Abstract
Metal oxide nanoparticles (NPs), such as TiO2 and CuO, are widely applied in an increasing number of products and applications, and therefore their release to the aquatic ecosystems is unavoidable. However, little is known about joint toxicity of different NPs on tissues of aquatic organisms, such as fish. This study was conducted to assess the uptake and depuration of Cu following exposure to CuO NPs in the presence of TiO2 NPs in the liver, intestine, muscle, and gill of common carp, Cyprinus carpio. Carps with a mean total length of 23 ± 1.5 cm and mean weight of 13 ± 1.3 g were divided into 6 groups of 15 each (1 control group) and exposed to TiO2 NPs, CuO NPs, and a mixture of TiO2 and CuO NPs for periods of 20 days for uptake and 10 days for depuration. The determination of total Cu concentration was carried out by an ICP–OES. The order of Cu uptake in different tissues of the carps was liver > gill > muscle > intestine in both levels of CuO NPs alone; results showed that the total Cu concentrations in the presence of TiO2 nanoparticles were increased and were in the sequence of liver > gill > intestine > muscle. In depuration period, Cu concentrations were decreased in all treatments in the sequence of gill > intestine > muscle > liver. Uptake of Cu in different tissues of common carp increased with increasing concentration and time and was tissues- and time-dependent. In conclusion, this study suggested that the uptake of Cu in the tissues of common carp increased in the joint presence of TiO2 NPs.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Titanium dioxide (n-TiO2) is one of the most commonly used nanoparticles (NPs) in consumer products and various applications, including sun screens, catalytic reactions, surface coatings, cosmetics, personal care products, paints, building materials, embedded in glass, and other products (Shahmoradi et al. 2011; Aitken et al. 2006; Wiesner et al. 2006). Moreover, nanocopper oxide (CuO-NPs) is widely used in various industrial and commercial applications, such as wood preservation, gas sensors, facial spray, bioactive coatings, antimicrobial textiles, hospital equipment, heat transfer nanofluids, and other products (Li et al. 2007; Sau et al. 2010; Ebrahimnia-Bajestan et al. 2011). The toxicity studies of CuO NPs were assessed in several test organisms, including bacteria (Baek and An 2011; Heinlaan et al. 2008), aquatic crustaceans and algae (Aruoja et al. 2009; Hu et al. 2014), and fish (Chen et al. 2011; Villarreal et al. 2014). These studies reported that CuO nanoparticles exhibit cytotoxic effects. Moreover, Cu-NPs, as other metal-based NPs, could pose a toxicity threat and biological damages because of their ability to produce ROS (reactive oxygen species) directly and ROS can induce DNA strand breaks and affect gene expression (Chang et al. 2012). Compared with other metal oxide NPs, such as TiO2 and ZnO NPs, limited information is available about the potential toxicity effects of CuO NPs on aquatic organisms, such as fish and their fate in the aquatic ecosystems, especially concerning effects of long-term exposure and interactive effects with other materials in the environment. Although the toxicity of the single compounds might be well known, the interaction may happen during the process of absorption, distribution, metabolic transformation, and excretion or depending among other things on the affinity to target sites at cellular level (Martin et al. 2007).
Some nanoparticles may facilitate potential transport of the toxic chemicals in the environment by their large surface area, crystalline structure, and reactivity (Zhang and Masciangioli 2003). However, few studies have assessed the interactive toxicity effect of NPs with each other as well as their interactions with other pollutants on the aquatic organisms to indicate how and to what extent emerging nanoparticles may facilitate the transport of other nanoparticles and toxic chemicals in the environment. For example, findings of Zhang et al. (2007) illustrated that carps exposed to cadmium in the presence of TiO2 NPs accumulated 146 % more Cd than controls. In another study, Zou et al. (2014) reported that the existence of TiO2 NPs in various illumination modes changed the surface chemistry of Ag NPs and then led to different toxicity effects. They pointed out that the TiO2 NPs reduce the environmental risks of Ag NPs in natural light, but in continuous light, TiO2 NPs enhance the environmental risks of Ag NPs. In a similar study, Zhang et al. (2013) reported that the toxic effects of Cu ions in zebrafish in the joint presence of cadmium Telluride quantum dots (CdTe QDs) were dose-dependent with greater toxicity at increasing dose and the joint toxicities of the two toxicants were synergistic. In addition, more studies are needed to assess the toxicological effects of long-term exposure to NPs in the presence of other pollutants to understand the potential risks of environmental pollution by NPs in the aquatic ecosystems.
The common carp, Cyprinus carpio, is a widespread species in the aquatic ecosystems, an economically important fish, and approximately 10 % of annual freshwater aquaculture production globally (Aydın and Köprücü 2005; Lee et al. 2012). This species can provide interesting data in relation with monitoring the quality of the aquatic systems. Many studies have recommended that carp can be considered one of the most suitable models to assess the nonfatal effects of pollutants because of: (1) the dominance of this species in the aquatic systems; (2) having a better capacity for resistance against pollutants rather than other laboratory fish, such as zebrafish and Japanese medaka; and (3) one of the suitable fish models for ecotoxicological studies according to OECD standard (Lee et al. 2012; OECD 1993; Gul et al. 2012; Hedayati et al. 2014). Therefore, the purpose of this study was to determine the Cu uptake and elimination in the gill, intestine, liver, and muscles of common carp (C. carpio) following co-existence of CuO NPs and TiO2 NPs under controlled condition.
