/10.1002/jbt.20417
Pirsaheb M, Azadi NA, Miglietta ML et al (2019) Toxicological effects of transition metal-doped titanium dioxide nanoparticles on goldfish (Carassius auratus) and common carp (Cyprinus carpio). Chemosphere 215:904–915. https://doi.org/10.1016/j.chemosphere.2018.10.111
Kappus H (1985) Lipid peroxidation: mechanisms, analysis, enzymology and biological relevance. Oxidative Stress 273:273–310. https://doi.org/10.1016/b978-0-12-642760-8.50016-8
Govindasamy R, Rahuman AA (2012) Histopathological studies and oxidative stress of synthesized silver nanoparticles in Mozambique tilapia (Oreochromis mossambicus). J Environ Sci (China) 24:1091–1098. https://doi.org/10.1016/S1001-0742(11)60845-0
Zhu X, Zhu L, Lang Y, Chen Y (2008) Oxidative stress and growth inhibition in the freshwater fish Carassius auratus induced by chronic exposure to sublethal fullerene aggregates. Environ Toxicol Chem 27:1979–1985. https://doi.org/10.1897/07-573.1
Atli G, Alptekin Ö, Tükel S, Canli M (2006) Response of catalase activity to Ag+, Cd2+, Cr6+, Cu2+ and Zn2+ in five tissues of freshwater fish Oreochromis niloticus. Comp Biochem Physiol - C Toxicol Pharmacol 143:218–224. https://doi.org/10.1016/j.cbpc.2006.02.003
Hansen BH, Rømma S, Garmo A et al (2006) Antioxidative stress proteins and their gene expression in brown trout (Salmo trutta) from three rivers with different heavy metal levels. Comp Biochem Physiol - C Toxicol Pharmacol 143:263–274. https://doi.org/10.1016/j.cbpc.2006.02.010
Husak VV, Mosiichuk NM, Kubrak OI et al (2018) Acute exposure to copper induces variable intensity of oxidative stress in goldfish tissues. Fish Physiol Biochem 44:841–852. https://doi.org/10.1007/s10695-018-0473-5
Vieira MC, Torronteras R, Córdoba F, Canalejo A (2012) Acute toxicity of manganese in goldfish Carassius auratus is associated with oxidative stress and organ specific antioxidant responses. Ecotoxicol Environ Saf 78:212–217. https://doi.org/10.1016/j.ecoenv.2011.11.015
Fırat Ö, Bozat RC (2019) Assessment of biochemical and toxic responses induced by titanium dioxide nanoparticles in Nile tilapia Oreochromis niloticus. Hum Ecol Risk Assess 25:1438–1447. https://doi.org/10.1080/10807039.2018.1465338
Hao L, Wang Z, Xing B (2009) Effect of sub-acute exposure to TiO2 nanoparticles on oxidative stress and histopathological changes in Juvenile Carp (Cyprinus carpio). J Environ Sci (China) 21:1459–1466. https://doi.org/10.1016/s1001-0742(08)62440-7
Afifi M, Saddick S, Abu Zinada OA (2016) Toxicity of silver nanoparticles on the brain of Oreochromis niloticus and Tilapia zillii. Saudi J Biol Sci 23:754–760. https://doi.org/10.1016/j.sjbs.2016.06.008
Wu Y, Zhou Q (2013) Silver nanoparticles cause oxidative damage and histological changes in medaka (Oryzias latipes) after 14 days of exposure. Environ Toxicol Chem 32:165–173. https://doi.org/10.1002/etc.2038
Massarsky A, Abraham R, Nguyen KC et al (2014) Nanosilver cytotoxicity in rainbow trout (Oncorhynchus mykiss) erythrocytes and hepatocytes. Comp Biochem Physiol - C Toxicol Pharmacol 159:10–21. https://doi.org/10.1016/j.cbpc.2013.09.008
Lee B, Duong CN, Cho J, et al (2012) Toxicity of citrate-capped silver nanoparticles in common carp (Cyprinus carpio). J Biomed Biotechnol. 2012:262670. https://doi.org/10.1155/2012/262670
Monteiro DA, Rantin FT, Kalinin AL (2010) Inorganic mercury exposure: toxicological effects, oxidative stress biomarkers and bioaccumulation in the tropical freshwater fish matrinxã, Brycon amazonicus (Spix and Agassiz, 1829). Ecotoxicology 19:105–123. https://doi.org/10.1007/s10646-009-0395-1