Materials and Methods
Nanoparticles and Characterizations
TiO2 NPs (anatase/rutile, 99+ %, 20 nm) and CuO NPs (99+ %, 40 nm) used in this study were produced by U.S. Research Nanomaterials Inc. (Houston, TX), and purchased from Nanosany Co. (Mashhad, Iran). Complete characterization of both nanomaterials were provided by Nanosany Co. Briefly, morphology and mean unaggregated particle diameters of TiO2 and CuO nanoparticles were determined by transmission electron microscopy (TEM) and scanning electron micrographs (SEM); and the phase structure of nanoparticles was characterized by x-ray diffraction (XRD; Fig. 1). Moreover, particle suspensions were prepared by weighing dry CuO NPs and TiO2 NPs into the distillated water then ultra-sonication (50 W, 37 kHz) for three periods of 30 min each to increase their dispersion (Ultrasonic bath, Elmasonic P, Germany). As shown in Table 1, the hydrodynamic diameter and zeta potential of 100 mg L−1 suspension of TiO2 NPs and CuO NPs in double distilled water, measured by dynamic light scattering (DLS) using a Zetasizer Nano (ZS) model ZEN3600 (Malvern Instruments Ltd., Worcestershire, UK). The zeta potentials of the TiO2 NPs and CuO NPs were shown in Fig. 2. Absorption spectra of CuO NPs and TiO2 NPs was obtained using a UV–Vis spectrophotometer (DR 5000™ UV–Vis Spectrophotometer, Hach Com., US).
Test Organism and Experimental Condition
Common carps (C. carpio) with a mean total length of 23 ± 1.5 cm and mean weight of 13 ± 1.3 g were obtained from a local aquaculture farm in the north of Iran and before beginning of the experiments were acclimatized in 1000 L tanks for 1 month supplied with continuously aerated tap water (24 ± 1.1 °C) under a 12-h light and a 12- h darkness. Fish were fed with commercially available fish food at a rate of 3 % body weight per day. Fish were deprived of food for 1 day prior to toxicity experiment. The characteristics of the water used for the common carp exposures were: pH 7.5 ± 0.5, conductivity 600 ± 10 µS/cm, hardness 5ºdGH, temperature 26.0 ± 1 °C, and dissolved oxygen content (DO) 6.0 ± 0.6 mg L−1.
Acute Toxicity
For the future chronic experiments, a non-lethal concentration of TiO2 NPs (10 mg L−1) was chosen based on the published toxicity studies (Linhua et al. 2009; Lee et al. 2012). In the case of CuO NPs, because of the diversity of existing data, an acute toxicity tests was conducted according to OECD 203 test guideline (OECD 1993) to determine the appropriated concentrations of CuO NPs for chronic experiments. After conducting series of range finding tests, for main test, fish were exposed to 15, 20, 25, 30, 35, 40, 45, 50, and 55 mg L−1 CuO NPs for 96 h. Accordingly, the 96-h LC50 of CuO NPs was 49.6 mg L−1 as calculated using probit method.
Chronic Toxicity
After determining the 96-h LC50 for CuO NPs, two sublethal concentrations, including 2.5 and 5.0 mg L−1 CuO NPs, which were approximately equal to 1/20th and 1/10th of LC50 concentrations were selected to study their toxicity alone or in combination with 10 mg L−1 TiO2 nanoparticles. Briefly, fish was divided into 6 groups of 15 each in 55-L glass aquariums to be exposed to different treatment in triplicate: first group was the control (without adding any chemicals), whereas the second group was exposed to 10 mg L−1 TiO2 NPs; third and fourth groups were exposed to 2.5 and 5.0 mg L−1 CuO NPs, respectively; and fifth and sixth groups were exposed to mixtures of 10 mg L−1 TiO2 NPs + 2.5 mg L−1 CuO NPs and 10 mg L−1 TiO2 NPs + 5.0 mg L−1 CuO NPs, respectively. Fish were exposed to the above-mentioned sublethal concentrations of NPs for two 10-days periods (20 days accumulation period, sampling at 10th and 20th days). At the end of accumulation periods, the remaining fish were transferred to clean water (depuration period) for another 10 days. To minimize decreases in the CuO and TiO2 concentrations during the experiments due to precipitation, 50 % percent of the water of each aquarium was renewed every day. During the testing period, the fish were fed every day.
Tissue Digestion
In this study, testing period was divided into two sections, including 20 days of bioaccumulation and sampling on 10th and 20th days, plus 10 days of depuration and sampling on 30th day. After the expiry of bioaccumulation and depuration phases, three fish each from the respectively marked experimental group, as well as control group, were sacrificed. The gill, intestine, liver, and muscles were selected as target organs. The wet tissue specimens were digested in nitric-perchloric acid (2:1; Ip et al. 2005). One gram of wet tissue was accurately weighed into 150-mL Erlenmeyer flasks, 10 mL of nitric acid (65 %) was added to each sample, and the samples were left overnight to be slowly digested (Hoshyari et al. 2012 and Nowrouzi et al. 2012a). Then, 5 ml of perchloric acid (70 %) was added to each sample. Digestions were performed on a water bath (Bain Marie) at 100 °C, for approximately 6 h or until the solutions were clear. After that, the digested samples were diluted with distilled water to 25 mL. Concentration of total Cu was measured using an ICP–OES, and total Cu concentration, in each organ, was presented as µg/g wet weight (ww). Moreover, the concentrations of copper during the exposure tests were determined and are shown in Fig. 3.
Statistical Analysis
Fish risk assessment was done by calculating bioconcentration factor (BCF) as the ratio of element uptake constant from water and elimination rate constant (Carter et al. 2014). The BCF determined on the basis of the rates of uptake and depuration. This equation is one of the options in the OECD 305 guideline for calculating the BCF; the other being the ratio of the concentration in fish and the concentration in water at steady state (OECD 1996).
where k 1 = first-order rate constant for uptake into fish (day−1), k 2 = first-order rate constant for depuration/elimination from fish (day−1), [C water] = concentration in water (mg L−1), [C fish] = concentration in fish (mg kg−1 wet weight).
Moreover, the magnitude of bioaccumulation (MB) is the amount of accumulation of in fish tissues in comparison with control group. The magnitude of bioaccumulation (MB) in fish can be calculated by the following equation:
where C fish is the total metal concentration in treatment group, C control is the total metal concentration in control group. Data analysis was carried out using the SPSS statistical package (version 16). The values are reported as mean ± SD. Statistical differences for the values of CuO NPs in the different groups and tissues of common carp were determined using two-way analysis of variance (ANOVA). Ethical considerations and animal rights in this paper were considered and the study was approved by Ethics Committee of the Kurdistan University of Medical Sciences (MUK.REC.1393.98).
Results
Characterizations of Nanoparticles
The characteristics of TiO2 nanoparticles were 20 m2 g−1 for specific surface area (SSA), 0.48 % for loss of weight in drying, 0.99 % loss of weight on ignition, 5.5–6.0 for pH, and 0.46 g cm−3 for bulk density. Also, other characteristics of CuO nanoparticles were 20 m2 g−1 for specific surface area (SSA), 6.4 g cm−3 for True density, and 0.79 g cm−3 for bulk density. As shown in Table 1, polydispersity index (PDI) and the hydrodynamic diameter of the TiO2 NPs, CuO NPs (2.5 mg L−1), CuO NPs (5.0 mg L−1), mixture of TiO2 NPs & CuO NPs (2.5 mg L−1) and TiO2 NPs & CuO NPs (5.0 mg L−1) determined by DLS method were 0.57 and 22.2 nm, 0.20 and 26.68 nm, 0.08 and 35.07 nm, 0.41 and 42.71 nm, and 0.42 and 61.53 nm, respectively. Results indicated that the presence of TiO2 NPs increased the hydrodynamic diameter, zeta potential, and polydispersity index of CuO NPs compared with the CuO NPs alone (Table 1). Results of zeta potential are summarized in Fig. 2, as shown the zeta potential of the TiO2 NPs, CuO NPs (2.5 mg L−1), CuO NPs (5.0 mg L−1), mixture of TiO2 NPs and CuO NPs (2.5 mg L−1), and TiO2 NPs & CuO NPs (5.0 mg L−1) determined by DLS method were −14.18, −5.07, −1.46, −21.5, and −4.66 (mV), respectively. Moreover, results of UV–Vis absorption peak (max) positions for different NPs after 30 min showed an absorption band in UV region with λ max around 315 nm for TiO2 NPs, 318–320 nm for CuO NPs and 335 nm for mixture of TiO2 NPs and CuO NPs (Table 2).
Bioaccumulation and Depuration of CuO NPs
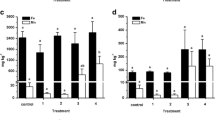
The nanoparticles are adsorption by fish gill and nano-like spots on the gill surface are visible (Fig. 4). This condition in some of different treatments was observed with increasing concentrations of nanoparticles and number of days. Amounts of Cu uptake and elimination in different groups of exposed fish to nanoparticles and control group are shown in Table 3. The order of Cu uptake in different parts of carp was liver > gill > muscle > intestine in both levels of CuO NPs alone; while, results showed that the Cu concentrations in the organs of carp in presence of TiO2 nanoparticles were decreased in the sequence liver > gill > intestine > muscle. In elimination period, the order of Cu depuration in different parts of carp was gill > intestine > muscle > liver. Uptake of Cu displayed a general tissues- and days-dependent pattern in all tissues. Results indicated from the beginning until day 10 of exposure, amount of Cu in all tissues showed an increasing trend and this increase continued until day 20 of exposure. In depuration period, from days 20 through 30, concentration of Cu in tissues was reduced, but it was still higher than the control treatment (except CuO NPs (2.5 mg L−1) in gill). Depuration of the CuO NPs also indicated considerable differences in terms of both the tissues and the days (p < 0.05; Table 3). Using two-way ANOVA statistical analysis the effect of different tissues, different groups and days in the accumulation of Cu were studied. Results indicated that the Cu concentrations differed significantly among tissues (p < 0.05), whereas Cu accumulation in the different groups was not significant (p > 0.05) after 10 and 20 days’ exposure to the NPs (Table 3).
Co-exposure Effects
The results of this study showed that in the uptake period, Cu concentrations in the mixture group (CuO NPs + TiO2 NPs) compared with the CuO NPs alone were higher (except CuO NPs (2.5 mg L−1) in gill), whereas in the elimination stage, the concentration of total Cu in CuO NPs and TiO2 NPs mixture was lower than the CuO NPs alone. The co-existence effect of TiO2 NPs and CuO NPs led to increase in uptake of total Cu in different tissues of common carp in the mixture of TiO2 NPs and CuO NPs compared with CuO NPs alone.
The levels of magnitude of bioaccumulation, bioconcentration factor (BCF), uptake rate (k 1), and eliminate rate (k 2) constants of total Cu in the selected tissues of common carp are illustrated in Table 4. The levels of magnitude of bioaccumulation in both groups of mixture of CuO NPs + TiO2 NPs (1/10th and 1/20th) compared with the control group were higher and the concentrations were 56 (in gill) and 59 (in intestine) times more than those of the control group. Eventually, the highest bioconcentration factor (BCF) and uptake rate of total Cu occurred in the liver followed by the gill, intestine, and muscle. The uptake of Cu in different tissues of carp in the joint presence of TiO2 NPs was increased (p < 0.05; Table 3). Moreover, the level of BCF and uptake rates of Cu in the groups of CuO NPs and TiO2 mixture were higher than those in the groups of CuO NPs alone (Table 4).
Discussion
Nanoparticle Characterization
The polydispersity indices of all samples were less than 0.4, which indicate the distribution moderate of the nanoparticles. The present results showed that the λ max for treatments containing CuO NPs changed from 318 nm toward higher wavelengths, plus all the λ max in the mixture of TiO2 NPs and CuO NPs were longer than those in the CuO NPs alone. Moreover, hydrodynamic diameter of CuO NPs increased in the presence of TiO2 NPs and could be responsible for absorption capacity of CuO NPs by TiO2 NPs. According to our results by DLS, hydrodynamic diameter of CuO NPs in the suspension mixture with TiO2 NPs was concentration-dependent, 2.5 and 5.0 mg L−1 of CuO NPs with mixture of TiO2 NPs at nominal concentration of 100 mg L−1 had an average hydrodynamic diameter of 42.7 and 61.5 nm, much larger than the size measured in CuO NPs alone, due to CuO aggregation with TiO2 NPs. For oxide NPs, electrostatic repulsion is an important stabilization mechanism, and the NPs tend to aggregate as the surface charge approaches neutral (Ghosh et al. 2008). The zeta potential of the particles was range −4–−21 mV in the Suspensions. According to findings of Yu et al. (2015) and Li et al. (2016), when the zeta potential is between −30 and 30 mV, the suspension is unlikely to be stable and is prone to aggregate. This is the reason behind the obvious agglomeration of TiO2 NPs and CuO NPs. Aeration used in the toxicity tests might decrease aggregation and sedimentation of NPs to a certain extent.