Chae YJ, Pham CH, Lee J et al (2009) Evaluation of the toxic impact of silver nanoparticles on Japanese medaka (Oryzias latipes). Aquat Toxicol 94:320–327. https://doi.org/10.1016/j.aquatox.2009.07.019
Al-Abdan MA, Bin-Jumah MN, Alarifi S (2020) Exploration of cadmium dioxide nanoparticles on bioaccumulation, oxidative stress, and carcinogenic potential in Oreochromis mossambicus L. Oxid Med Cell Longev 2020:1–11. https://doi.org/10.1155/2020/5407159
Alak G, Ucar A, Parlak V et al (2020) Antioxidant potential of ulexite in zebrafish brain: assessment of oxidative DNA damage, apoptosis, and response of antioxidant defense system. Biol Trace Elem Res 199:1092–1099. https://doi.org/10.1007/s12011-020-02231-7
Hoseinifar SH, Yousefi S, Van Doan H et al (2021) Oxidative stress and antioxidant defense in fish: the implications of probiotic, prebiotic, and synbiotics. Rev Fish Sci Aquac 29:198–217. https://doi.org/10.1080/23308249.2020.1795616
Reddy UA, Prabhakar PV, Mahboob M (2017) Biomarkers of oxidative stress for in vivo assessment of toxicological effects of iron oxide nanoparticles. Saudi J Biol Sci 24:1172–1180. https://doi.org/10.1016/j.sjbs.2015.09.029
Jifa W, Yu Z, Xiuxian S, You W (2006) Response of integrated biomarkers of fish (Lateolabrax japonicus) exposed to benzo[a]pyrene and sodium dodecylbenzene sulfonate. Ecotoxicol Environ Saf 65:230–236. https://doi.org/10.1016/j.ecoenv.2005.08.002
Arora S, Jain J, Rajwade JM, Paknikar KM (2008) Cellular responses induced by silver nanoparticles: In vitro studies. Toxicol Lett 179:93–100. https://doi.org/10.1016/j.toxlet.2008.04.009
Hajirezaee S, Mohammadi G, Naserabad SS (2020) The protective effects of vitamin C on common carp (Cyprinus carpio) exposed to titanium oxide nanoparticles (TiO2-NPs). Aquaculture 518:734734. https://doi.org/10.1016/j.aquaculture.2019.734734
Abdelazim AM, Saadeldin IM, Swelum AAA et al (2018) Oxidative stress in the muscles of the fish Nile tilapia caused by zinc oxide nanoparticles and its modulation by vitamins C and E. Oxid Med Cell Longev 2018:6926712. https://doi.org/10.1155/2018/6926712
Cristina Soare L, Păunescu A, Cristina Maria P (2019) The morphophysiological, histological, and biochemical response of some nontarget organisms to the stress induced by the pesticides in the environment. In: Larramendy, Soloneski (ed) Pesticides — use and misuse and their impact in the environment. IntechOpen. https://doi.org/10.5772/intechopen.84332
Elgendy MY, Abumourad IK, Ali SEM et al (2017) Health status and genotoxic effects of metal pollution in Tilapia zillii and Solea vulgaris from polluted aquatic habitats. Int J Zool Res 13:54–63. https://doi.org/10.3923/ijzr.2017.54.63
Ossana NA, Baudou FG, Castañé PM et al (2019) Histological, genotoxic, and biochemical effects on Cnesterodon decemmaculatus (Jenyns 1842) (Cyprinodontiformes, Poeciliidae): early response bioassays to assess the impact of receiving waters. J Toxicol 2019:1–13. https://doi.org/10.1155/2019/4687685
Nero V, Farwell A, Lister A et al (2006) Gill and liver histopathological changes in yellow perch (Perca flavescens) and goldfish (Carassius auratus) exposed to oil sands process-affected water. Ecotoxicol Environ Saf 63:365–377. https://doi.org/10.1016/j.ecoenv.2005.04.014
Tchounwou PB, Yedjou CG, Patlolla AK, Sutton DJ (2012) Heavy metal toxicity and the environment. EXS 101:133–164. https://doi.org/10.1007/2F978-3-7643-8340-4_6
Butchiram MS, Vijaya Kumar M, Tilak KS (2013) Studies on the histopathological changes in selected tissues of fish Labeo rohita exposed to phenol. J Environ Biol 34:247–251