Bioaccumulations of CuO NPs
One of the important factors for the toxicity of nanoparticles is bioaccumulation potential and depuration by the tissues of fish. Bioaccumulation of nanoparticles reflects an increase in the concentration of nanoparticles in fish organs over time, compared with the nanoparticle’s concentration in the environment. The results of the present study indicated that the bioaccumulation of different groups of nanoparticles in the organs of carp was time- and concentration-dependent. Several studies have been carried out in relation to bioaccumulation and depuration of environmental pollutants by fish under controlled laboratory conditions. For example, Jang et al. (2014) and Consoer et al. (2014) reported the uptake and elimination kinetics of silver nanoparticles and perfluorooctanoate (PFOA) in the organs of common carp and in the rainbow trout, respectively. In a similar study, Mansouri et al. (2012) illustrated bioaccumulation and elimination of nickel in the organs of black fish (Capoeta fusca) under controlled condition. These studies have shown that the accumulation of chemicals in the tissues depends on the concentration of pollutants in water, duration of exposure, organs, and environmental factors such as salinity, pH, hardness, and temperature.
The waterborne chemicals can accumulate in fish from three potential sites including the gill epithelia, digestive tract (dietary exposure and drinking), and the skin (Pedlar and Klaverkamp 2002; Handy et al. 2008). According to our results, some light black blocks accumulated on the surface of common carp gill, which were suspected as mixture of CuO NPs and TiO2 NPs aggregates, show that these NPs might directly enter into the fish body through the injured epithelial cell membrane and induce the undesirable toxic effects. Besides, previous research work by Kamunde et al. (2001) and Handy et al. (2002) showed that copper is accumulated mainly in the liver, the gill, the gut, and least in the carcass. From our results, it is obvious that the livers and gills accumulated higher levels of CuO NPs in the presence of TiO2 NPs than the muscle of common carp, which reflects the affinity of these metal oxides to be up taken by these tissues and are known to be target organs for Cu (Farkas et al. 2003; Zhao et al. 2011). In a similar study, Shaw et al. (2012) indicated that exposure to Cu (20 g L−1) for 10 days caused measurable increases in the total Cu levels in the liver of rainbow trout, but with no detectable increases in the muscle, brain, or spleen. In addition, similar observations are reported in the study of Al-Bairuty et al. (2016) at the Cu accumulation with the liver being the only internal organ to show an elevated tissue Cu accumulation by the end of the experiment. For teleost fish in freshwater, Cu is absorbed across the gill and transferred to the liver as the central compartment for Cu homeostasis (Grosell et al. 1997, 1998). The liver regulates the biliary excretion of Cu and therefore only limited amounts of Cu are passed on to other internal organs (Kamunde and Wood 2004). Because the liver is a major producer of metal binding proteins, the induction of low molecular weight metal binding proteins, such as metallothionein, can be closely related to the metal exposures and this metal taken up from the environment is possibly detoxified by its binding on to proteins (Palaniappan and Karthikeyan 2009); the results in the higher concentration of metal mixtures in the liver.
Gills are in direct contact with aquatic environment and are physiologically complex and vulnerable structures, making them target organs for waterborne toxicants (Nowrouzi et al. 2012b; Baramaki et al. 2012). Moreover, toxicity of waterborne Cu is well correlated with gill total Cu burden (Kamunde and Wood 2004). According to our results, next to liver, the gills accumulated the highest levels of CuO NPs concentrations. Moreover, Table 3 shows, CuO NPs concentrations in the presence of TiO2 NPs in the gills of common carp increased 56 times [TiO2 NPs + CuO NPs (LC)] and 53 times [TiO2 NPs + CuO NPs (HC)], after chronic exposure, respectively. Wang et al. (2015) studied 25 days exposure effect of Cu-NPs and CuSO4 on juvenile Epinephelus coioides. They found high concentration of copper in gills and suggested gill as a major organ to uptake Cu. They also reported that the accumulation of CuSO4 in the gill tissues was higher the accumulation of Cu-NPs. Zhao et al. (2011) found higher concentration of copper in the gill of common carp treated with CuO NPs for 30 days than those exposed to CuO bulk particles and soluble Cu++. Subsequent work by Griffitt et al. (2007) illustrated that the gill is the primary target of toxicity affected by Cu-NPs, although this research in the same study reported that the nano-Cu to be more acute toxic to juvenile zebrafish than dissolved Cu (Griffitt et al. 2008). In this experiment, low CuO NPs accumulation was observed in the muscle of common carp compared with other tissues, but it increased at CuO NPs in the presence of TiO2 NPs than CuO NPs and TiO2 NPs alone. Similarly, several previous studies illustrated lower Cu accumulation in the muscle of fish compared with other tissues (Kim et al. 2011; Pourkhabbaz and Mohseni 2013; Maleki et al. 2015). Lower concentrations of NPs in this tissue can be related to the lower metabolic activity of muscle tissue compared with the other tissues, such as gills, kidney, and liver (Majnoni et al. 2013; Squadrone et al. 2013). Furthermore, Kim et al. (2011) claim that the bioaccumulation of metals in the muscle tissue becomes significant only when the maximum storage capacity of the liver has been reached.
Depuration of CuO NPs
Like accumulation, elimination of metals from the fish tissues depends on several factors, such exposure time, concentration, environmental conditions, and interacting agents, as well as the organ concerned (Kim et al. 2006; Mansouri et al. 2012, 2013a). Metal elimination studies are important for health protection, allowing the determination of the self-cleansing ability of contaminated organisms (Kim et al. 2006). The route of Cu elimination from fish tissues are generally through urinary, branchial, biliary, and fecal excretion (Kim et al. 2011). However, metal accumulation is more rapid than metal elimination because of the presence of metal binding proteins in tissue (Kargin and Cogun 1999). According to our results, there was no significant difference between the Residual Cu concentrations in most tissues in recovery period with control group and the concentration of Cu in the tissues of common carp has been returned to the near primary levels in clean water, suggesting that longer time was required for complete elimination. So, recovery potential of fish tissues to exposure to environmental pollutants can be considered as one of the important factors in relation to chemical contaminations. In similar study, Ates et al. (2016) reported that the gill, intestine, and kidney of tilapia exposed to iron oxide nanoparticles were cleared out of the accumulated particles within 30-day elimination period. In another study, Zhang et al. (2015) illustrated that elimination of Fe2O3 and Fe3O4 NPs followed a first-order decay from exposed zebrafish, and all particles were eliminated within 24 days post-exposure. In the present experiment, gill and intestine tissues showed the fastest elimination rates compared with other tissues, and next to gills, intestine eliminated the highest level of different groups of nanoparticles. Also, results showed 56–59 times increase of accumulation in the intestinal tissue of common carp in mixture of TiO2 NPs and CuO NPs than the control group. In a similar study, Mansouri et al. (2013b) reported that the elimination rates in the gills of Capoeta fusca were higher than those of the liver after 15 days of depuration. Moreover, studies by Kalay and Canli (2000) and Mansouri et al. (2012) indicated that the gill tissue of freshwater fish is a more effective organ for metal elimination than either the liver or muscle. On the other hand, the cause of low level of CuO NPs excretion in the liver may be due to the direct accumulation of this nanoparticle via gills and intestine, and subsequently nanoparticles were rapidly transferred, distributed and accumulated, resulting in the high concentration of nanoparticles in liver for detoxification. However, Kim et al. (2011) illustrated that the Cu elimination rates in intestine was more than those in the other tissues, that could potentially result from the physiological role of this tissue in essential element homeostasis and in protein metabolism. In another study, Gündogdu et al. (2011) reported that the highest and lowest elimination of copper in different tissues of rainbow trout was in intestine and liver tissues, respectively. They also stated that the elimination of copper in different groups in the gill and muscle tissues was higher than that of the liver tissue.
Co-exposure Effects of NPs
Taken together, toxicities of TiO2 NPs alone have been well documented. However, knowledge of the combined effects of TiO2 NPs with other chemicals is limited. Existing evidence suggests that TiO2 NPs can absorb metal ions, including Pb, As, Cu, and Cd, in the solution (Liu et al. 2013). Meanwhile, interaction of TiO2 NPs and metal compounds also may result in the increased toxicity demonstrated by increased oxidative stress to cells and decreased LC50 in aquatic organisms. The results of this study revealed that the presence of TiO2 NPs increases the uptake of CuO NPs on common carp and the effect of TiO2 NPs and CuO co-existence leads to an increase in the bioaccumulation of CuO NPs in the tissues of common carp. The BCF and magnitude of bioaccumulation in the co-existing CuO NPs and TiO2 NPs are more than individual states, though this increase was low. Increasing the accumulation of CuO NPs in the presence of TiO2 NPs could be due to the adsorbed CuO NPs onto the TiO2 NPs and ingested and accumulated in the tissues of common carp, thereby causing toxic injury.
The results of Liu et al. (2013, 2015) indicated that TiO2 nanoparticles (NPs) increased copper bioaccumulation by 9.8 % in the tissues of Daphnia magna and reported that the co-existence of copper and TiO2 is more dangerous than copper alone in aquatic environments. Nano-TiO2 easily attached on the surface of the tissue cells and the adsorbed Cu2+ had direct contact with the fish. Neutral and unoccupied surface sites of TiO2 as Ti-(OH)(OH2), having both a surface hydroxyl and chemisorbed water (Stone et al. 1993), whereas TiOH−3 and TiOH species were also included (Kim and Chung 2001; Barakat 2005). It was obvious from their data that the TiO2 NPs combined with Cu2+ via the formation of O-Cu bonds, and these conjugated forms were expressed as Ti-(OH2)-OCu+, TiOCu−2 and TiOCu+ (Yang and Davis 1999; Kim and Chung 2001; Barakat 2005). Cu2+ induced cellular toxicity can be explained by the participation of Cu2+ in the formation of ROS. Cu2+ can be reduced to Cu+ in the presence of superoxide (\({\text{O}}_{2}^{ - }\)·), and Cu+ is capable of catalyzing the formation of hydroxyl radical (OH·) from hydrogen peroxide (H2O2; Gaetke and Chow 2003). Therefore, the nano-TiO2 may compete for free copper ions with sulfhydryl groups, causing the increased Cu in the tissues of common carp. However, this supposition requires further studies. Chen et al. (2007) shown that the biotoxicity of Cu2+ was correlated with the interactions between its adsorption and coordination with co-substrates. Fan et al. (2011) observed that the presence of TiO2 NPs at a level of 2 mg L−1 (at a concentration generally considered to be safe in the environment) remarkably increased the toxicity of Cu by increasing bioaccumulation of Cu in order to cause death of Daphnia magna. In addition, they found that Cu was adsorbed on to the TiO2 NPs when ingested and was accumulated in the animals, thereby causing an increase in toxic effects. In a similar study, Zhang et al. (2007) illustrated that the presence of natural sediment particles did not have significant influence on the accumulation of Cd in the tissues of common carp during the 25 days of exposure. However, the presence of TiO2 nanoparticles greatly enhanced the accumulation of Cd in the tissues of common carp. Moreover, in another study, Sun et al. (2007) reported that the arsenic accumulation in the stomach, intestine, and gill tissues of common carp was enhanced by the presence of TiO2 nanoparticles.