Mansouri B, Maleki A, Johari SA et al (2017) Histopathological effects of copper oxide nanoparticles on the gill and intestine of common carp (Cyprinus carpio) in the presence of titanium dioxide nanoparticles. Chem Ecol 33:295–308. https://doi.org/10.1080/02757540.2017.1301436
Rajkumar KS, Kanipandian N, Thirumurugan R (2016) Toxicity assessment on haemotology, biochemical and histopathological alterations of silver nanoparticles-exposed freshwater fish Labeo rohita. Appl Nanosci 6:19–29. https://doi.org/10.1007/s13204-015-0417-7
Cassar S, Adatto I, Freeman JL et al (2020) Use of zebrafish in drug discovery toxicology. Chem Res Toxicol 33:95–118. https://doi.org/10.1021/acs.chemrestox.9b00335
Chen J, Dong X, Xin Y, Zhao M (2011) Effects of titanium dioxide nano-particles on growth and some histological parameters of zebrafish (Danio rerio) after a long-term exposure. Aquat Toxicol 101:493–499. https://doi.org/10.1016/j.aquatox.2010.12.004
Jang MH, Kim WK, Lee SK et al (2014) Uptake, tissue distribution, and depuration of total silver in common carp (Cyprinus carpio) after aqueous exposure to silver nanoparticles. Environ Sci Technol 48:11568–11574. https://doi.org/10.1021/es5022813
Shi H, Magaye R, Castranova V, Zhao J (2013) Titanium dioxide nanoparticles: a review of current toxicological data. Part Fibre Toxicol 10:15. https://doi.org/10.1186/1743-8977-10-15
Vali S, Mohammadi G, Tavabe KR et al (2020) The effects of silver nanoparticles (Ag-NPs) sublethal concentrations on common carp (Cyprinus carpio): bioaccumulation, hematology, serum biochemistry and immunology, antioxidant enzymes, and skin mucosal responses. Ecotoxicol Environ Saf 194:110353. https://doi.org/10.1016/j.ecoenv.2020.110353
Hall AP, Elcombe CR, Foster JR et al (2012) Liver hypertrophy: a review of adaptive (adverse and non-adverse) changes-conclusions from the 3rd international ESTP expert workshop. Toxicol Pathol 40:971–994
Schramm M, Müller E, Triebskorn R (1998) Brown trout (Salmo trutta) liver ultrastructure as a biomarker for assessment of small stream pollution. Biomarkers 3:93–108. https://doi.org/10.1080/135475098231264
Bernet D, Schmidt H, Meier W et al (1999) Histopathology in fish: proposal for a protocol to assess aquatic pollution. J Fish Dis 22:25–34. https://doi.org/10.1046/j.1365-2761.1999.00134.x
Rodrigues S, Antunes SC, Nunes B, Correia AT (2019) Histopathological effects of the antibiotic erythromycin on the freshwater fish species Oncorhynchus mykiss. Ecotoxicol Environ Saf 181:1–10. https://doi.org/10.1016/j.ecoenv.2019.05.067
da Cunha RLD, de Brito-Gitirana L (2020) Effects of titanium dioxide nanoparticles on the intestine, liver, and kidney of Danio rerio. Ecotoxicol Environ Saf 203:111032. https://doi.org/10.1016/j.ecoenv.2020.111032
Huang CW, Li SW, Liao VHC (2019) Long-term sediment exposure to ZnO nanoparticles induces oxidative stress. Caenorhabditis elegans Environ Sci Nano 6:2602–2614. https://doi.org/10.1039/c9en00039a
Kim KS, Lee D, Song CG, Kang PM (2015) Reactive oxygen species-activated nanomaterials as theranostic agents. Nanomedicine 10:2709–2723.https://doi.org/10.2217/2Fnnm.15.108
Maharajan A, Kitto MR, Paruruckumani PS, Ganapiriya V (2016) Histopathology biomarker responses in Asian sea bass, Lates calcarifer (Bloch) exposed to copper. J Basic Appl Zool 77:21–30. https://doi.org/10.1016/j.jobaz.2016.02.001
Yao JW, Liu J, Kong XZ et al (2012) Induction of activation of the antioxidant response element and stabilization of Nrf2 by 3-(3-pyridylmethylidene)-2-indolinone (PMID) confers protection against oxidative stress-induced cell death. Toxicol Appl Pharmacol 259:227–235. https://doi.org/10.1016/j.taap.2011.12.027