Conclusions
Differences were observed in the bioaccumulation of CuO NPs in the gill, liver, intestine, and muscle of common carp exposed to co-existence of TiO2 NPs and CuO NPs compared with the single nanoparticles. It can be reported that the liver and gill are the target organs for accumulation and elimination of CuO NPs, respectively. Also, the accumulation and elimination of this nanoparticle in common carp depend on the organ, concentration, and time. It appears that the joint presence of TiO2 NPs can potentially increase the uptake of CuO NPs in the tissues of carp. However, to assess the behavior mechanisms of nanoparticles in presence of pollutants, further studies are encouraged.
References
Aitken RJ, Chaudhry MQ, Boxall ABA, Hull M (2006) Manufacture and use of nanomaterials: current status in the UK and global trends. Occup Med 56:300–306 Oxford
Al-Bairuty GA, Boyle D, Henry TB, Handy RD (2016) Sublethal effects of copper sulphate compared to copper nanoparticles in rainbow trout (Oncorhynchus mykiss) at low pH: physiology and metal accumulation. Aquat Toxicol 174:188–198
Aruoja V, Dubourguier HC, Kasemets K, Kahru A (2009) Toxicity of nanoparticles of CuO, ZnO and TiO2 to microalgae Pseudokirchneriella subcapitata. Sci Total Environ 407:1461–1468
Ates M, Demir V, Arslanc Z, Kaya H, Yılmaz S, Camas M (2016) Chronic exposure of tilapia (Oreochromis niloticus) to iron oxide nanoparticles: effects of particle morphology on accumulation, elimination, hematology and immune responses. Aquat Toxicol 177:22–32
Aydın R, Köprücü K (2005) Acute toxicity of diazinon on the common carp (Cyprinus carpio L) embryos and larvae. Pestic Biochem Physiol 82:220–225
Baek YW, An YJ (2011) Microbial toxicity of metal oxide nanoparticles (CuO, NiO, ZnO, and Sb2O3) to Escherichia coli, Bacillus subtilis and Streptococcus aureus. Sci Total Environ 409:1603–1608
Barakat MA (2005) Adsorption behavior of copper and cyanide ions at TiO2-solution interface. J Colloid Interface Sci 291:e345–e352
Baramaki R, Ebrahimpour M, Mansouri B, Rezaei MR, Babaei H (2012) Contamination of metals in tissues of Ctenopharyngodon idella and Perca fluviatilis, from Anzali Wetland, Iran. Bull Environ Contamin Toxicol 89:831–835
Carter LJ, Ashauer R, Ryan JJ, Boxall ABA (2014) Minimised bioconcentration tests: a useful tool for assessing chemical uptake into terrestrial and aquatic invertebrates. Environ Sci Technol 48:13497–13503
Chang Y-N, Zhang M, Xia L, Zhang J, Xing G (2012) The toxic effects and mechanisms of CuO and ZnO nanoparticles. Materials 5:2850–2871
Chen N, Hao JS, Wang Y, Su CY, Wu BF (2007) Single and binary-combined acute toxicity of heavy metal ion Hg2+, Cu2+, Cd2+, Ag+, Zn2+ and Pb2+ to Hydra. J Biol 24(3): 32–35
Chen D, Zhang D, Yu JC, Chan KM (2011) Effects of Cu2O nanoparticle and CuCl2 on zebrafish larvae and a liver cell-line. Aquat Toxicol 105:344–354
Consoer DM, Hoffman AD, Fitzsimmons PN, Kosian PA, Nichols JW (2014) Toxicokinetics of perfluorooctanoate (PFOA) in rainbow trout (Oncorhynchus mykiss). Aquat Toxicol 156:65–73
Ebrahimnia-Bajestan E, Niazmand H, Duangthongsuk W, Wongwises S (2011) Numerical investigation of effective parameters in convective heat transfer of nanofluids flowing under a laminar flow regime. Int J Heat Mass Trans 54:4376–4388
Fan W, Cui M, Liu H, Wang C, Shi Z et al (2011) NanoTiO2 enhances the toxicity of copper in natural water to Daphnia magna. Environ Pollut 159:729–734
Farkas A, Salanki J, Specziar A (2003) Age- and size-specific patterns of heavy metals in the organs of freshwater fish Abramis brama L. populating a low contaminated site. Water Res 37:959–964
Gaetke LM, Chow CK (2003) Copper toxicity, oxidative stress, and antioxidant nutrients. Toxicology 189:147–163
Ghosh S, Mashayekhi H, Pan B, Bhowmik P, Xing B (2008) Colloidal behavior of aluminum oxide nanoparticles as affected by pH and natural organic matter. Langmuir 24:12385–12391
Griffitt RJ, Weil R, Hyndman KA, Denslow ND, Powers K, Taylor D, Barber AS (2007) Exposure to copper nanoparticles causes gill injury and acute lethality in zebrafish (Danio rerio). Environ Sci Technol 41:8178–8186
Griffitt RJ, Luo J, Gao J, Bonzongo JC, Barber DS (2008) Effects of particle composition and species on toxicity of metallic nanomaterials in aquatic organisms. Environ Toxicol Chem 27:1972–1978
Grosell MH, Hogstrand C, Wood CM (1997) Cu uptake and turnover in both acclimated and non-acclimated rainbow trout (Oncorhynchus mykiss). Aquat Toxicol 38:257–276
Grosell M, Hansen HJM, Rosenkilde P, Hansen HJ (1998) Cu uptake, metabolism and elimination in fed and starved European eels (Anguilla anguilla) during adaptation to water-borne Cu exposure. Comp Biochem Physiol C 120:295–305
Gul A, Benli ACK, Ayhan A, Memmi BK, Selvi M, Sepici-Dincel A et al (2012) Sublethal propoxur toxicity to juvenile common carp (Cyprinus carpio L., 1758): biochemical, hematological, histopathology, and Genotoxicity effects. Environ Toxicol Chem 31:2085–2095
Gündogdu A, Harmantepe FB, Karsli Z, Dogan G (2011) Elimination of copper in tissues and organs of rainbow trout (Oncorhynchus mykiss, Walbaum, 1792) following dietary exposure. Ital J Anim Sci 10:1–10