Kobayashi M, Li L, Iwamoto N et al (2009) The antioxidant defense system Keap1-Nrf2 comprises a multiple sensing mechanism for responding to a wide range of chemical compounds. Mol Cell Biol 29:493–502. https://doi.org/10.1128/mcb.01080-08
Wu P, Liu Y, Jiang WD et al (2017) A comparative study on antioxidant system in fish hepatopancreas and intestine affected by choline deficiency: different change patterns of varied antioxidant enzyme genes and nrf2 signaling factors. PLoS ONE 12:e0169888. https://doi.org/10.1371/journal.pone.0169888
Imai H, Nakagawa Y (2003) Biological significance of phospholipid hydroperoxide glutathione peroxidase (PHGPx, GPx4) in mammalian cells. Free Radic Biol Med 34:145–169. https://doi.org/10.1016/S0891-5849(02)01197-8
Neefjes VME, Evelo CTA, Baars LGM, Blanco CE (1999) Erythrocyte glutathione S transferase as a marker of oxidative stress at birth. Arch Dis Child Fetal Neonatal Ed 81:F130–F133. https://doi.org/10.1136/fn.81.2.F130
Yeo MK, Park HG (2012) Gene expression in zebrafish embryos following exposure to Cu-doped TiO2 and pure TiO2 nanometer-sized photocatalysts. Mol Cell Toxicol 8:127–137. https://doi.org/10.1007/s13273-012-0016-6
Van der Oost R, Beyer J, Vermeulen NPE (2003) Fish bioaccumulation and biomarkers in environmental risk assessment: a review. Environ Toxicol Pharmacol 13:57–149. https://doi.org/10.1016/S1382-6689(02)00126-6
Oliva M, Gravato C, Guilhermino L et al (2014) EROD activity and cytochrome P4501A induction in liver and gills of Senegal sole Solea senegalensis from a polluted Huelva Estuary (SW Spain). Comp Biochem Physiol Part - C Toxicol Pharmacol 166:134–144. https://doi.org/10.1016/j.cbpc.2014.07.010
Valko M, Leibfritz D, Moncol J et al (2007) Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol 39:44–84. https://doi.org/10.1016/j.biocel.2006.07.001
Nakajima H, Nakajima-Takagi Y, Tsujita T et al (2011) Tissue-restricted expression of Nrf2 and its target genes in zebrafish with gene-specific variations in the induction profiles. PLoS ONE 6:e26884. https://doi.org/10.1371/journal.pone.0026884
Wu G, Fang YZ, Yang S et al (2004) Glutathione metabolism and its implications for health. J Nutr 134:489–492. https://doi.org/10.1093/jn/134.3.489
Ma Q (2013) Role of Nrf2 in oxidative stress and toxicity. Annu Rev Pharmacol Toxicol 53:401–426. https://doi.org/10.1146/annurev-pharmtox-011112-140320
Nabavi SF, Barber AJ, Spagnuolo C et al (2016) Nrf2 as molecular target for polyphenols: a novel therapeutic strategy in diabetic retinopathy. Crit Rev Clin Lab Sci 53:293–312. https://doi.org/10.3109/10408363.2015.1129530
Zhang XD, Zhu YF, Cai LS, Wu TX (2008) Effects of fasting on the meat quality and antioxidant defenses of market-size farmed large yellow croaker (Pseudosciaena crocea). Aquaculture 280:136–139. https://doi.org/10.1016/j.aquaculture.2008.05.010
Zheng JL, Zhu QL, Wu CW et al (2016) Zinc acclimation mitigated high zinc induced oxidative stress by enhancing antioxidant defenses in large yellow croaker Pseudosciaena crocea. Aquat Toxicol 172:21–29. https://doi.org/10.1016/j.aquatox.2015.12.009
Pillet M, Castaldo G, De Weggheleire S et al (2019) Limited oxidative stress in common carp (Cyprinus carpio, L., 1758) exposed to a sublethal tertiary (Cu, Cd and Zn) metal mixture. Comp Biochem Physiol Part - C Toxicol Pharmacol 218:70–80. https://doi.org/10.1016/j.cbpc.2019.01.003
Wu P, Jiang WD, Liu Y et al (2014) Effect of choline on antioxidant defenses and gene expressions of Nrf2 signaling molecule in the spleen and head kidney of juvenile Jian carp (Cyprinus carpio var. Jian). Fish Shellfish Immunol 38:374–382. https://doi.org/10.1016/j.fsi.2014.03.032