Handy RD, Eddy FB, Baines H (2002) Sodium-dependent copper uptake across epithelia: a review of rationale with experimental evidence from gill and intestine. Biochim Biophys Acta Biomembr 1566:104–115
Handy RD, Kammer FVD, Lead JR, Hassellov M, Owen R, Crane M (2008) The ecotoxicology and chemistry of manufactured nanoparticles. Ecotoxicology 17:287–314
Hedayati A, Vajargah MF, Mohamadi Yalsuyi A, Abarghoei S, Hajiahmadyan M (2014) Acute toxicity test of pesticide abamectin on common carp (Cyprinus carpio). J Coast Life Med 2:841–844
Heinlaan M, Ivask A, Blinova I, Dubourguier HC, Kahru A (2008) Toxicity of nanosized and bulk ZnO, CuO and TiO2 to bacteria Vibrio fischeri and crustaceans Daphnia magna and Thamnocephalus platyurus. Chemosphere 71:1308–1316
Hoshyari E, Pourkhabbaz A, Mansouri B (2012) Contaminations of metal in tissues of Siberian gull (Larus heuglini): gender, age, and tissue differences. Bull Environ Contamin Toxicol 89:102–106
Hu W, Culloty S, Darmody G, Lynch S, Davenport J, Ramirez-Garcia S et al (2014) Toxicity of copper oxide nanoparticles in the blue mussel, Mytilus edulis: a redox proteomic investigation. Chemosphere 108:289–299
Ip CM, Li XD, Zhang G, Wong CSC, Zhang WL (2005) Heavy metal and Pb isotopic compositions of aquatic organisms in the Pearl River Estuary, South China. Environ Pollut 138:494–504
Jang MH, Kim WK, Lee SK, Henry TB, Park JW (2014) Uptake, tissue distribution, and depuration of total silver in common carp (Cyprinus carpio) after aqueous exposure to silver nanoparticles. Environ Sci Tech 14:11568–11574
Kalay M, Canli M (2000) Elimination of essential (Cu, Zn) and non-essential (Cd, Pb) metals from tissues of a freshwater fish Tilapia zillii. Turk J Zool 24:429–436
Kamunde CN, Wood CM (2004) Environmental chemistry, physiology homeostasis, toxicology, and environmental regulation of copper, an essential element in freshwater fish. Aust J Ecotoxicol 10:1–20
Kamunde CN, Grosell M, Lott JNA, Wood CM (2001) Copper metabolism and gut morphology in rainbow trout (Oncorhynchus mykiss) during chronic sublethal dietary copper exposure. Can J Fish Aquat Sci 58:293–305
Kargin F, Cogun HY (1999) Metal interactions during accumulation and elimination of zinc and cadmium in tissues of the fish water fish, Tilapia nilonitica. Bull Environ Contam Toxicol 63:511–519
Kim MS, Chung JG (2001) A study on the adsorption characteristics of orthophosphates on rutile-type titanium dioxide in aqueous solutions. J Colloid Interface Sci 233:e31–e37
Kim SG, Eom KH, Kim SS, Jin HG, Kang JC (2006) Kinetics of Cd accumulation and elimination in tissues of juvenile rockfish (Sebastes schlegeli) exposed to dietary Cd. Mar Environ Res 62:327–340
Kim SG, Jang SW, Lee YJ, Kim SS (2011) Cu accumulation and elimination in the tissues of the olive flounder Paralichthys olivaceus. Fish Aquat Sci 14:210–217
Lee B, Duong CN, Cho J, Lee J, Kim K, Seo Y et al (2012) Toxicity of citrate-capped silver nanoparticles in common carp (Cyprinus carpio). J Biotechnol 2012:1–14
Li Y, Liang J, Tao Z, Chen J (2007) CuO particles and plates: synthesis and gas-sensor application. Mater Res Bull 43:2380–2385
Li X, Zhou S, Fan W (2016) Effect of Nano-Al2O3 on the toxicity and oxidative stress of copper towards Scenedesmus obliquus. Int J Environ Res Public Health 13:575–580
Liu K, Lin X, Zhao J (2013) Toxic effects of the interaction of titanium dioxide nanoparticles with chemicals or physical factors. Int J Nanomed 8:2509–2520
Liu L, Fan W, Lu H, Xiao W (2015) Effects of the interaction between TiO2 with different percentages of exposed 001 facets and Cu2+ on biotoxicity in Daphnia magna. Sci Rep. doi:10.1038/srep11121
Linhua H, Zhenyu W, Baoshan X (2009) Effect of sub-acute exposure to TiO2 nanoparticles on oxidative stress and histopathological changes in juvenile carp (Cyprinus carpio). J Environ Sci 21:1459–1466
Majnoni F, Mansouri B, Rezaei MR, Hamidian AH (2013) Contaminations of metals in tissues of Common crap, Cyprinus carpio and Silver crap, Hypophthalmichthys molitrix from Zarivar wetland, western Iran. Arch Polish Fish 21:11–18
Maleki A, Azadi N, Mansouri B, Majnoni F, Rezaei Z, Gharibi F (2015) Health risk assessment of trace elements in two fish species from the Sanandaj Gheshlagh Reservoir, Iran. Toxicol Environ Health Sci 7:43–49
Mansouri B, Ebrahimpour M, Babaei H (2012) Bioaccumulation and elimination of nickel in the organs of black fish (Capoeta fusca). Toxicol Ind Health 28:361–368
Mansouri B, Baramaki R, Pourkhabbaz A, Zareh M, Hamidian AH (2013a) Bioaccumulation and depuration of copper in the kidney and liver of freshwater fish Capoeta fusca. Iran J Toxicol 7:808–814
Mansouri B, Ebrahimpour M, Pourkhabbaz A, Babaei H, Hamidian AH (2013b) Bioaccumulation and elimination rate of cobalt in Capoeta fusca under controlled conditions. Chem Spec Bioavail 25:52–56
Martin R, Wilson LF, Peter GB, Gary RH, Jill S, Richard JS, Vicki S (2007) Nanoparticle interactions with zinc and iron: implications for toxicology and inflammation. Toxicol Appl Pharm 225:80–89
Nowrouzi M, Mansouri B, Hamidian AH, Ebrahimi T, Kardoni F (2012a) Comparison of the levels of metals in feathers of three bird species from southern Iran. Bull Environ Contam Toxicol 89:1082–1086
Nowrouzi M, Mansouri B, Hamidian AH, Zarei I, Mansouri A (2012b) Metal contents in tissues of two fish species from Qeshm Island, Iran. Bull Environ Contam Toxicol 89:1004–1008
Organisation for Economic Co-operation and Development (OECD) (1993) OECD guidelines for testing of chemicals. OECD, Organization for Economic, Paris
Organisation for Economic Co-operation and Development (OECD) (1996) OECD guidelines for testing of chemicals. Proposal for updating guideline 305. Bioconcentration: Flow-through fish test. OECD, Paris
Palaniappan PLRM, Karthikeyan S (2009) Bioaccumulation and depuration of chromium in the selected organs and whole body tissues of freshwater fish Cirrhinus mrigala individually and in binary solutions with nickel. J Environ Sci 21:229–236
Pedlar RM, Klaverkamp JF (2002) Accumulation and distribution of dietary arsenic in lake whitefish (Coregonus clupeaformis). Aquat Toxicol 57:153–166
Pourkhabbaz A, Mohseni Z (2013) The study of bioaccumulation and elimination of copper in blackfish (Capoeta fusca) organs from the east Qanats of Iran. Vet J 4:29–37 In Persian
Sau TK, Rogach AL, Jackel F, Klar TA, Feldmann J (2010) Properties and applications of colloidal nonspherical noble metal nanoparticles. Adv Mater 22:1805–1825
Shahmoradi B, Maleki A, Byrappa K (2011) Photocatalytic degradation of Amaranth and Brilliant Blue FCF dyes using in situ modified tungsten doped TiO2 hybrid nanoparticles. Catal Sci Technol 1:1216–1223
Shaw BJ, Al-Bairuty G, Handy RD (2012) Effects of waterborne copper nanoparticles and copper sulphate on rainbow trout, (Oncorhynchus mykiss): physiology and accumulation. Aquat Toxicol 116–117:90–101
Squadrone S, Prearo M, Brizio P, Gavinelli S, Pellegrino M, Scanzia T, Guarise S, Bendetto A, Abete MC (2013) Heavy metals distribution in muscle, liver, kidney and gills of European catfih (Silurus glanis) from Italian Rivers. Chemosphere 90:358–365
Stone AT, Torrents A, Smolen J, Vasudevan D, Hadley J (1993) Adsorption of organic compounds possessing ligand donor groups at the oxide/water interface. Environ Sci Tech 27:e895–e909
Sun H, Zhang X, Niu Q, Chen Y, Crittenden JC (2007) Enhanced accumulation of arsenate in carp in the presence of titanium dioxide nanoparticles. Water Air Soil Pollut 178:245–254
Villarreal FD, Das GK, Abid A, Kennedy IM, Kultz D (2014) Sublethal effects of CuO nanoparticles on Mozambique Tilapia (Oreochromis mossambicus) are modulated by environmental salinity. PLoS One 9:88723–88737
Wang T, Long X, Liu Z, Cheng Y, Yan S (2015) Effect of copper nanoparticles and copper sulphate on oxidation stress, cell apoptosis and immune responses in the intestines of juvenile Epinephelus coioides. Fish Shellfish Immunol 44:674–682
Wiesner MR, Lowry GV, Alvarez P, Dionysiou D, Biswas P (2006) Assessing the risks of manufactured nanomaterials. Environ Sci Technol 40:4336–4345
Yang JK, Davis AP (1999) Competitive adsorption of Cu (II)-EDTA and Cd(II)-EDTA onto TiO2. J Colloid Interface Sci 216:e77–e85
Yu R, Fang X, Somasundaran P, Chandran K (2015) Short-term effects of TiO2, CeO2, and Zno nanoparticles on metabolic activities and gene expression of Nitrosomonas europaea. Chemosphere 128:207–215
Zhang WX, Masciangioli T (2003) Environmental technologies at the nanoscales. Environ Sci Technol 37:102–108
Zhang X, Sun H, Zhang Z, Niu Q, Chen Y, Crittenden JC (2007) Enhanced bioaccumulation of cadmium in carp in the presence of titanium dioxide nanoparticles. Chemosphere 67:160–166
Zhang W, Miao Y, Lin K, Chen L, Dong Q, Huang C (2013) Toxic effects of copper ion in zebrafish in the joint presence of CdTe QDs. Environ Pollut 176:158–164
Zhang Y, Zhu L, Zhou Y, Chen J (2015) Accumulation and elimination of iron oxide nanomaterials in zebrafish (Danio rerio) upon chronic aqueous exposure. J Environ Sci 30:223–230
Zhao J, Wang Z, Liu X, Xie X, Zhang K, Xing B (2011) Distribution of CuO nanoparticles in juvenile carp (Cyprinus carpio) and their potential toxicity. J Hazard Mater 197:304–310
Zou X, Shi J, Zhang, H (2014) Coexistence of silver and titanium dioxide nanoparticles: enhancing or reducing environmental risks? Aquat Toxicol 154:168–175
Acknowledgments
The data provided in this study was taken from first author Ph.D. Dissertation project. This work was supported by the Kurdistan University of Medical Sciences under Grant number 14/33858. The contribution of the Environmental Health Research Center of Kurdistan University of Medical Sciences is also sincerely appreciated.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mansouri, B., Maleki, A., Johari, S.A. et al. Copper Bioaccumulation and Depuration in Common Carp (Cyprinus carpio) Following Co-exposure to TiO2 and CuO Nanoparticles. Arch Environ Contam Toxicol 71, 541–552 (2016). https://doi.org/10.1007/s00244-016-0313-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00244-016-0313-5