Li Z-H, Li P, Shi Z-C (2015) Responses of the hepatic glutathione antioxidant defense system and related gene expression in juvenile common carp after chronic treatment with tributyltin. Ecotoxicology 24:700–705. https://doi.org/10.1007/s10646-014-1416-2
Hoseinifar SH, Ahmadi A, Khalili M et al (2017) The study of antioxidant enzymes and immune-related genes expression in common carp (Cyprinus carpio) fingerlings fed different prebiotics. Aquac Res 48:5447–5454. https://doi.org/10.1111/are.13359
Xing H, Zhang Z, Yao H et al (2014) Effects of atrazine and chlorpyrifos on cytochrome P450 in common carp liver. Chemosphere 104:244–250. https://doi.org/10.1016/j.chemosphere.2014.01.002
Zhang C, Zhang J, Fan W et al (2019) Effects of dietary Lactobacillus delbrueckii on growth performance, body composition, digestive and absorptive capacity, and gene expression of common carp (Cyprinus carpio Huanghe var). Aquac Nutr 25:166–175. https://doi.org/10.1111/anu.12840
Acknowledgements
The author Rajkumar Krishnasamy Sekar gratefully acknowledges the Department of Science and Technology (DST), Govt. of India, for providing fellowship under the DST-INSPIRE Fellowship (IF140546) scheme. The authors thank National Centre for Alternatives to Animal Experiments (NCAAE) under UGC-CPEPA scheme, Government of India (F.No.2-1/2013(NS/PE)), for RTqPCR studies in this work. The authors highly acknowledge the UGC-SAP-DRS-II (F.3-9/2013(SAP-II)), Department of Science and Technology Fund for Improvement of Science and Technology Infrastructure (DST-FIST)-Level-I (stage-II) (Ref. No. SR/FST/LSI-647/2015(C) Date.11.08.2016) and Department of Science and Technology Promotion of University Research and Scientific Excellence (DST PURSE Phase—II) (Ref. No. SR/PURSE PHASE 2/16(G)/&16(C) Date. 21.02.2017) for the instrumentation facility to the Department of Animal Science, Bharathidasan University, Tiruchirappalli 620 024, Tamil Nadu, India. The authors also thank “RUSA, 2.0—Biological Sciences, Bharathidasan University.”
Author information
Authors and Affiliations
Contributions
Investigation, methodology, data curation, writing — original draft: Rajkumar Krishnasamy Sekar; methodology, resources: Ramkumar Arunachalam, Murugadas Anbazhagan, and Sivagaami Palaniyappan; formal analysis, validation, writing — review and editing: Srinivasan Veeran and Arun Sridhar; conceptualization, project administration, supervision, validation, visualization, writing — review and editing: Thirumurugan Ramasamy. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Ethical Approval
Institutional Animal Ethical Committee (IAEC) of Bharathidasan University, Tiruchirappalli 620 024, Tamil Nadu, India, has approved this research work (Ref. No: BDU/IAEC/P28/2018).
Consent for Publication
All authors read and approved the final manuscript and have complete access to study data and agree with this manuscript publication.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original version of this article was revised. equations 1 and 2 should be: 1. \(\mathrm{MCV }(\mathrm{cu}.\mathrm{ microns}) =\mathrm{ Hct}/\mathrm{RBC }(10^6/\mathrm{ml})\) 2. \(\mathrm{MCH }(\mathrm{pg}) = [\mathrm{Hb }(\mathrm{g}/\mathrm{l}) \times 10]/\mathrm{RBC }(10^6/\mathrm{ml})\)
Rights and permissions
About this article
Cite this article
Krishnasamy Sekar, R., Arunachalam, R., Anbazhagan, M. et al. Accumulation, Chronicity, and Induction of Oxidative Stress Regulating Genes Through Allium cepa L. Functionalized Silver Nanoparticles in Freshwater Common Carp (Cyprinus carpio). Biol Trace Elem Res 201, 904–925 (2023). https://doi.org/10.1007/s12011-022-03164-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-022-03164-z